Eukaryotic Translation Initiation Factor 4A Down-Regulation Mediates Interleukin-24-Induced Apoptosis through Inhibition of Translation
Abstract
:1. Introduction
2. Results
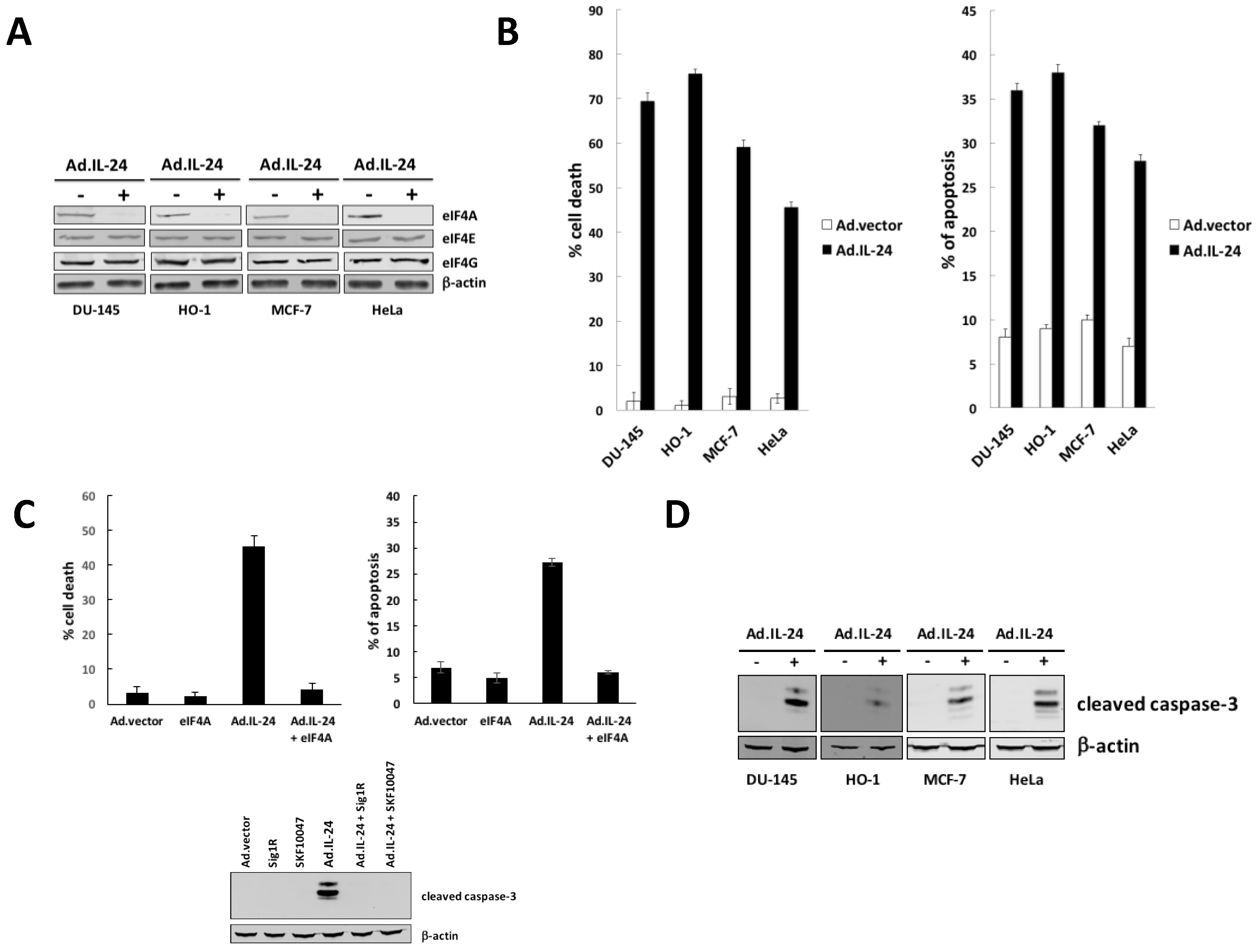
2.1. IL-24-Dependent Inhibition of eIF4A Is Necessary to Mediate Apoptosis
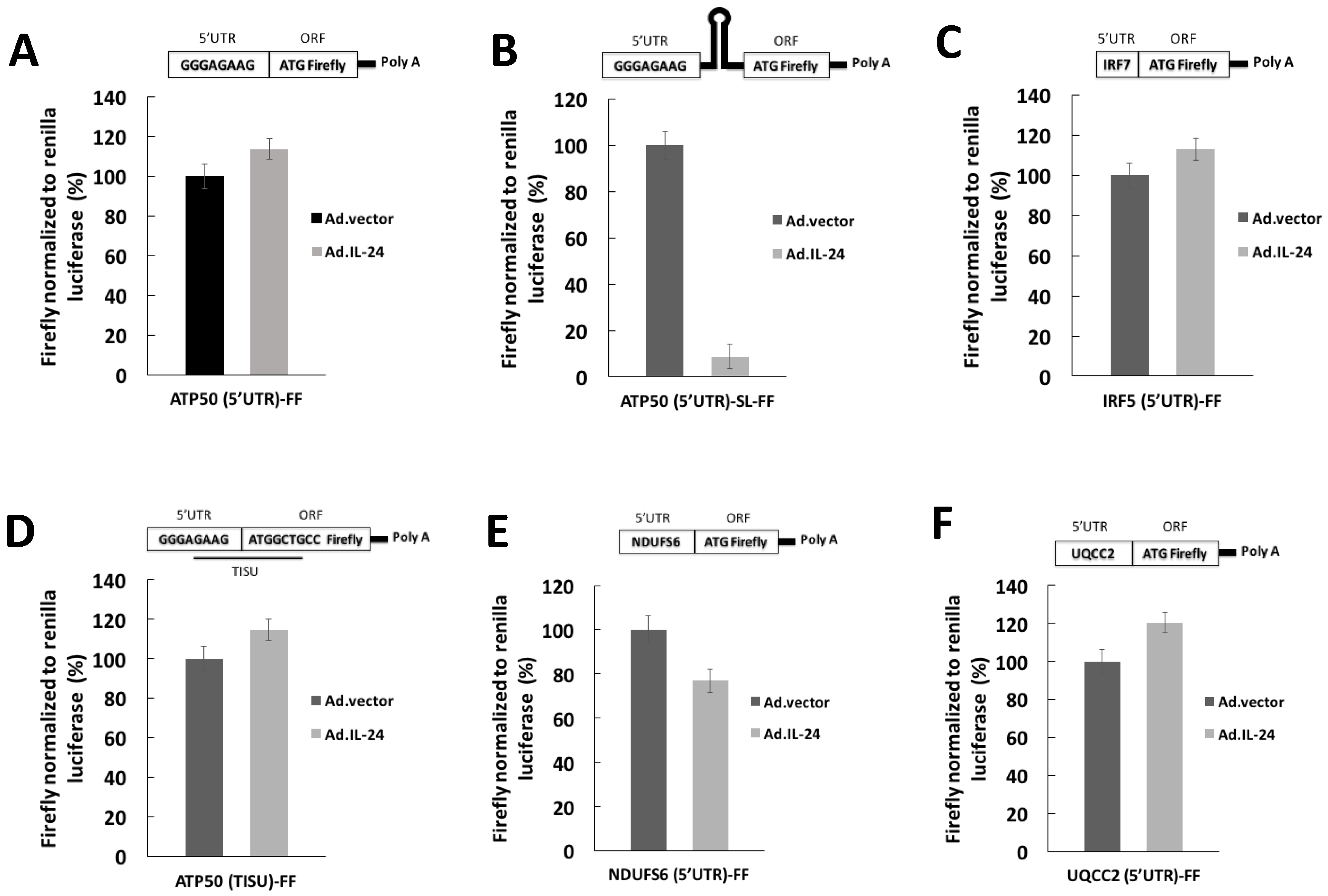
2.2. IL-24 Appears to Affects Translation of mRNAs with Long, but Not Short, 5′UTRs
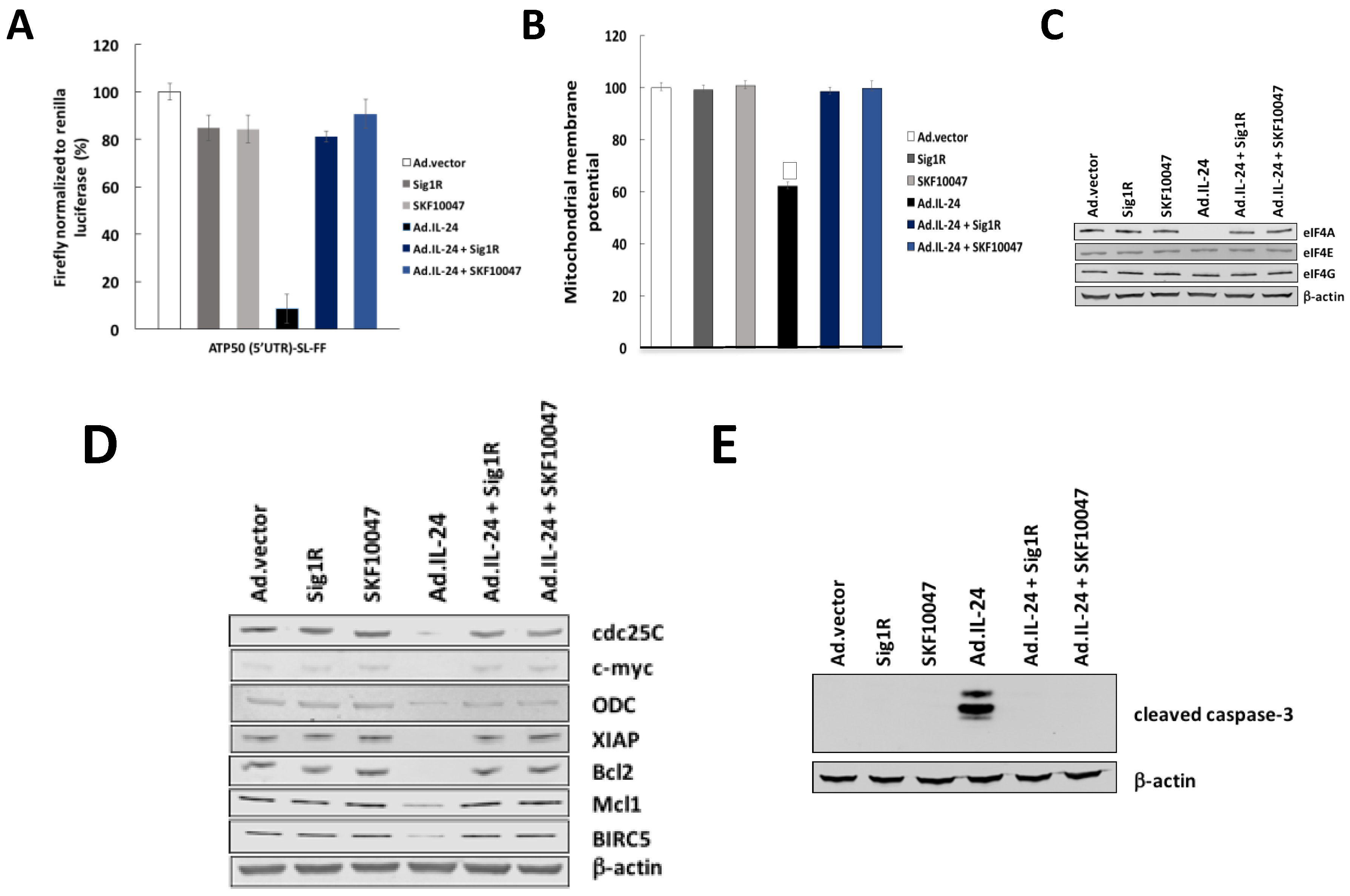
2.3. IL-24 Appears to Reduces Translation of mRNAs Harboring Structured 5′UTRs
2.4. IL-24 Treatment Leads to Mitochondrial Dysfunction
2.5. IL-24-Dependent Sigma 1 Receptor (Sig1R) Mediate eIF4A Down-Regulation, Translation of mRNAs Harboring Structured 5′UTRs and Mitochondrial Dysfunction
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Reagents
4.2. Western Blot Analysis
4.3. Generation of Luciferase Reporters and Luciferase Assays
4.4. Annexin V Binding Assay
4.5. RT-PCR
4.6. Mitochondrial Membrane Potential
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Acknowledgments
Grant Support
Conflicts of Interest
References
- Hinnebusch, A.G.; Ivanov, I.P.; Sonenberg, N. Translational control by 5′-untranslated regions of eukaryotic mRNAs. Science 2016, 352, 1413–1416. [Google Scholar] [CrossRef] [PubMed]
- Ruggero, D. Translational control in cancer etiology. Cold Spring Harb. Perspect. Biol. 2013, 5. [Google Scholar] [CrossRef] [PubMed]
- Silvera, D.; Formenti, S.C.; Schneider, R.J. Translational control in cancer. Nat. Rev. Cancer 2010, 10, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Hinnebusch, A.G. The scanning mechanism of eukaryotic translation initiation. Annu. Rev. Biochem. 2014, 83, 779–812. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.; Hershey, J.W. Identification and quantitation of levels of protein synthesis initiation factors in crude HeLa cell lysates by two-dimensional polyacrylamide gel electrophoresis. J. Biol. Chem. 1983, 258, 7228–7235. [Google Scholar] [PubMed]
- Galicia-Vazquez, G.; Cencic, R.; Robert, F.; Agenor, A.Q.; Pelletier, J. A cellular response linking eIF4AI activity to eIF4AII transcription. RNA 2012, 18, 1373–1384. [Google Scholar] [CrossRef] [PubMed]
- Lomakin, I.B.; Hellen, C.U.; Pestova, T.V. Physical association of eukaryotic initiation factor 4G (eIF4G) with eIF4A strongly enhances binding of eIF4G to the internal ribosomal entry site of encephalomyocarditis virus and is required for internal initiation of translation. Mol. Cell. Biol. 2000, 20, 6019–6029. [Google Scholar] [CrossRef] [PubMed]
- Sonenberg, N. ATP/Mg2+-dependent cross-linking of cap binding proteins to the 5′ end of eukaryotic mRNA. Nucleic Acids Res. 1981, 9, 1643–1656. [Google Scholar] [CrossRef] [PubMed]
- Malka-Mahieu, H.; Newman, M.; Desaubry, L.; Robert, C.; Vagner, S. Molecular Pathways: The eIF4F Translation Initiation Complex-New Opportunities for Cancer Treatment. Clin. Cancer Res. 2017, 23, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Persaud, L.; Zhong, X.; Alvarado, G.; Do, W.; Dejoie, J.; Zybtseva, A.; Aktas, B.H.; Sauane, M. eIF2alpha phosphorylation mediates IL24-induced apoptosis through inhibition of translation. Mol. Cancer Res. 2017, 15, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, R.; Ioannides, C.G.; Roth, J.A.; Chada, S. Adenovirus-mediated interleukin (IL)-24 immunotherapy for cancer. Methods Mol. Biol. 2010, 651, 241–270. [Google Scholar] [PubMed]
- Cunningham, C.C.; Chada, S.; Merritt, J.A.; Tong, A.; Senzer, N.; Zhang, Y.; Mhashilkar, A.; Parker, K.; Vukelja, S.; Richards, D.; et al. Clinical and local biological effects of an intratumoral injection of MDA-7 (IL24; INGN 241) in patients with advanced carcinoma: A phase I study. Mol. Ther. 2005, 11, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Tong, A.W.; Nemunaitis, J.; Su, D.; Zhang, Y.; Cunningham, C.; Senzer, N.; Netto, G.; Rich, D.; Mhashilkar, A.; Parker, K.; et al. Intratumoral injection of INGN 241, a nonreplicating adenovector expressing the melanoma-differentiation associated gene-7 (MDA-7/IL24): Biologic outcome in advanced cancer patients. Mol. Ther. 2005, 11, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Menezes, M.E.; Bhoopathi, P.; Pradhan, A.K.; Emdad, L.; Das, S.K.; Guo, C.; Wang, X.Y.; Sarkar, D.; Fisher, P.B. Role of MDA-7/IL-24 a Multifunction Protein in Human Diseases. Adv. Cancer Res. 2018, 138, 143–182. [Google Scholar] [PubMed]
- Persaud, L.; De Jesus, D.; Brannigan, O.; Richiez-Paredes, M.; Huaman, J.; Alvarado, G.; Riker, L.; Mendez, G.; Dejoie, J.; Sauane, M. Mechanism of Action and Applications of Interleukin 24 in Immunotherapy. Int. J. Mol. Sci. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Sauane, M.; Su, Z.Z.; Gupta, P.; Lebedeva, I.V.; Dent, P.; Sarkar, D.; Fisher, P.B. Autocrine regulation of mda-7/IL-24 mediates cancer-specific apoptosis. Proc. Natl. Acad. Sci. USA 2008, 105, 9763–9768. [Google Scholar] [CrossRef] [PubMed]
- Sauane, M.; Su, Z.Z.; Dash, R.; Liu, X.; Norris, J.S.; Sarkar, D.; Lee, S.G.; Allegood, J.C.; Dent, P.; Spiegel, S.; et al. Ceramide plays a prominent role in MDA-7/IL-24-induced cancer-specific apoptosis. J. Cell. Physiol. 2010, 222, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Do, W.; Herrera, C.; Mighty, J.; Shumskaya, M.; Redenti, S.M.; Sauane, M. Sigma 1 Receptor plays a prominent role in IL-24-induced cancer-specific apoptosis. Biochem. Biophys. Res. Commun. 2013, 439, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Bina, S.; Hosseini, S.Y.; Shenavar, F.; Hosseini, E.; Mortazavi, M. The effect of RGD/NGR peptide modification of melanoma differentiation-associated gene-7/interleukin-24 on its receptor attachment, an in silico analysis. Cancer Biother. Radiopharm. 2017, 32, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Lin, Z.; Cao, Y.; Chen, Y.; Xu, Z.; Qin, Z. Targeting EIF4F complex in non-small cell lung cancer cells. Oncotarget 2017, 8, 55731–55735. [Google Scholar] [CrossRef] [PubMed]
- Heerma van Voss, M.R.; van Diest, P.J.; Raman, V. Targeting RNA helicases in cancer: The translation trap. Biochim. Biophys. Acta 2017, 1868, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Gandin, V.; Masvidal, L.; Hulea, L.; Gravel, S.P.; Cargnello, M.; McLaughlan, S.; Cai, Y.; Balanathan, P.; Morita, M.; Rajakumar, A.; et al. nanoCAGE reveals 5′ UTR features that define specific modes of translation of functionally related MTOR-sensitive mRNAs. Genome Res. 2016, 26, 636–648. [Google Scholar] [CrossRef] [PubMed]
- Matassa, D.S.; Amoroso, M.R.; Agliarulo, I.; Maddalena, F.; Sisinni, L.; Paladino, S.; Romano, S.; Romano, M.F.; Sagar, V.; Loreni, F.; et al. Translational control in the stress adaptive response of cancer cells: A novel role for the heat shock protein TRAP1. Cell Death Dis. 2013, 4, e851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, T.P.; Su, T.C.; Nakamura, Y.; Tsai, S.Y. The Sigma-1 Receptor as a Pluripotent Modulator in Living Systems. Trends Pharmacol. Sci. 2016, 37, 262–278. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.; Robichaud, N.; Hulea, L.; Sonenberg, N.; Pelletier, J.; Topisirovic, I. Targeting the translation machinery in cancer. Nat. Rev. Drug Discov. 2015, 14, 261–278. [Google Scholar] [CrossRef] [PubMed]
- De la Parra, C.; Walters, B.A.; Geter, P.; Schneider, R.J. Translation initiation factors and their relevance in cancer. Curr. Opin. Genet. Dev. 2017, 48, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, L.M.; Tandoc, K.; Topisirovic, I.; Furic, L. Cross-talk between protein synthesis, energy metabolism and autophagy in cancer. Curr. Opin. Genet. Dev. 2017, 48, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Mamane, Y.; Petroulakis, E.; LeBacquer, O.; Sonenberg, N. mTOR, translation initiation and cancer. Oncogene 2006, 25, 6416–6422. [Google Scholar] [CrossRef] [PubMed]
- Sonenberg, N. Translation factors as effectors of cell growth and tumorigenesis. Curr. Opin. Cell Biol. 1993, 5, 955–960. [Google Scholar] [CrossRef]
- Cencic, R.; Pelletier, J. Hippuristanol—A potent steroid inhibitor of eukaryotic initiation factor 4A. Translation (Austin) 2016, 4, e1137381. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Pelletier, J. Targeting the eIF4A RNA helicase as an anti-neoplastic approach. Biochim. Biophys. Acta 2015, 1849, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Parsyan, A.; Svitkin, Y.; Shahbazian, D.; Gkogkas, C.; Lasko, P.; Merrick, W.C.; Sonenberg, N. mRNA helicases: The tacticians of translational control. Nat. Rev. Mol. Cell Biol. 2011, 12, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Steinberger, J.; Chu, J.; Maiga, R.I.; Sleiman, K.; Pelletier, J. Developing anti-neoplastic biotherapeutics against eIF4F. Cell. Mol. Life Sci. 2017, 74, 1681–1692. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Ozel, D.; Qiao, Y.; Harbinski, F.; Chen, L.; Denoyelle, S.; He, X.; Zvereva, N.; Supko, J.G.; Chorev, M.; et al. Chemical genetics identify eIF2alpha kinase heme-regulated inhibitor as an anticancer target. Nat. Chem. Biol. 2011, 7, 610–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denoyelle, S.; Chen, T.; Chen, L.; Wang, Y.; Klosi, E.; Halperin, J.A.; Aktas, B.H.; Chorev, M. In vitro inhibition of translation initiation by N,N’-diarylureas-potential anti-cancer agents. Bioorg. Med. Chem. Lett. 2012, 22, 402–409. [Google Scholar] [CrossRef] [PubMed]


| Name | Sequence |
|---|---|
| ATP5O 5’UTR-FF (forward) | 5’-TACCGAGCTCGGATCCAAGGGAGAAGATGGAAGACGCCAA-3’ |
| ATP5O 5’UTR-FF (reverse) | 5’-TTGGCGTCTTCCATCTTCTCCCTTGGATCCGAGCTCGGTA-3’ |
| UQCC2 5’UTR-FF (forward) | 5’-TACCGAGCTCGGATCCAAGGGGCCCAAGATGGAAGACGCCAA-3’ |
| UQCC2 5’UTR-FF (reverse) | 5’-TTGGCGTCTTCCATCTTGGGCCCCTTGGATCCGAGCTCGGTA-3’ |
| NDUFS6 5’UTR-FF (forward) | 5’-TACCGAGCTCGGATCCAAGGGTCAAAGGCCAGCGGCGCAAAATGGAAGACGCCAA-3’ |
| NDUFS6 5’UTR-FF (reverse) | 5’-TTGGCGTCTTCCATTTTGCGCCGCTGGCCTTTGACCCTTGGATCCGAGCTCGGTA-3’ |
| ATP5O (TISU)-FF (forward) | 5’-CGGATCCAAGGGAGAAGATGGCTGCCGCCAAAAACATAAAGAAAGG-3’ |
| ATP5O (TISU)-FF (reverse) | 5’-CCTTTCTTTATGTTTTTGGCGGCAGCCATCTTCTCCCTTGGATCCG-3’ |
| ATP5O (5’UTR)-SL-FF (forward) | 5’-AGCTAAGCTTGGGAGAAGGTCCACCACGGCCGATATCACGGCCGTGGTGGACGGATCCGACT-3’ |
| ATP5O (5’UTR)-SL-FF (reverse) | 5’-GACTGGATCCGTCCACCACGGCCGTGATATCGGCCGTGGTGGACCTTCTCCCAAGCTTAGCT-3’ |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, X.; Persaud, L.; Muharam, H.; Francis, A.; Das, D.; Aktas, B.H.; Sauane, M. Eukaryotic Translation Initiation Factor 4A Down-Regulation Mediates Interleukin-24-Induced Apoptosis through Inhibition of Translation. Cancers 2018, 10, 153. https://doi.org/10.3390/cancers10050153
Zhong X, Persaud L, Muharam H, Francis A, Das D, Aktas BH, Sauane M. Eukaryotic Translation Initiation Factor 4A Down-Regulation Mediates Interleukin-24-Induced Apoptosis through Inhibition of Translation. Cancers. 2018; 10(5):153. https://doi.org/10.3390/cancers10050153
Chicago/Turabian StyleZhong, Xuelin, Leah Persaud, Hilal Muharam, Ashleigh Francis, Dibash Das, Bertal Huseyin Aktas, and Moira Sauane. 2018. "Eukaryotic Translation Initiation Factor 4A Down-Regulation Mediates Interleukin-24-Induced Apoptosis through Inhibition of Translation" Cancers 10, no. 5: 153. https://doi.org/10.3390/cancers10050153




