Crosstalk between ERα and Receptor Tyrosine Kinase Signalling and Implications for the Development of Anti-Endocrine Resistance
Abstract
:1. Introduction
2. Results
2.1. Levels of Anti-Endocrine Resistance in Breast Cancer Cell Lines
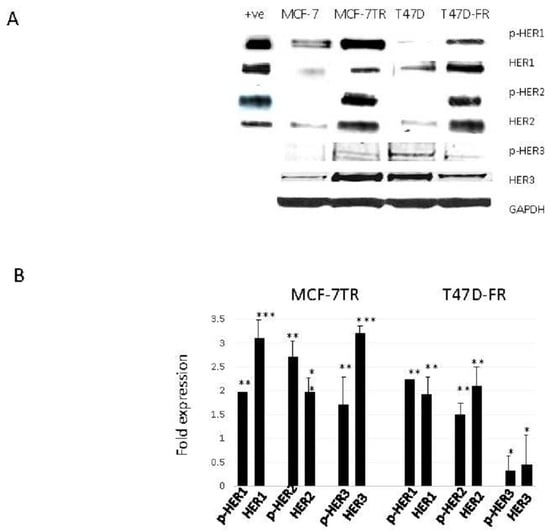
2.2. Development of Anti-Endocrine-Resistant Breast Cancer Cells Gives Rise to Changes in Expression of HER Receptors
2.3. Anti-Endocrine-Resistant Cells Show Collateral Sensitivity to TKI Therapies
2.4. HER1 and HER2 Expression in Endocrine-Resistant Cell Lines Following TKI Treatment (Confocal Microscopy)
2.5. TKI Therapy Amplifies p27kip1 in the Presence of Anti-Endocrine Therapies as a Mechanism of Overcoming Drug Resistance
2.6. The Impact of TKI Treatment on ERα Expression in Drug-Resistant Cells
2.7. The Co-Administration of TKI and Anti-Oestrogens Enhances Cell Death and Overcomes Anti-Endocrine Resistance
2.8. Treatment of Anti-Endocrine-Resistant Cell Lines with Anti-Oestrogens and TKIs Produced Increases in the Sub-G1 Component of the Cell Cycle
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture
4.3. Cytotoxicity Testing
4.4. Western Blot Analysis
4.5. Real-Time Quantitative PCR Assay
4.6. Optical and Fluorescence Confocal Microscopy (Confocol Laser Scanning Microscopy)
4.7. Annexin V with Propidium Iodide Methodology for Apoptosis with Flow Cytometry
4.8. Cell-Cycle Analysis (Flow Cytometry)
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zilli, M.; Grassadonia, A.; Tinari, N.; DI Giacobbe, A.; Gildetti, S.; Giampietro, J.; Natoli, C.; Iacobelli, S. Molecular mechanisms of endocrine resistance and their implication in the therapy of breast cancer. Biochim. Biophys. Acta 2009, 1795, 62–81. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, R.I.; Hutcheson, I.R.; Jones, H.E.; Hiscox, S.E.; Giles, M.; Taylor, K.M.; Gee, J.M. Growth factor signalling in endocrine and anti-growth factor resistant breast cancer. Rev. Endocr. Metab. Disord. 2007, 8, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Arpino, G.; Green, S.J.; Allred, D.C.; Lew, D.; Martino, S.; Osborne, C.K.; Elledge, R.M. HER-2 amplification, HER-1 expression, and tamoxifen response in estrogen receptor-positive metastatic breast cancer: A southwest oncology group study. Clin. Cancer Res. 2004, 10, 5670–5676. [Google Scholar] [CrossRef] [PubMed]
- Moulder, S.L.; Yakes, F.M.; Muthuswamy, S.K.; Bianco, R.; Simpson, J.F.; Arteaga, C.L. Epidermal growth factor receptor (HER1) tyrosine kinase inhibitor Zd1839 (Iressa) inhibits HER2/Neu (ERBB2)-overexpressing breast cancer cells in vitro and in vivo. Cancer Res. 2001, 61, 8887–8895. [Google Scholar] [PubMed]
- Johnston, S.R.; Dowsett, M. Aromatase inhibitors for breast cancer: Lessons from the laboratory. Nat. Rev. Cancer 2003, 3, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Knowlden, J.M.; Hutcheson, I.R.; Jones, H.E.; Madden, T.; Gee, J.M.; Harper, M.E.; Barrow, D.; Wakeling, A.E.; Nicholson, R.I. Elevated levels of epidermal growth factor receptor/C-ERBB2 heterodimers mediate an autocrine growth regulatory pathway in tamoxifen-resistant MCF-7 cells. Endocrinology 2003, 144, 1032–1044. [Google Scholar] [CrossRef] [PubMed]
- Massarweh, S.; Schiff, R. Unraveling the mechanisms of endocrine resistance in breast cancer: New therapeutic opportunities. Clin. Cancer Res. 2007, 13, 1950–1954. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, M.C.; Detre, S.; Johnston, S.; Mohsin, S.K.; Shou, J.; Allred, D.C.; Schiff, R.; Osborne, C.K.; Dowsett, M. Molecular changes in tamoxifen-resistant breast cancer: Relationship between Estrogen Receptor, HER-2, and P38 Mitogen-Activated Protein Kinase. J. Clin. Oncol. 2005, 23, 2469–2476. [Google Scholar] [CrossRef] [PubMed]
- Van Tine, B.A.; Crowder, R.J.; Ellis, M.J. ER and Pi3k independently modulate endocrine resistance in ER-positive breast cancer. Cancer Discov. 2011, 1, 287–288. [Google Scholar] [CrossRef] [PubMed]
- Shou, J.; Massarweh, S.; Osborne, C.K.; Wakeling, A.E.; Ali, S.; Weiss, H.; Schiff, R. Mechanisms of Tamoxifen resistance: Increased estrogen receptor-HER2/Neu cross-talk in ER/HER2-positive breast cancer. J. Natl. Cancer Inst. 2004, 96, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Gee, J.M.; Robertson, J.F.; Ellis, I.O.; Nicholson, R.I. Phosphorylation of Erk1/2 mitogen-activated protein kinase is associated with poor response to anti-hormonal therapy and decreased patient survival in clinical breast cancer. Int. J. Cancer 2001, 95, 247–254. [Google Scholar] [CrossRef]
- Musgrove, E.A.; Sutherland, R.L. Biological determinants of endocrine resistance in breast cancer. Nat. Rev. Cancer 2009, 9, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Mcgowan, E.M.; Alling, N.; Jackson, E.A.; Haass, N.K.; Allen, J.D.; Martinello-Wilks, R. Evaluation of cell cycle arrest in estrogen responsive MCF-7 breast cancer cells: Pitfalls of the MTS assay. PLoS ONE 2011, 6, e20623. [Google Scholar] [CrossRef] [PubMed]
- Arpino, G.; Wiechmann, L.; Osborne, C.K.; Schiff, R. Crosstalk between the Estrogen Receptor and the HER tyrosine kinase receptor family: Molecular mechanism and clinical implications for endocrine therapy resistance. Endocr. Rev. 2008, 29, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Osborne, C.K.; Schiff, R. Mechanisms of endocrine resistance in breast cancer. Ann. Rev. Med. 2011, 62, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.R.; Pietras, R.J. Estrogen receptors outside the nucleus in breast cancer. Breast Cancer Res. Treat. 2008, 108, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Morrison, G.; Fu, X.; Shea, M.; Nanda, S.; Giuliano, M.; Wang, T.; Klinowska, T.; Osborne, C.K.; Rimawi, M.F.; Schiff, R. Therapeutic potential of the dual EGFR/HER2 inhibitor AZD8931 in circumventing endocrine resistance. Breast Cancer Res. Treat. 2014, 144, 263–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massarweh, S.; Osborne, C.K.; Creighton, C.J.; Qin, L.; Tsimelzon, A.; Huang, S.; Weiss, H.; Rimawi, M.; Schiff, R. Tamoxifen resistance in breast tumors is driven by growth factor receptor signaling with repression of classic estrogen receptor genomic function. Cancer Res. 2008, 68, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Germer, K.; Wu, T.; Wang, J.; Luo, J.; Wang, S.C.; Wang, Q.; Zhang, X. Cross-talk between HER2 and MED1 regulates tamoxifen resistance of human breast cancer cells. Cancer Res. 2012, 72, 5625–5634. [Google Scholar] [CrossRef] [PubMed]
- Rimawi, M.F.; Aleixo, S.B.; Rozas, A.A.; De Matos Neto, J.N.; Caleffi, M.; Figueira, A.C.; Souza, S.C.; Reiriz, A.B.; Gutierrez, C.; Arantes, H.; et al. A neoadjuvant, randomized, open label phase II trial of Afatinib versus Trastuzumab versus Lapatinib in patients with locally advanced HER2-positive breast cancer. Clin. Breast Cancer 2015, 15, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Kong, A.; Calleja, V.; Leboucher, P.; Harris, A.; Parker, P.J.; Larijani, B. HER2 oncogenic function escapes EGFR tyrosine kinase inhibitors via activation of alternative HER receptors in breast cancer cells. PLoS ONE 2008, 3, e2881. [Google Scholar] [CrossRef] [PubMed]
- Thrane, S.; Lykkesfeldt, A.E.; Larsen, M.S.; Sorensen, B.S.; Yde, C.W. Estrogen receptor A is the major driving factor for growth in tamoxifen-resistant breast cancer and supported by HER/ERK signalling. Breast Cancer Res. Treat. 2013, 139, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Diaz, M.R.; Yee, D. Fulvestrant regulates epidermal growth factor (EGF) family ligands to activate EGF Receptor (EGFR) signaling in breast cancer cells. Breast Cancer Res. Treat. 2013, 139, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Kirkegaard, T.; Hansen, S.K.; Larsen, S.L.; Reiter, B.E.; Sorensen, B.S.; Lykkesfeldt, A.E. T47d breast cancer cells switch from ER/HER to HER/C-Src signaling upon acquiring resistance to the antiestrogen fulvestrant. Cancer Lett. 2014, 344, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Leary, A.F.; Drury, S.; Detre, S.; Pancholi, S.; Lykkesfeldt, A.E.; Martin, L.A.; Dowsett, M.; Johnston, S.R. Lapatinib restores hormone sensitivity with differential effects on estrogen receptor signaling in cell models of human epidermal growth factor receptor 2-negative breast cancer with acquired endocrine resistance. Clin. Cancer Res. 2010, 16, 1486–1497. [Google Scholar] [CrossRef] [PubMed]
- Chu, I.; Blackwell, K.; Chen, S.; Slingerland, J. The dual ERBB1/ERBB2 inhibitor, Lapatinib (Gw572016), cooperates with tamoxifen to inhibit both cell proliferation- and estrogen-dependent gene expression in antiestrogen-resistant breast cancer. Cancer Res. 2005, 65, 18–25. [Google Scholar] [PubMed]
- Ravaioli, A.; Monti, F.; Regan, M.M.; Maffini, F.; Mastropasqua, M.G.; Spataro, V.; Castiglione-Gertsch, M.; Panzini, I.; Gianni, L.; Goldhirsch, A.; et al. P27 and Skp2 immunoreactivity and its clinical significance with endocrine and chemo-endocrine treatments in node-negative early breast cancer. Ann. Oncol. 2008, 19, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Fukushima, H.; Inuzuka, H.; Wan, L.; Liu, P.; Gao, D.; Sarkar, F.H.; Wei, W. SKP2 is a promising therapuetic target in breast cancer. Front. Oncol. 2012, 1, 57. [Google Scholar] [CrossRef] [PubMed]
- Davidovich, S.; Ben-Ishak, O.; Shapira, M.; Futerman, B.; Hershko, D.D. Over-expression of SKP2 is associated with resistance to preoperative doxorubicin-based chemotherapy in primary breast cancer. Breast Cancer Res. 2008, 10, R63. [Google Scholar] [CrossRef] [PubMed]
- Suzawa, K.; Toyooka, S.; Sakaguchi, M.; Morita, M.; Yamamoto, H.; Tomida, S.; Ohtsuka, T.; Watanabe, M.; Hashida, S.; Maki, Y.; et al. Antitumour effect of afatinib as a human epidermal growth factor receptor 2 targeted therapy in lung cancers harboring HER2 oncogenic alterations. Cancer Sci. 2016, 107, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Samaddar, J.S.; Gaddy, V.T.; Duplantier, J.; Thandavan, S.P.; Shah, M.; Smith, M.J.; Schoenlein, P.V. A role for macroautophagy in protection against 4-hydroxytamoxifen-induced cell death and the development of antioestrogen resistance. Mol. Cancer Ther. 2008, 7, 2977–2987. [Google Scholar] [CrossRef] [PubMed]
- Kisanga, E.R.; Gjerde, J.; Guerrieri-Gonzaga, A.; Pigatto, F.; Pesci-Feltri, A.; Robertson, C.; Serrano, D.; Pelosi, G.; Decensi, A.; Lien, E.A. Tamoxifen and Metabolite concentrations in serum and breast cancer tissue during three dose regimens in a randomized preoperative trial. Clin. Cancer Res. 2004, 10, 2336–2343. [Google Scholar] [CrossRef] [PubMed]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montaser, R.Z.; Coley, H.M. Crosstalk between ERα and Receptor Tyrosine Kinase Signalling and Implications for the Development of Anti-Endocrine Resistance. Cancers 2018, 10, 209. https://doi.org/10.3390/cancers10060209
Montaser RZ, Coley HM. Crosstalk between ERα and Receptor Tyrosine Kinase Signalling and Implications for the Development of Anti-Endocrine Resistance. Cancers. 2018; 10(6):209. https://doi.org/10.3390/cancers10060209
Chicago/Turabian StyleMontaser, Rugaia Z., and Helen M. Coley. 2018. "Crosstalk between ERα and Receptor Tyrosine Kinase Signalling and Implications for the Development of Anti-Endocrine Resistance" Cancers 10, no. 6: 209. https://doi.org/10.3390/cancers10060209