CAF-1 Subunits Levels Suggest Combined Treatments with PARP-Inhibitors and Ionizing Radiation in Advanced HNSCC
Abstract
:1. Introduction
2. Results
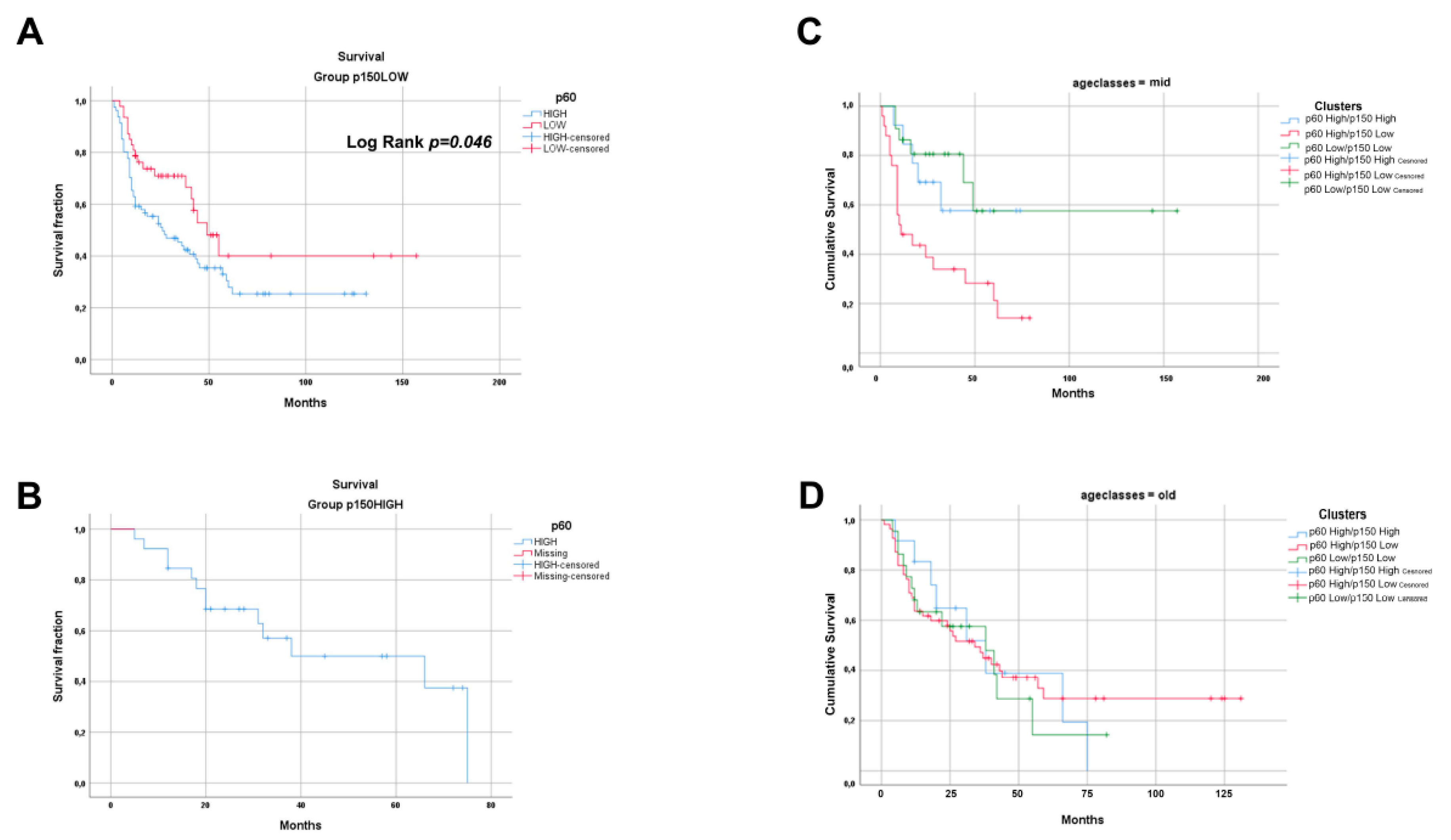
2.1. Immunohistochemistry Expression of CAF-1/p60 and p150 Subunits in OSCC and OPSCC Tumour Samples
2.2. The Silencing of the CAF-1 Subunits Increases the Sensitivity to Ionizing Radiation in HPV-Negative and HPV-Positive Head and Neck Cancer Cell Lines
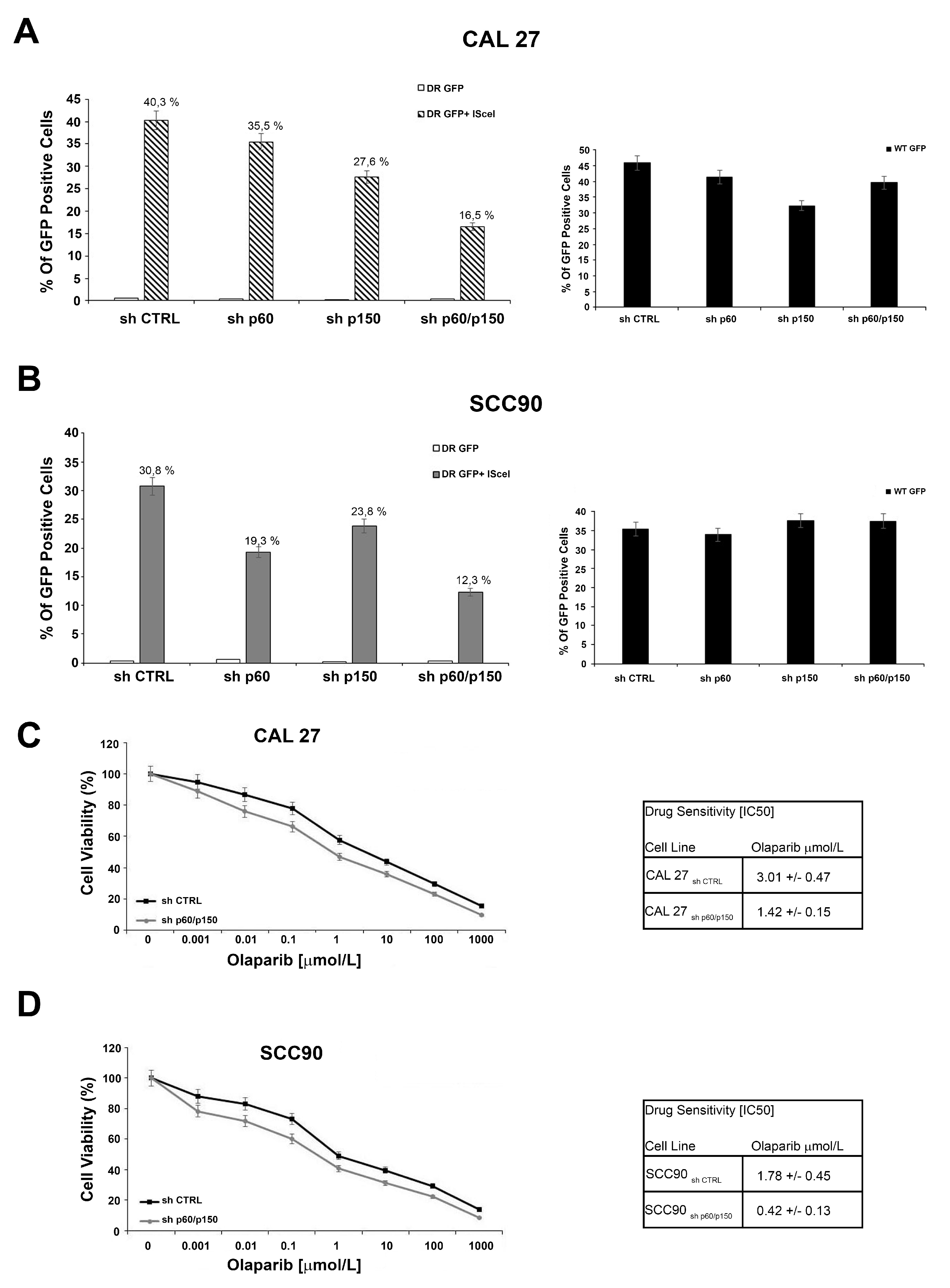
2.3. The Silencing of the Subunits of CAF-1 Leads to Defect in DNA Repair Mediated by Homologous Recombination and Sensitizes OSCC and OPSCC Cells to PARP-Inhibitors
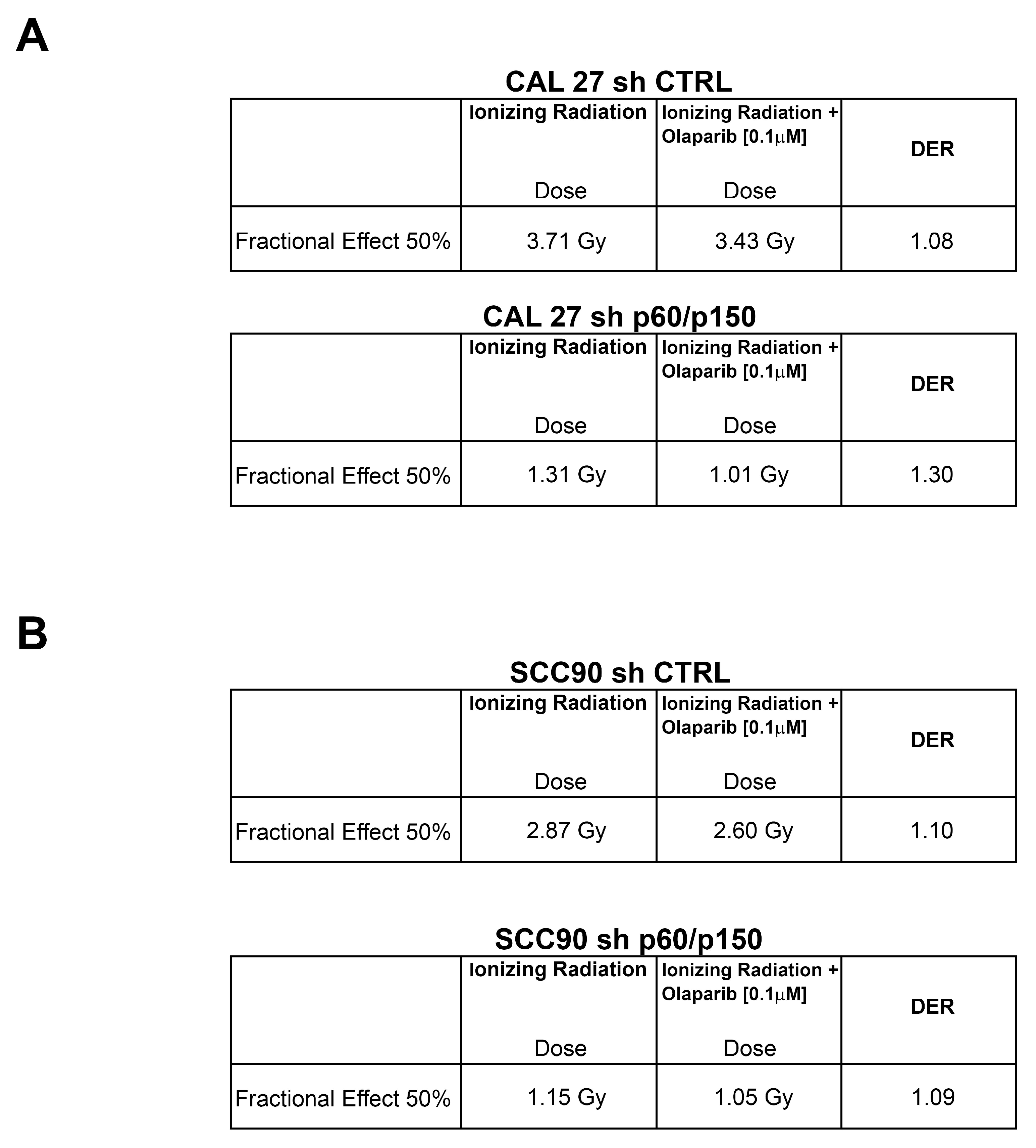
2.4. Treatment with PARP-Inhibitors Increases the Radiosensitivity in HPV-Negative Head and Neck Cancer Cells
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Drugs
4.2. Real Time PCR
4.3. Western Blotting and Antibodies
4.4. Plasmids and Transfection
4.5. Sensitivity Test and Design for Drug Combination
4.6. Colony Forming Assay
4.7. TMA and IHC
4.8. Statistical Analysis
4.9. HR Reporter Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Pontes, F.S.; Carneiro, J.T., Jr.; Fonseca, F.P.; da Silva, T.S.; Pontes, H.A.; Pinto Ddos, S., Jr. Squamous cell carcinoma of the tongue and floor of the mouth: Analysis of survival rate and independent prognostic factors in the Amazon region. J. Craniofac. Surg. 2011, 22, 925–930. [Google Scholar] [CrossRef]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-tieulent, J.; Jemal, A. Global Cancer Statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef]
- Hashibe, M.; Brennan, P.; Chuang, S.C.; Boccia, S.; Castellsague, X.; Chen, C.; Curado, M.P.; Dal Maso, L.; Daudt, A.W.; Fabianova, E.; et al. Interaction between tobacco and alcohol use and the risk of head and neck cancer: Pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer Epidemiol. Biomark. Prev. 2009, 18, 541–550. [Google Scholar] [CrossRef]
- Mallen-St Clair, J.; Alani, M.; Wang, M.B.; Srivastan, E.S. Human papillomavirus in oropharyngeal cancer: The changing face of a disease. Biochim. Biophys. Acta 2016, 1866, 141–150. [Google Scholar] [CrossRef]
- Windon, M.J.; D’Souza, G.; Rettig, E.M.; Westra, W.H.; van Zante, A.; Wang, S.J.; Ryan, W.R.; Mydlarz, W.K.; Ha, P.K.; Miles, B.A.; et al. Increasing prevalence of human papillomavirus-positive oropharyngeal cancers among older adults. Cancer 2018, 124, 2993–2999. [Google Scholar] [CrossRef] [Green Version]
- Hecht, S.S. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat. Rev. Cancer 2003, 3, 733–744. [Google Scholar] [CrossRef]
- Kadaja, M.; Isok-Paas, H.; Laos, T.; Ustav, E.; Ustav, M. Mechanism of genomic instability in cells infected with the high-risk human papillomaviruses. PLoS Pathog. 2009, 5, e1000397. [Google Scholar] [CrossRef]
- Chatterjee, N.; Walker, G.C. Mechanisms of DNA damage, repair, and mutagenesis. Environ. Mol. Mutagen. 2017, 58, 235–263. [Google Scholar] [CrossRef] [Green Version]
- Merolla, F.; Mascolo, M.; Ilardi, G.; Siano, M.; Russo, D.; Graziano, V.; Celetti, A.; Staibano, S. Nucleotide Excision Repair and head and neck cancers. Front. Biosci. (Landmark Ed.) 2016, 21, 55–69. [Google Scholar] [Green Version]
- Wood, R.D.; Mitchell, M.; Lindahl, T. Human DNA repair genes, 2005. Mutat. Res. 2005, 577, 275–283. [Google Scholar] [CrossRef]
- Verreault, A.; Kaufman, P.D.; Kobayashi, R.; Stillman, B. Nucleosome assembly by a complex of CAF-1 and acetylated histones H3/H4. Cell 1996, 87, 95–104. [Google Scholar] [CrossRef]
- Ridgway, P.; Almouzni, G. CAF-1 and the inheritance of chromatin states: At the crossroads of DNA replication and repair. J. Cell Sci. 2000, 113 Pt 15, 2647–2658. [Google Scholar]
- Taddei, A.; Roche, D.; Sibarita, J.B.; Turner, B.M.; Almouzni, G. Duplication and maintenance of heterochromatin domains. J. Cell Biol. 1999, 147, 1153–1166. [Google Scholar] [CrossRef]
- Moggs, J.G.; Grandi, P.; Quivy, J.P.; Jonsson, Z.O.; Hubscher, U.; Becker, P.B.; Almouzni, G. A CAF-1-PCNA-mediated chromatin assembly pathway triggered by sensing DNA damage. Mol. Cell. Biol. 2000, 20, 1206–1218. [Google Scholar] [CrossRef]
- Hoek, M.; Myers, M.P.; Stillman, B. An analysis of CAF-1-interacting proteins reveals dynamic and direct interactions with the KU complex and 14-3-3 proteins. J. Biol. Chem. 2011, 286, 10876–10887. [Google Scholar] [CrossRef]
- Kaufman, P.D.; Kobayashi, R.; Kessler, N.; Stillman, B. The p150 and p60 subunits of chromatin assembly factor, I. A molecular link between newly synthesized histones and DNA replication. Cell 1995, 81, 1105–1114. [Google Scholar] [CrossRef]
- Smith, S.; Stillman, B. Purification and characterization of CAF-I, a human cell factor required for chromatin assembly during DNA replication in vitro. Cell 1989, 58, 15–25. [Google Scholar] [CrossRef]
- Nabatiyan, A.; Krude, T. Silencing of chromatin assembly factor 1 in human cells leads to cell death and loss of chromatin assembly during DNA synthesis. Mol. Cell. Biol. 2004, 24, 2853–2862. [Google Scholar] [CrossRef]
- Staibano, S.; Mignogna, C.; Lo Muzio, L.; Mascolo, M.; Salvatore, G.; Di Benedetto, M.; Califano, L.; Rubini, C.; De Rosa, G. Chromatin assembly factor-1 (CAF-1)-mediated regulation of cell proliferation and DNA repair: A link with the biological behaviour of squamous cell carcinoma of the tongue? Histopathology 2007, 50, 911–919. [Google Scholar] [CrossRef]
- Staibano, S.; Mascolo, M.; Mancini, F.P.; Kisslinger, A.; Salvatore, G.; Di Benedetto, M.; Chieffi, P.; Altieri, V.; Prezioso, D.; Ilardi, G.; et al. Overexpression of chromatin assembly factor-1 (CAF-1) p60 is predictive of adverse behaviour of prostatic cancer. Histopathology 2009, 54, 580–589. [Google Scholar] [CrossRef]
- Mascolo, M.; Vecchione, M.L.; Ilardi, G.; Scalvenzi, M.; Molea, G.; Di Benedetto, M.; Nugnes, L.; Siano, M.; De Rosa, G.; Staibano, S. Overexpression of Chromatin Assembly Factor-1/p60 helps to predict the prognosis of melanoma patients. BMC Cancer 2010, 10, 63. [Google Scholar] [CrossRef]
- Staibano, S.; Mascolo, M.; Rocco, A.; Lo Muzio, L.; Ilardi, G.; Siano, M.; Pannone, G.; Vecchione, M.L.; Nugnes, L.; Califano, L.; et al. The proliferation marker Chromatin Assembly Factor-1 is of clinical value in predicting the biological behaviour of salivary gland tumours. Oncol. Rep. 2011, 25, 13–22. [Google Scholar] [CrossRef]
- Mascolo, M.; Ilardi, G.; Romano, M.F.; Celetti, A.; Siano, M.; Romano, S.; Luise, C.; Merolla, F.; Rocco, A.; Vecchione, M.L.; et al. Overexpression of chromatin assembly factor-1 p60, Poly(ADP-ribose) polymerase 1 and nestin predicts metastasizing behaviour of oral cancer. Histopathology 2012, 61, 1089–1105. [Google Scholar] [CrossRef]
- Mesolella, M.; Iorio, B.; Landi, M.; Cimmino, M.; Ilardi, G.; Iengo, M.; Mascolo, M. Overexpression of chromatin assembly factor-1/p60 predicts biological behaviour of laryngeal carcinomas. Acta Otorhinolaryngol. Ital. 2017, 37, 17–24. [Google Scholar] [CrossRef]
- Bochner, B.H.; Hansel, D.E.; Efstathiou, J.A.; Konety, B.; Lee, C.; Mckiernan, J.M.; Plimack, E.R.; Reuter, V.E.; Sridhar, S.; Vikram, R.; et al. AJCC Cancer Staging Manual, 8th ed.; American Joint Committee on Cancer: Chicago, IL, USA, 2017. [Google Scholar] [CrossRef]
- Gaillard, P.; Martini, E.; Kaufman, P. Chromatin assembly coupled to DNA repair: A new role for chromatin assembly factor, I. Cell 1996, 86, 887–896. [Google Scholar] [CrossRef]
- Polo, S.E.; Theocharis, S.E.; Grandin, L.; Gambotti, L.; Antoni, G.; Savignoni, A.; Asselain, B.; Patsouris, E.; Almouzni, G. Clinical significance and prognostic value of chromatin assembly factor-1 overexpression in human solid tumours. Histopathology 2010, 57, 716–724. [Google Scholar] [CrossRef] [Green Version]
- Volk, A.; Crispino, J.D. The role of the chromatin assembly complex (CAF-1) and its p60 subunit (CHAF1b) in homeostasis and disease. Biochim. Biophys. Acta 2015, 1849, 979–986. [Google Scholar] [CrossRef] [Green Version]
- Cerrato, A.; Morra, F.; Celetti, A. Use of poly ADP-ribose polymerase [PARP] inhibitors in cancer cells bearing DDR defects: The rationale for their inclusion in the clinic. J. Exp. Clin. Cancer Res. 2016, 35, 179. [Google Scholar] [CrossRef]
- Palazzo, L.; Ahel, I. PARPs in genome stability and signal transduction: Implications for cancer therapy. Biochem. Soc. Trans. 2018, 46, 1681–1695. [Google Scholar] [CrossRef]
- Roeske, J.C.; Nunez, L.; Hoggarth, M.; Labay, E.; Weichselbaum, R.R. Characterization of the theorectical radiation dose enhancement from nanoparticles. Technol. Cancer Res. Treat. 2007, 6, 395–401. [Google Scholar] [CrossRef]
- Johnson, F.L. Management of advanced premalignant laryngeal lesions. Curr. Opin. Otolaryngol. Head Neck Surg. 2003, 11, 462–466. [Google Scholar] [CrossRef]
- Schuller, D.E.; Wilson, H.E.; Smith, R.E.; Batley, F.; James, A.D. Preoperative reductive chemotherapy for locally advanced carcinoma of the oral cavity, oropharynx, and hypopharynx. Cancer 1983, 51, 15–19. [Google Scholar] [CrossRef]
- De Tayrac, M.; Saikali, S.; Aubry, M.; Bellaud, P.; Boniface, R.; Quillien, V.; Mosser, J. Prognostic significance of EDN/RB, HJURP, p60/CAF-1 and PDLI4, four new markers in high-grade gliomas. PLoS ONE 2013, 8, e73332. [Google Scholar] [CrossRef]
- Molofsky, A.V.; Pardal, R.; Morrison, S.J. Diverse mechanisms regulate stem cell self renewal. Curr. Opin. Cell Biol. 2004, 16, 700–707. [Google Scholar] [CrossRef]
- Tabor, M.P.; Brakenhoff, R.H.; Ruijter-Schippers, H.J.; Van Der Wal, J.E.; Snow, G.B.; Leemans, C.R.; Braakhuis, B.J. Multiple head and neck tumours frequently originate from a single preneoplastic lesion. Am. J. Pathol. 2002, 161, 1051–1060. [Google Scholar] [CrossRef]
- Zukerberg, L. The molecular basis of dysplasia. Semin. Diagn. Pathol. 2002, 19, 48–53. [Google Scholar]
- Russo, D.; Merolla, F.; Varricchio, S.; Salzano, G.; Zarrilli, G.; Mascolo, M.; Strazzullo, V.; Di Crescenzo, R.M.; Celetti, A.; Ilardi, G. Epigenetics of oral and oropharyngeal cancers. Biomed. Rep. 2018, 9, 275–283. [Google Scholar] [CrossRef]
- Rosai, J.; Carcangiu, M.L.; DeLellis, R.A. Atlas of Tumour Pathology-Tumours of the Larynx, 3rd ed.; Armed Force Institute of Pathology: Washington, DC, USA, 1992. [Google Scholar]
- Gillison, M.L.; Koch, W.M.; Capone, R.B.; Spafford, M.; Westra, W.H.; Wu, L.; Zahurak, M.L.; Daniel, R.W.; Viglione, M.; Symer, D.E.; et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J. Natl. Cancer Inst. 2000, 92, 709–720. [Google Scholar] [CrossRef]
- Gillison, M.L.; D’Souza, G.; Westra, W.; Sugar, E.; Xiao, W.; Begum, S.; Viscidi, R. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J. Natl. Cancer Inst. 2008, 100, 407–420. [Google Scholar] [CrossRef]
- D’Souza, G.; Kreimer, A.R.; Viscidi, R.; Pawlita, M.; Fakhry, C.; Koch, W.M.; Westra, W.H.; Gillison, M.L. Case-control study of human papillomavirus and oropharyngeal cancer. N. Engl. J. Med. 2007, 356, 1944–1956. [Google Scholar] [CrossRef]
- Applebaum, K.M.; Furniss, C.S.; Zeka, A.; Posner, M.R.; Smith, J.F.; Bryan, J.; Eisen, E.A.; Peters, E.S.; McClean, M.D.; Kelsey, K.T. Lack of association of alcohol and tobacco with HPV16-associated head and neck cancer. J. Natl. Cancer Inst. 2007, 99, 1801–1810. [Google Scholar] [CrossRef]
- Hunt, J.L.; Barnes, L.; Lewis, J.S.J.; Mahfouz, M.E.; Slootweg, P.J.; Thompson, L.D.R.; Cardesa, A.; Devaney, K.O.; Gnepp, D.R.; Westra, W.H.; et al. Molecular diagnostic alterations in squamous cell carcinoma of the head and neck and potential diagnostic applications. Eur. Arch. Otorhinolaryngol. 2014, 271, 211–223. [Google Scholar] [CrossRef]
- Clauditz, T.S.; Gontarewicz, A.; Lebok, P.; Tsourlakis, M.-C.; Grob, T.J.; Munscher, A.; Sauter, G.; Bokemeyer, C.; Knecht, R.; Wilczak, W. Epidermal growth factor receptor (EGFR) in salivary gland carcinomas: Potentials as therapeutic target. Oral Oncol. 2012, 48, 991–996. [Google Scholar] [CrossRef]
- Clauditz, T.S.; Reiff, M.; Gravert, L.; Gnoss, A.; Tsourlakis, M.-C.; Munscher, A.; Sauter, G.; Bokemeyer, C.; Knecht, R.; Wilczak, W. Human epidermal growth factor receptor 2 (HER2) in salivary gland carcinomas. Pathology 2011, 43, 459–464. [Google Scholar] [CrossRef]
- Goffin, J.R.; Zbuk, K. Epidermal growth factor receptor: Pathway, therapies, and pipeline. Clin. Ther. 2013, 35, 1282–1303. [Google Scholar] [CrossRef]
- Ang, K.K.; Berkey, B.A.; Tu, X.; Zhang, H.-Z.; Katz, R.; Hammond, E.H.; Fu, K.K.; Milas, L. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 2002, 62, 7350–7356. [Google Scholar]
- Mjelle, R.; Hegre, S.A.; Aas, P.A.; Slupphaug, G.; Drabløs, F.; Saetrom, P.; Krokan, H.E. Cell cycle regulation of human DNA repair and chromatin remodelling genes. DNA Repair (Amst) 2015, 30, 53–67. [Google Scholar] [CrossRef]
- Henikoff, S. Versatile assembler. Nature 2003, 423, 814–815. [Google Scholar] [CrossRef]
- Polo, S.E.; Theocharis, S.E.; Klijanienko, J.; Savignoni, A.; Asselain, B.; Vielh, P.; Almouzni, G. Chromatin assembly factor-1, a marker of clinical value to distinguish quiescent from proliferating cells. Cancer Res. 2004, 64, 2371–2381. [Google Scholar] [CrossRef]
- Renan, M.J. How many mutations are required for tumourigenesis? Implications from human cancer data. Mol. Carcinog. 1993, 7, 139–146. [Google Scholar]
- Mascolo, M.; Ilardi, G.; Merolla, F.; Russo, D.; Vecchione, M.L.; de Rosa, G.; Staibano, S. Tissue microarray-based evaluation of Chromatin Assembly Factor-1 (CAF-1)/p60 as tumour prognostic marker. Int. J. Mol. Sci. 2013, 13, 11044–11062. [Google Scholar] [CrossRef]
- Lord, C.J.; Ashworth, A. BRCAness revisited. Nat. Rev. Cancer 2016, 16, 110–120. [Google Scholar] [CrossRef]
- Cerrato, A.; Merolla, F.; Morra, F.; Celetti, A. CCDC6: The identity of a protein known to be partner in fusion. Int. J. Cancer 2018, 142, 1300–1308. [Google Scholar] [CrossRef]
- Leone, V.; Langella, C.; Esposito, F.; Arra, C.; Palma, G.; Rea, D.; Paciello, O.; Merolla, F.; De Biase, D.; Papparella, S.; et al. Ccdc6 knock-in mice develop thyroid hyperplasia associated to an enhanced CREB1 activity. Oncotarget 2015, 6, 15628–15638. [Google Scholar] [CrossRef] [Green Version]
- Lord, C.J.; Tutt, A.N.J.; Ashworth, A. Synthetic lethality and cancer therapy: Lessons learned from the development of PARP inhibitors. Annu. Rev. Med. 2015, 66, 455–470. [Google Scholar] [CrossRef]
- Wurster, S.; Hennes, F.; Parplys, A.C.; Seelbach, J.I.; Mansour, W.Y.; Zielinski, A.; Petersen, C.; Clauditz, T.S.; Münscher, A.; Friedl, A.A.; et al. PARP1 inhibition radiosensitizes HNSCC cells deficient in homologous recombination by disabling the DNA replication fork elongation response. Oncotarget 2016, 7, 9732–9734. [Google Scholar] [CrossRef]
- Nickson, C.M.; Moori, P.; Carter, R.J.; Rubbi, C.P.; Parsons, J.L. Misregulation of DNA damage repair pathways in HPV-positive head and neck squamous cell carcinoma contributes to cellular radiosensitivity. Oncotarget 2017, 8, 29963–29975. [Google Scholar] [CrossRef] [Green Version]
- Mascitti, M.; Rubini, C.; De Michele, F.; Balercia, P.; Girotto, R.; Troiano, G.; Lo Muzio, L.; Santarelli, A. American Joint Committee on Cancer staging system 7th edition versus 8th edition: Any improvement for patients with squamous cell carcinoma of the tongue? Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 126, 415–423. [Google Scholar] [CrossRef]
- Laemli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Towbin, H.; Staehelin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef] [PubMed]
- Kossatz, S.; Weber, W.A.; Reiner, T. Optical Imaging of PARP1 in Response to Radiation in Oral Squamous Cell Carcinoma. PLoS ONE 2016, 11, e0147752. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Russo, D.; Merolla, F.; Mascolo, M.; Ilardi, G.; Romano, S.; Varricchio, S.; Napolitano, V.; Celetti, A.; Postiglione, L.; Di Lorenzo, P.P.; et al. FKBP51 Immunohistochemical Expression: A New Prognostic Biomarker for OSCC? Int. J. Mol. Sci. 2017, 18, 443. [Google Scholar] [CrossRef]
- Mascolo, M.; Ayala, F.; Ilardi, G.; Balato, A.; Lembo, S. Chromatin Assembly Factor-1/p60 overexpression: A potential index of psoriasis severity. Eur. J. Dermatol. 2014, 24, 509–511. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]
- Morra, F.; Merolla, F.; D’Abbiero, D.; Ilardi, G.; Campione, S.; Monaco, R.; Guggino, G.; Ambrosio, F.; Staibano, S.; Cerrato, A.; et al. Analysis of CCDC6 as a novel biomarker for the clinical use of PARP1 inhibitors in malignant pleural mesothelioma. Lung Cancer 2019, 135, 56–65. [Google Scholar] [CrossRef]
- Morra, F.; Merolla, F.; Criscuolo, D.; Insabato, L.; Giannella, R.; Ilardi, G.; Cerrato, A.; Visconti, R.; Staibano, S.; Celetti, A. CCDC6 and USP7 expression levels suggest novel treatment options in high-grade urothelial bladder cancer. J. Exp. Clin. Cancer Res. 2019, 38, 90. [Google Scholar] [CrossRef]
- Jasin, M. Genetic manipulation of genomes with rare-cutting endonucleases. Trends Genet. 1996, 12, 224–228. [Google Scholar] [CrossRef]



| Study Population | Frequency | % | |
|---|---|---|---|
| Stage | I | 15 | 9.7 |
| II | 30 | 19.5 | |
| III | 18 | 11.7 | |
| IVA | 56 | 36.4 | |
| IVB | 12 | 7.8 | |
| Missing | 23 | 14.9 | |
| Sex | F | 72 | 46.8 |
| M | 82 | 53.2 | |
| Age | Mean | 63.8 | |
| Median | 64 | ||
| Std Dev | 13.1 | ||
| Range | 57 | ||
| Min | 33 | ||
| Max | 90 | ||
| p60 score | HIGH | 107 | 69.5 |
| LOW | 47 | 30.5 | |
| p150 score | HIGH | 26 | 16.9 |
| LOW | 128 | 83.1 | |
| p60/p150 combined score | p60HIGH/p150HIGH | 26 | 16.9 |
| p60HIGH/p150LOW | 81 | 52.6 | |
| p60LOW/p150LOW | 47 | 30.5 | |
| Tumour site | NOP | 112 | 72.7 |
| OP | 42 | 27.3 | |
| HPV (p16) | NEG | 34 | 81 |
| POS | 8 | 19 | |
| F-up (months) | Mean | 32.92 | |
| Median | 24 | ||
| Mode | 12 | ||
| Range | 156 | ||
| Min | 1 | ||
| Max | 157 | ||
| Tot | 154 | 100 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morra, F.; Merolla, F.; Picardi, I.; Russo, D.; Ilardi, G.; Varricchio, S.; Liotti, F.; Pacelli, R.; Palazzo, L.; Mascolo, M.; et al. CAF-1 Subunits Levels Suggest Combined Treatments with PARP-Inhibitors and Ionizing Radiation in Advanced HNSCC. Cancers 2019, 11, 1582. https://doi.org/10.3390/cancers11101582
Morra F, Merolla F, Picardi I, Russo D, Ilardi G, Varricchio S, Liotti F, Pacelli R, Palazzo L, Mascolo M, et al. CAF-1 Subunits Levels Suggest Combined Treatments with PARP-Inhibitors and Ionizing Radiation in Advanced HNSCC. Cancers. 2019; 11(10):1582. https://doi.org/10.3390/cancers11101582
Chicago/Turabian StyleMorra, Francesco, Francesco Merolla, Ida Picardi, Daniela Russo, Gennaro Ilardi, Silvia Varricchio, Federica Liotti, Roberto Pacelli, Luca Palazzo, Massimo Mascolo, and et al. 2019. "CAF-1 Subunits Levels Suggest Combined Treatments with PARP-Inhibitors and Ionizing Radiation in Advanced HNSCC" Cancers 11, no. 10: 1582. https://doi.org/10.3390/cancers11101582





