Engineered Extracellular Vesicles as a Reliable Tool in Cancer Nanomedicine
Abstract
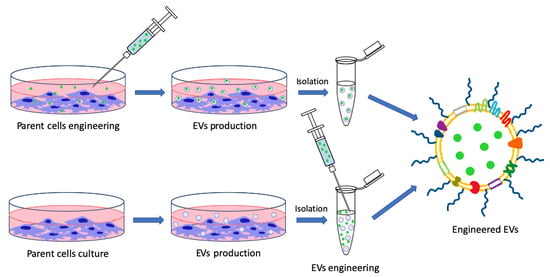
:1. Introduction
2. EVs’ Post Isolation Direct Engineering
2.1. Chemico-Physical Functionalization
2.2. Loading Nanotechnological Modification into EVs
3. EVs’ Indirect Nanotechnological Modification through Parent Cells’ Engineering
3.1. Indirect Surface Functionalization
3.2. Indirect Loading
4. Conclusions and Future Outlook
Author Contributions
Funding
Conflicts of Interest
References
- D’Anca, M.; Fenoglio, C.; Serpente, M.; Arosio, B.; Cesari, M.; Scarpini, E.A.; Galimberti, D. Exosome determinants of physiological aging and age-related neurodegenerative diseases. Front. Aging Neurosci. 2019, 11, 232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giusti, I.; Di Francesco, M.; D’Ascenzo, S.; Palmerini, M.G.; Macchiarelli, G.; Carta, G.; Dolo, V. Ovarian cancer-derived extracellular vesicles affect normal human fibroblast behavior. Cancer Biol. Ther. 2018, 19, 722–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilaria, G.; Marianna Di, F.; Vincenza, D. Extracellular vesicles in glioblastoma: Role in biological processes and in therapeutic applications. Curr. Cancer Drug Targets 2017, 17, 221–235. [Google Scholar] [CrossRef]
- Palumbo, P.; Lombardi, F.; Augello, F.R.; Giusti, I.; Luzzi, S.; Dolo, V.; Cifone, M.G.; Cinque, B. NOS2 inhibitor 1400W induces autophagic flux and influences extracellular vesicle profile in human glioblastoma U87MG cell line. Int. J. Mol. Sci. 2019, 20, 3010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rome, S.; Forterre, A.; Mizgier, M.L.; Bouzakri, K. Skeletal muscle-released extracellular vesicles: State of the art. Front. Physiol. 2019, 10, 929. [Google Scholar] [CrossRef] [PubMed]
- Ruivo, C.F.; Adem, B.; Silva, M.; Melo, S.A. The biology of cancer exosomes: Insights and new perspectives. Cancer Res. 2017, 77, 6480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rybak, K.; Robatzek, S. Functions of extracellular vesicles in immunity and virulence. Plant Physiol. 2019, 179, 1236–1247. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, T.; Kosaka, N.; Ochiya, T. Latest advances in extracellular vesicles: From bench to bedside. Sci. Technol. Adv. Mater. 2019, 20, 746–757. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Dang, G.; Lü, S.; Liu, H.; Ma, X.; Han, L.; Deng, J.; Miao, Y.; Li, X.; Shao, F.; et al. T-cell-derived extracellular vesicles regulate B-cell IgG production via pyruvate kinase muscle isozyme 2. FASEB J. 2019, 33. [Google Scholar] [CrossRef] [Green Version]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Witwer, K.W.; Théry, C. Extracellular vesicles or exosomes? On primacy, precision, and popularity influencing a choice of nomenclature. J. Extracell. Vesicles 2019, 8, 1648167. [Google Scholar] [CrossRef] [PubMed]
- Willms, E.; Cabañas, C.; Mäger, I.; Wood, M.J.A.; Vader, P. Extracellular vesicle heterogeneity: Subpopulations, isolation techniques, and diverse functions in cancer progression. Front. Immunol. 2018, 9, 738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213. [Google Scholar] [CrossRef] [PubMed]
- Caruso, S.; Poon, I.K.H. Apoptotic cell-derived extracellular vesicles: More than just debris. Front. Immunol. 2018, 9, 1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauser, P.; Wang, S.; Didenko, V.V. Apoptotic bodies: Selective detection in extracellular vesicles. In Signal Transduction Immunohistochemistry: Methods and Protocols; Kalyuzhny, A.E., Ed.; Springer New York: New York, NY, USA, 2017; pp. 193–200. [Google Scholar] [CrossRef]
- Antimisiaris, S.G.; Mourtas, S.; Marazioti, A. Exosomes and exosome-inspired vesicles for targeted drug delivery. Pharmaceutics 2018, 10, 218. [Google Scholar] [CrossRef] [Green Version]
- Meldolesi, J. Exosomes and ectosomes in intercellular communication. Curr. Biol. 2018, 28, R435–R444. [Google Scholar] [CrossRef] [Green Version]
- Théry, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef]
- Kalra, H.; Drummen, G.P.C.; Mathivanan, S. Focus on extracellular vesicles: Introducing the next small big thing. Int. J. Mol. Sci. 2016, 17, 170. [Google Scholar] [CrossRef] [Green Version]
- He, C.; Zheng, S.; Luo, Y.; Wang, B. Exosome theranostics: Biology and translational medicine. Theranostics 2018, 8, 237–255. [Google Scholar] [CrossRef]
- Jaiswal, R.; Sedger, L.M. Intercellular vesicular transfer by exosomes, microparticles and oncosomes—Implications for cancer biology and treatments. Front. Oncol. 2019, 9, 125. [Google Scholar] [CrossRef] [Green Version]
- Maacha, S.; Bhat, A.A.; Jimenez, L.; Raza, A.; Haris, M.; Uddin, S.; Grivel, J.-C. Extracellular vesicles-mediated intercellular communication: Roles in the tumor microenvironment and anti-cancer drug resistance. Mol. Cancer 2019, 18, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, L.; Lam, E.W.F.; Sun, Y. Extracellular vesicles in the tumor microenvironment: Old stories, but new tales. Mol. Cancer 2019, 18, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, B.H.; Weaver, A.M. Exosome secretion promotes chemotaxis of cancer cells. Cell Adh. Migr. 2017, 11, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Wang, L.; Dai, T.; Jin, K.; Zhang, Z.; Wang, S.; Xie, F.; Fang, P.; Yang, B.; Huang, H.; et al. Tumor-derived exosomes antagonize innate antiviral immunity. Nat. Immunol. 2018, 19, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.; Lee, T.H.; Spinelli, C.; Chennakrishnaiah, S.; D’Asti, E.; Rak, J. Extracellular vesicle communication pathways as regulatory targets of oncogenic transformation. Semin. Cell Dev. Biol. 2017, 67, 11–22. [Google Scholar] [CrossRef]
- Tirinato, L.; Pagliari, F.; Limongi, T.; Marini, M.; Falqui, A.; Seco, J.; Candeloro, P.; Liberale, C.; Di Fabrizio, E. An overview of lipid droplets in cancer and cancer stem cells. Stem Cells Int. 2017, 2017, 1656053. [Google Scholar] [CrossRef]
- Romagnoli, G.G.; Zelante, B.B.; Toniolo, P.A.; Migliori, I.K.; Barbuto, J.A.M. Dendritic cell-derived exosomes may be a tool for cancer immunotherapy by converting tumor cells into immunogenic targets. Front. Immunol. 2015, 5. [Google Scholar] [CrossRef] [Green Version]
- Pitt, J.M.; André, F.; Amigorena, S.; Soria, J.-C.; Eggermont, A.; Kroemer, G.; Zitvogel, L. Dendritic cell-derived exosomes for cancer therapy. J. Clin. Investig. 2016, 126, 1224–1232. [Google Scholar] [CrossRef]
- Markov, O.; Oshchepkova, A.; Mironova, N. Immunotherapy based on dendritic cell-targeted/-derived extracellular vesicles—A novel strategy for enhancement of the anti-tumor immune response. Front. Pharmacol. 2019, 10, 1152. [Google Scholar] [CrossRef] [Green Version]
- Munich, S.; Sobo-Vujanovic, A.; Buchser, W.J.; Beer-Stolz, D.; Vujanovic, N.L. Dendritic cell exosomes directly kill tumor cells and activate natural killer cells via TNF superfamily ligands. Oncoimmunology 2012, 1, 1074–1083. [Google Scholar] [CrossRef] [Green Version]
- Viaud, S.; Théry, C.; Ploix, S.; Tursz, T.; Lapierre, V.; Lantz, O.; Zitvogel, L.; Chaput, N. Dendritic cell-derived exosomes for cancer immunotherapy: What’s next? Cancer Res. 2010, 70, 1281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Yin, Y.; Lai, R.C.; Lim, S.K. Immunotherapeutic potential of extracellular vesicles. Front. Immunol. 2014, 5, 518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syn, N.L.; Wang, L.; Chow, E.K.-H.; Lim, C.T.; Goh, B.-C. Exosomes in cancer nanomedicine and immunotherapy: Prospects and challenges. Trends Biotechnol. 2017, 35, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, A.; Yoshioka, Y.; Yamamoto, Y.; Ishikawa, M.; Ikeda, S.-I.; Kato, T.; Kiyono, T.; Takeshita, F.; Kajiyama, H.; Kikkawa, F.; et al. Malignant extracellular vesicles carrying MMP1 mRNA facilitate peritoneal dissemination in ovarian cancer. Nat. Commun. 2017, 8, 14470. [Google Scholar] [CrossRef] [Green Version]
- Ostrowski, M.; Carmo, N.B.; Krumeich, S.; Fanget, I.; Raposo, G.; Savina, A.; Moita, C.F.; Schauer, K.; Hume, A.N.; Freitas, R.P.; et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat. Cell Biol. 2010, 12, 19–30. [Google Scholar] [CrossRef] [Green Version]
- Baietti, M.F.; Zhang, Z.; Mortier, E.; Melchior, A.; Degeest, G.; Geeraerts, A.; Ivarsson, Y.; Depoortere, F.; Coomans, C.; Vermeiren, E.; et al. Syndecan–syntenin–ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 2012, 14, 677–685. [Google Scholar] [CrossRef]
- Khawar, M.B.; Abbasi, M.H.; Siddique, Z.; Arif, A.; Sheikh, N. An update on novel therapeutic warfronts of extracellular vesicles (EVs) in cancer treatment: Where we are standing right now and where to go in the future. Oxid. Med. Cell Longev. 2019, 2019, 9702562. [Google Scholar] [CrossRef]
- Kosaka, N.; Yoshioka, Y.; Fujita, Y.; Ochiya, T. Versatile roles of extracellular vesicles in cancer. J. Clin. Investig. 2016, 126, 1163–1172. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Su, C. Design strategies and application progress of therapeutic exosomes. Theranostics 2019, 9, 1015–1028. [Google Scholar] [CrossRef]
- Munagala, R.; Aqil, F.; Jeyabalan, J.; Gupta, R.C. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016, 371, 48–61. [Google Scholar] [CrossRef] [Green Version]
- Smyth, T.J.; Redzic, J.S.; Graner, M.W.; Anchordoquy, T.J. Examination of the specificity of tumor cell derived exosomes with tumor cells in vitro. Biochim. Biophys. Acta 2014, 1838, 2954–2965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Zheng, Y.; Zhao, M. Exosome-based cancer therapy: Implication for targeting cancer stem cells. Front. Pharmacol. 2017, 7, 533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, J.P.; Holme, M.N.; Stevens, M.M. Re-engineering extracellular vesicles as smart nanoscale therapeutics. ACS Nano 2017, 11, 69–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, L.-M.; Quan, L.; Liu, J. Tracking exosomes in vitro and in vivo to elucidate their physiological functions: Implications for diagnostic and therapeutic nanocarriers. ACS Appl. Nano Mater. 2018, 1, 2438–2448. [Google Scholar] [CrossRef]
- Chiba, M.; Kubota, S.; Sato, K.; Monzen, S. Exosomes released from pancreatic cancer cells enhance angiogenic activities via dynamin-dependent endocytosis in endothelial cells in vitro. Sci. Rep. 2018, 8, 11972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smyth, T.; Kullberg, M.; Malik, N.; Smith-Jones, P.; Graner, M.W.; Anchordoquy, T.J. Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J. Control. Release 2015, 199, 145–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franzen, C.A.; Simms, P.E.; Van Huis, A.F.; Foreman, K.E.; Kuo, P.C.; Gupta, G.N. Characterization of uptake and internalization of exosomes by bladder cancer cells. Biomed. Res. Int. 2014, 2014, 619829. [Google Scholar] [CrossRef] [Green Version]
- Tian, T.; Wang, Y.; Wang, H.; Zhu, Z.; Xiao, Z. Visualizing of the cellular uptake and intracellular trafficking of exosomes by live-cell microscopy. J. Cell. Biochem. 2010, 111, 488–496. [Google Scholar] [CrossRef]
- Smyth, T.; Petrova, K.; Payton, N.M.; Persaud, I.; Redzic, J.S.; Graner, M.W.; Smith-Jones, P.; Anchordoquy, T.J. Surface functionalization of exosomes using click chemistry. Bioconjug. Chem. 2014, 25, 1777–1784. [Google Scholar] [CrossRef] [Green Version]
- Hein, C.D.; Liu, X.-M.; Wang, D. Click chemistry, a powerful tool for pharmaceutical sciences. Pharmacol. Res. 2008, 25, 2216–2230. [Google Scholar] [CrossRef] [Green Version]
- Kooijmans, S.A.A.; Fliervoet, L.A.L.; van der Meel, R.; Fens, M.; Heijnen, H.F.G.; van Bergen En Henegouwen, P.M.P.; Vader, P.; Schiffelers, R.M. PEGylated and targeted extracellular vesicles display enhanced cell specificity and circulation time. J. Control. Release 2016, 224, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Sapra, P.; Moase, E. Use of the post-insertion method for the formation of ligand-coupled liposomes. Cell. Mol. Biol. Lett. 2002, 7, 889–894. [Google Scholar] [PubMed]
- Choi, E.S.; Song, J.; Kang, Y.Y.; Mok, H. Mannose-modified serum exosomes for the elevated uptake to murine dendritic cells and lymphatic accumulation. Macromol. Biosci. 2019, 19, e1900042. [Google Scholar] [CrossRef]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Yuan, D.; Deygen, I.; Klyachko, N.L.; Kabanov, A.V.; Batrakova, E.V. Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: In vitro and in vivo evaluations. Nanomedicine 2018, 14, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Mahajan, V.; Deygen, I.; Klyachko, N.L.; Inskoe, E.; Piroyan, A.; Sokolsky, M.; Okolie, O.; et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 655–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pi, F.; Binzel, D.W.; Lee, T.J.; Li, Z.; Sun, M.; Rychahou, P.; Li, H.; Haque, F.; Wang, S.; Croce, C.M.; et al. Nanoparticle orientation to control RNA loading and ligand display on extracellular vesicles for cancer regression. Nat. Nanotechnol. 2018, 13, 82–89. [Google Scholar] [CrossRef]
- Jo, J.; Okazaki, A.; Nagane, K.; Yamamoto, M.; Tabata, Y. Preparation of cationized polysaccharides as gene transfection carrier for bone marrow-derived mesenchymal stem cells. J. Biomater. Sci. Polym. Ed. 2010, 21, 185–204. [Google Scholar] [CrossRef] [Green Version]
- Nakase, I.; Futaki, S. Combined treatment with a pH-sensitive fusogenic peptide and cationic lipids achieves enhanced cytosolic delivery of exosomes. Sci. Rep. 2015, 5, 10112. [Google Scholar] [CrossRef]
- Nakase, I.; Kogure, K.; Harashima, H.; Futaki, S. Application of a fusiogenic peptide GALA for intracellular delivery. Methods Mol. Biol. (Clifton, NJ) 2011, 683, 525–533. [Google Scholar] [CrossRef]
- Liang, Y.; Eng, W.S.; Colquhoun, D.R.; Dinglasan, R.R.; Graham, D.R.; Mahal, L.K. Complex N-linked glycans serve as a determinant for exosome/microvesicle cargo recruitment. J. Biol. Chem. 2014, 289, 32526–32537. [Google Scholar] [CrossRef] [Green Version]
- Williams, C.; Pazos, R.; Royo, F.; González, E.; Roura-Ferrer, M.; Martinez, A.; Gamiz, J.; Reichardt, N.-C.; Falcón-Pérez, J.M. Assessing the role of surface glycans of extracellular vesicles on cellular uptake. Sci. Rep. 2019, 9, 11920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batista, B.S.; Eng, W.S.; Pilobello, K.T.; Hendricks-Munoz, K.D.; Mahal, L.K. Identification of a conserved glycan signature for microvesicles. J. Proteome Res. 2011, 10, 4624–4633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimoda, A.; Tahara, Y.; Sawada, S.I.; Sasaki, Y.; Akiyoshi, K. Glycan profiling analysis using evanescent-field fluorescence-assisted lectin array: Importance of sugar recognition for cellular uptake of exosomes from mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2017, 491, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.G.; Balmana, M.; Macedo, J.A.; Pocas, J.; Fernandes, A.; de-Freitas-Junior, J.C.M.; Pinho, S.S.; Gomes, J.; Magalhaes, A.; Gomes, C.; et al. Glycosylation in cancer: Selected roles in tumour progression, immune modulation and metastasis. Cell. Immunol. 2018, 333, 46–57. [Google Scholar] [CrossRef] [Green Version]
- Varki, A.; Kannagi, E.; Toole, B.; Stanley, P. Glycosylation changes in cancer. In Essentials of Glycobiology, 3rd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2017. [Google Scholar] [CrossRef]
- Royo, F.; Cossio, U.; Ruiz de Angulo, A.; Llop, J.; Falcon-Perez, J.M. Modification of the glycosylation of extracellular vesicles alters their biodistribution in mice. Nanoscale 2019, 11, 1531–1537. [Google Scholar] [CrossRef] [Green Version]
- Dusoswa, S.A.; Horrevorts, S.K.; Ambrosini, M.; Kalay, H.; Paauw, N.J.; Nieuwland, R.; Pegtel, M.D.; Würdinger, T.; Van Kooyk, Y.; Garcia-Vallejo, J.J. Glycan modification of glioblastoma-derived extracellular vesicles enhances receptor-mediated targeting of dendritic cells. J. Extracell. Vesicles 2019, 8, 1648995. [Google Scholar] [CrossRef] [Green Version]
- Santegoets, K.C.M.; Gielen, P.R.; Büll, C.; Schulte, B.M.; Kers-Rebel, E.D.; Küsters, B.; Bossman, S.A.J.F.H.; ter Laan, M.; Wesseling, P.; Adema, G.J. Expression profiling of immune inhibitory Siglecs and their ligands in patients with glioma. Cancer Immunol. Immunother. 2019, 68, 937–949. [Google Scholar] [CrossRef] [Green Version]
- Hung, M.E.; Leonard, J.N. Stabilization of exosome-targeting peptides via engineered glycosylation. J. Biol. Chem. 2015, 290, 8166–8172. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Gao, Y.; Gong, C.; Wang, Z.; Xia, Q.; Gu, F.; Hu, C.; Zhang, L.; Guo, H.; Gao, S. A33 antibody-functionalized exosomes for targeted delivery of doxorubicin against colorectal cancer. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1973–1985. [Google Scholar] [CrossRef]
- Ye, Z.; Zhang, T.; He, W.; Jin, H.; Liu, C.; Yang, Z.; Ren, J. Methotrexate-loaded extracellular vesicles functionalized with therapeutic and targeted peptides for the treatment of glioblastoma multiforme. ACS Appl. Mater. Interfaces 2018, 10, 12341–12350. [Google Scholar] [CrossRef]
- Zhu, Q.; Ling, X.; Yang, Y.; Zhang, J.; Li, Q.; Niu, X.; Hu, G.; Chen, B.; Li, H.; Wang, Y.; et al. Embryonic stem cells-derived exosomes endowed with targeting properties as chemotherapeutics delivery vehicles for glioblastoma therapy. Adv. Sci. 2019, 6, 1801899. [Google Scholar] [CrossRef] [PubMed]
- Tamura, R.; Uemoto, S.; Tabata, Y. Augmented liver targeting of exosomes by surface modification with cationized pullulan. Acta Biomater. 2017, 57, 274–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakase, I.; Noguchi, K.; Aoki, A.; Takatani-Nakase, T.; Fujii, I.; Futaki, S. Arginine-rich cell-penetrating peptide-modified extracellular vesicles for active macropinocytosis induction and efficient intracellular delivery. Sci. Rep. 2017, 7, 1991. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Busch, D.J.; Vershel, C.P.; Stachowiak, J.C. Multifunctional transmembrane protein ligands for cell-specific targeting of plasma membrane-derived vesicles. Small 2016, 12, 3837–3848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Ren, Y.; Mu, J.; Egilmez, N.K.; Zhuang, X.; Deng, Z.; Zhang, L.; Yan, J.; Miller, D.; Zhang, H.-G. Grapefruit-derived nanovectors use an activated leukocyte trafficking pathway to deliver therapeutic agents to inflammatory tumor sites. Cancer Res. 2015, 75, 2520–2529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betzer, O.; Perets, N.; Angel, A.; Motiei, M.; Sadan, T.; Yadid, G.; Offen, D.; Popovtzer, R. In vivo neuroimaging of exosomes using gold nanoparticles. ACS Nano 2017, 11, 10883–10893. [Google Scholar] [CrossRef]
- Tian, Y.; Li, S.; Song, J.; Ji, T.; Zhu, M.; Anderson, G.J.; Wei, J.; Nie, G. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 2014, 35, 2383–2390. [Google Scholar] [CrossRef]
- Bunggulawa, E.J.; Wang, W.; Yin, T.; Wang, N.; Durkan, C.; Wang, Y.; Wang, G. Recent advancements in the use of exosomes as drug delivery systems. J. Nanobiotechnol. 2018, 16, 81. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Martin, P.; Fogarty, B.; Brown, A.; Schurman, K.; Phipps, R.; Yin, V.P.; Lockman, P.; Bai, S. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in danio rerio. Pharmacol. Res. 2015, 32, 2003–2014. [Google Scholar] [CrossRef]
- Illes, B.; Hirschle, P.; Barnert, S.; Cauda, V.; Engelke, H. Exosome-coated metal–organic framework nanoparticles: An efficient drug delivery platform. Chem. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Saari, H.; Lázaro-Ibáñez, E.; Viitala, T.; Vuorimaa-Laukkanen, E.; Siljander, P.; Yliperttula, M. Microvesicle- and exosome-mediated drug delivery enhances the cytotoxicity of Paclitaxel in autologous prostate cancer cells. J. Control. Release 2015, 220, 727–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellavia, D.; Raimondo, S.; Calabrese, G.; Forte, S.; Cristaldi, M.; Patinella, A.; Memeo, L.; Manno, M.; Raccosta, S.; Diana, P.; et al. Interleukin 3-receptor targeted exosomes inhibit in vitro and in vivo Chronic Myelogenous Leukemia cell growth. Theranostics 2017, 7, 1333–1345. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, D.; Nam, H.; Moon, S.; Kwon, Y.J.; Lee, J.B. Engineered extracellular vesicles and their mimetics for clinical translation. Methods 2019, 19, 30221-x. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yu, Z.-L.; Wu, M.; Ren, J.-G.; Xia, H.-F.; Sa, G.-L.; Zhu, J.-Y.; Pang, D.-W.; Zhao, Y.-F.; Chen, G. Magnetic and folate functionalization enables rapid isolation and enhanced tumor-targeting of cell-derived microvesicles. ACS Nano 2017, 11, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J.A. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef]
- Wahlgren, J.; Karlson, T.D.L.; Brisslert, M.; Vaziri Sani, F.; Telemo, E.; Sunnerhagen, P.; Valadi, H. Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Res. 2012, 40, e130. [Google Scholar] [CrossRef] [Green Version]
- Lamichhane, T.N.; Raiker, R.S.; Jay, S.M. Exogenous DNA loading into extracellular vesicles via electroporation is size-dependent and enables limited gene delivery. Mol. Pharmacol. 2015, 12, 3650–3657. [Google Scholar] [CrossRef] [Green Version]
- Hood, J.L.; Scott, M.J.; Wickline, S.A. Maximizing exosome colloidal stability following electroporation. Anal. Biochem 2014, 448, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Sato, Y.T.; Umezaki, K.; Sawada, S.; Mukai, S.-A.; Sasaki, Y.; Harada, N.; Shiku, H.; Akiyoshi, K. Engineering hybrid exosomes by membrane fusion with liposomes. Sci. Rep. 2016, 6, 21933. [Google Scholar] [CrossRef] [Green Version]
- Haney, M.J.; Klyachko, N.L.; Harrison, E.B.; Zhao, Y.; Kabanov, A.V.; Batrakova, E.V. TPP1 Delivery to lysosomes with extracellular vesicles and their enhanced brain distribution in the animal model of batten disease. Adv. Healthc. Mater. 2019, 8, 1801271. [Google Scholar] [CrossRef]
- Khongkow, M.; Yata, T.; Boonrungsiman, S.; Ruktanonchai, U.R.; Graham, D.; Namdee, K. Surface modification of gold nanoparticles with neuron-targeted exosome for enhanced blood-brain barrier penetration. Sci. Rep. 2019, 9, 8278. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.-C.; Gao, J.-Q. Exosomes as novel bio-carriers for gene and drug delivery. Int. J. Pharmacol. 2017, 521, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, T.N.; Jeyaram, A.; Patel, D.B.; Parajuli, B.; Livingston, N.K.; Arumugasaamy, N.; Schardt, J.S.; Jay, S.M. Oncogene knockdown via active loading of small RNAs into extracellular vesicles by sonication. Cell. Mol. Bioeng. 2016, 9, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Haney, M.J.; Klyachko, N.L.; Zhao, Y.; Gupta, R.; Plotnikova, E.G.; He, Z.; Patel, T.; Piroyan, A.; Sokolsky, M.; Kabanov, A.V.; et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J. Control. Release 2015, 207, 18–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuhrmann, G.; Serio, A.; Mazo, M.; Nair, R.; Stevens, M.M. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J. Control. Release 2015, 205, 35–44. [Google Scholar] [CrossRef]
- Zhang, D.; Qin, X.; Wu, T.; Qiao, Q.; Song, Q.; Zhang, Z. Extracellular vesicles based self-grown gold nanopopcorn for combinatorial chemo-photothermal therapy. Biomaterials 2019, 197, 220–228. [Google Scholar] [CrossRef]
- Ha, D.; Yang, N.; Nadithe, V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: Current perspectives and future challenges. Acta Pharm. Sin. B 2016, 6, 287–296. [Google Scholar] [CrossRef] [Green Version]
- Podolak, I.; Galanty, A.; Sobolewska, D. Saponins as cytotoxic agents: A review. Phytochem. Rev. 2010, 9, 425–474. [Google Scholar] [CrossRef] [Green Version]
- Pascucci, L.; Coccè, V.; Bonomi, A.; Ami, D.; Ceccarelli, P.; Ciusani, E.; Viganò, L.; Locatelli, A.; Sisto, F.; Doglia, S.M.; et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: A new approach for drug delivery. J. Control. Release 2014, 192, 262–270. [Google Scholar] [CrossRef]
- Agrawal, A.K.; Aqil, F.; Jeyabalan, J.; Spencer, W.A.; Beck, J.; Gachuki, B.W.; Alhakeem, S.S.; Oben, K.; Munagala, R.; Bondada, S.; et al. Milk-derived exosomes for oral delivery of paclitaxel. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1627–1636. [Google Scholar] [CrossRef]
- Aubertin, K.; Silva, A.K.A.; Luciani, N.; Espinosa, A.; Djemat, A.; Charue, D.; Gallet, F.; Blanc-Brude, O.; Wilhelm, C. Massive release of extracellular vesicles from cancer cells after photodynamic treatment or chemotherapy. Sci. Rep. 2016, 6, 35376. [Google Scholar] [CrossRef] [PubMed]
- Goh, W.J.; Lee, C.K.; Zou, S.; Woon, E.C.; Czarny, B.; Pastorin, G. Doxorubicin-loaded cell-derived nanovesicles: An alternative targeted approach for anti-tumor therapy. Int. J. Nanomed. 2017, 12, 2759–2767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoneda, A.; Lendorf, M.E.; Couchman, J.R.; Multhaupt, H.A.B. Breast and ovarian cancers: A survey and possible roles for the cell surface heparan sulfate proteoglycans. J. Histochem. Cytochem. 2012, 60, 9–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadla, M.; Palazzolo, S.; Corona, G.; Caligiuri, I.; Canzonieri, V.; Toffoli, G.; Rizzolio, F. Exosomes increase the therapeutic index of doxorubicin in breast and ovarian cancer mouse models. Nanomedicine 2016, 11, 2431–2441. [Google Scholar] [CrossRef] [PubMed]
- Toffoli, G.; Hadla, M.; Corona, G.; Caligiuri, I.; Palazzolo, S.; Semeraro, S.; Gamini, A.; Canzonieri, V.; Rizzolio, F. Exosomal doxorubicin reduces the cardiac toxicity of doxorubicin. Nanomedicine 2015, 10, 2963–2971. [Google Scholar] [CrossRef] [PubMed]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A review of its’ effects on human health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Aqil, F.; Munagala, R.; Jeyabalan, J.; Agrawal, A.K.; Gupta, R. Exosomes for the enhanced tissue bioavailability and efficacy of curcumin. AAPS J. 2017, 19, 1691–1702. [Google Scholar] [CrossRef]
- Zhang, H.-G.; Kim, H.; Liu, C.; Yu, S.; Wang, J.; Grizzle, W.E.; Kimberly, R.P.; Barnes, S. Curcumin reverses breast tumor exosomes mediated immune suppression of NK cell tumor cytotoxicity. Biochim. Biophys. Acta 2007, 1773, 1116–1123. [Google Scholar] [CrossRef] [Green Version]
- Farooqi, A.A.; Rehman, Z.U.; Muntane, J. Antisense therapeutics in oncology: Current status. OncoTargets Ther. 2014, 7, 2035–2042. [Google Scholar] [CrossRef] [Green Version]
- Brazzale, C.; Canaparo, R.; Racca, L.; Foglietta, F.; Durando, G.; Fantozzi, R.; Caliceti, P.; Salmaso, S.; Serpe, L. Enhanced selective sonosensitizing efficacy of ultrasound-based anticancer treatment by targeted gold nanoparticles. Nanomedicine 2016, 11, 3053–3070. [Google Scholar] [CrossRef] [Green Version]
- Limongi, T.; Canta, M.; Racca, L.; Ancona, A.; Tritta, S.; Vighetto, V.; Cauda, V. Improving dispersal of therapeutic nanoparticles in the human body. Nanomedicine 2019, 14, 797–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Gu, F.X.; Chan, J.M.; Wang, A.Z.; Langer, R.S.; Farokhzad, O.C. Nanoparticles in medicine: Therapeutic applications and developments. Clin. Pharmacol. Ther. 2008, 83, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Vogt, S.; Stadlmayr, G.; Grillari, J.; Rüker, F.; Wozniak-Knopp, G. Engineering of surface proteins in extracellular vesicles for tissue-specific targeting. Curr. Top. Biochem. Eng. 2019. [Google Scholar] [CrossRef] [Green Version]
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Barile, L.; Vassalli, G. Exosomes: Therapy delivery tools and biomarkers of diseases. Pharmacol. Ther. 2017, 174, 63–78. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, A.; Amreddy, N.; Babu, A.; Panneerselvam, J.; Mehta, M.; Muralidharan, R.; Chen, A.; Zhao, Y.D.; Razaq, M.; Riedinger, N.; et al. Nanosomes carrying doxorubicin exhibit potent anticancer activity against human lung cancer cells. Sci. Rep. 2016, 6, 38541. [Google Scholar] [CrossRef]
- Cheng, G.; Li, W.; Ha, L.; Han, X.; Hao, S.; Wan, Y.; Wang, Z.; Dong, F.; Zou, X.; Mao, Y.; et al. Self-assembly of extracellular vesicle-like metal–organic framework nanoparticles for protection and intracellular delivery of biofunctional proteins. J. Am. Chem. Soc. 2018, 140, 7282–7291. [Google Scholar] [CrossRef]
- Piffoux, M.; Silva, A.K.A.; Lugagne, J.-B.; Hersen, P.; Wilhelm, C.; Gazeau, F. Extracellular vesicle production loaded with nanoparticles and drugs in a trade-off between loading, yield and purity: Towards a personalized drug delivery system. Adv. Biosyst. 2017, 1, 1700044. [Google Scholar] [CrossRef]
- Bose, R.J.C.; Uday Kumar, S.; Zeng, Y.; Afjei, R.; Robinson, E.; Lau, K.; Bermudez, A.; Habte, F.; Pitteri, S.J.; Sinclair, R.; et al. Tumor cell-derived extracellular vesicle-coated nanocarriers: An efficient theranostic platform for the cancer-specific delivery of Anti-miR-21 and imaging agents. ACS Nano 2018, 12, 10817–10832. [Google Scholar] [CrossRef]
- Dumontel, B.; Susa, F.; Limongi, T.; Canta, M.; Racca, L.; Chiodoni, A.; Garino, N.; Chiabotto, G.; Centomo, M.L.; Pignochino, Y.; et al. ZnO nanocrystals shuttled by extracellular vesicles as effective Trojan nano-horses against cancer cells. Nanomedicine 2019, 14. [Google Scholar] [CrossRef] [Green Version]
- Sun, D.; Zhuang, X.; Xiang, X.; Liu, Y.; Zhang, S.; Liu, C.; Barnes, S.; Grizzle, W.; Miller, D.; Zhang, H.-G. A novel nanoparticle drug delivery system: The anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol. Ther. 2010, 18, 1606–1614. [Google Scholar] [CrossRef] [PubMed]
- Aqil, F.; Kausar, H.; Agrawal, A.K.; Jeyabalan, J.; Kyakulaga, A.-H.; Munagala, R.; Gupta, R. Exosomal formulation enhances therapeutic response of celastrol against lung cancer. Exp. Mol. Pathol. 2016, 101, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, M.; Villa, A.; Rizzi, N.; Kuryk, L.; Rinner, B.; Cerullo, V.; Yliperttula, M.; Mazzaferro, V.; Ciana, P. Extracellular vesicles enhance the targeted delivery of immunogenic oncolytic adenovirus and paclitaxel in immunocompetent mice. J. Control. Release 2019, 294, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Faruqu, F.N.; Wang, J.T.; Xu, L.; McNickle, L.; Chong, E.M.; Walters, A.; Gurney, M.; Clayton, A.; Smyth, L.A.; Hider, R.; et al. Membrane radiolabelling of exosomes for comparative biodistribution analysis in immunocompetent and immunodeficient mice—A novel and universal approach. Theranostics 2019, 9, 1666–1682. [Google Scholar] [CrossRef]
- Greco, K.A.; Franzen, C.A.; Foreman, K.E.; Flanigan, R.C.; Kuo, P.C.; Gupta, G.N. PLK-1 Silencing in bladder cancer by siRNA delivered with exosomes. Urology 2016, 91, 241.e1–241.e7. [Google Scholar] [CrossRef]
- Pomatto, M.A.C.; Bussolati, B.; D’Antico, S.; Ghiotto, S.; Tetta, C.; Brizzi, M.F.; Camussi, G. Improved loading of plasma-derived extracellular vesicles to encapsulate antitumor miRNAs. Mol. Ther. Methods Clin. Dev. 2019, 13, 133–144. [Google Scholar] [CrossRef] [Green Version]
- Mentkowski, K.I.; Snitzer, J.D.; Rusnak, S.; Lang, J.K. Therapeutic potential of engineered extracellular vesicles. AAPS J. 2018, 20, 50. [Google Scholar] [CrossRef] [Green Version]
- Zomer, A.; Maynard, C.; Verweij, F.J.; Kamermans, A.; Schäfer, R.; Beerling, E.; Schiffelers, R.M.; de Wit, E.; Berenguer, J.; Ellenbroek, S.I.J.; et al. In vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell 2015, 161, 1046–1057. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, R.M. Stromal-cell and cancer-cell exosomes leading the metastatic exodus for the promised niche. Breast Cancer Res. 2013, 15, 310. [Google Scholar] [CrossRef] [Green Version]
- Melo, S.A.; Luecke, L.B.; Kahlert, C.; Fernandez, A.F.; Gammon, S.T.; Kaye, J.; LeBleu, V.S.; Mittendorf, E.A.; Weitz, J.; Rahbari, N.; et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 2015, 523, 177. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.S.; Kim, Y.; Zhang, W.; Song, I.H.; Tung, C.-H. Facile metabolic glycan labeling strategy for exosome tracking. Biochim. Biophys. Acta 2018, 1862, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.P.; Tannous, B.A.; Breakefield, X.O. Noninvasive in vivo monitoring of extracellular vesicles. In Bioluminescent Imaging: Methods and Protocols; Badr, C.E., Ed.; Humana Press: Totowa, NJ, USA, 2014; pp. 249–258. [Google Scholar] [CrossRef] [Green Version]
- Lai, C.P.; Mardini, O.; Ericsson, M.; Prabhakar, S.; Maguire, C.; Chen, J.W.; Tannous, B.A.; Breakefield, X.O. Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano 2014, 8, 483–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, Y.; Nishikawa, M.; Shinotsuka, H.; Matsui, Y.; Ohara, S.; Imai, T.; Takakura, Y. Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. J. Biotechnol. 2013, 165, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Takahashi, Y.; Nishikawa, M.; Kato, K.; Morishita, M.; Yamashita, T.; Matsumoto, A.; Charoenviriyakul, C.; Takakura, Y. Macrophage-dependent clearance of systemically administered B16BL6-derived exosomes from the blood circulation in mice. J. Extracell. Vesicles 2015, 4, 26238. [Google Scholar] [CrossRef] [PubMed]
- Morishita, M.; Takahashi, Y.; Nishikawa, M.; Ariizumi, R.; Takakura, Y. Enhanced class I tumor antigen presentation via cytosolic delivery of exosomal cargos by tumor-cell-derived exosomes displaying a pH-sensitive fusogenic peptide. Mol. Pharmacol. 2017, 14, 4079–4086. [Google Scholar] [CrossRef] [PubMed]
- Limoni, S.K.; Moghadam, M.F.; Moazzeni, S.M.; Gomari, H.; Salimi, F. Engineered exosomes for targeted transfer of siRNA to HER2 positive breast cancer cells. Appl. Biochem. Biotechnol. 2019, 187, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-H.; Forterre, A.V.; Zhao, J.; Frimannsson, D.O.; Delcayre, A.; Antes, T.J.; Efron, B.; Jeffrey, S.S.; Pegram, M.D.; Matin, A.C. Anti-HER2 scFv-directed extracellular vesicle-mediated mRNA-based gene delivery inhibits growth of HER2-positive human breast tumor xenografts by prodrug activation. Mol. Cancer Ther. 2018, 17, 1133–1142. [Google Scholar] [CrossRef] [Green Version]
- Hartman, Z.C.; Wei, J.; Glass, O.K.; Guo, H.; Lei, G.; Yang, X.-Y.; Osada, T.; Hobeika, A.; Delcayre, A.; Le Pecq, J.-B.; et al. Increasing vaccine potency through exosome antigen targeting. Vaccine 2011, 29, 9361–9367. [Google Scholar] [CrossRef] [Green Version]
- Rountree, R.B.; Mandl, S.J.; Nachtwey, J.M.; Dalpozzo, K.; Do, L.; Lombardo, J.R.; Schoonmaker, P.L.; Brinkmann, K.; Dirmeier, U.; Laus, R.; et al. Exosome targeting of tumor antigens expressed by cancer vaccines can improve antigen immunogenicity and therapeutic efficacy. Cancer Res. 2011, 71, 5235. [Google Scholar] [CrossRef] [Green Version]
- Kooijmans, S.A.A.; Aleza, C.G.; Roffler, S.R.; van Solinge, W.W.; Vader, P.; Schiffelers, R.M. Display of GPI-anchored anti-EGFR nanobodies on extracellular vesicles promotes tumour cell targeting. J. Extracell. Vesicles 2016, 5, 31053. [Google Scholar] [CrossRef]
- Yamamoto, T.; Teramura, Y.; Itagaki, T.; Arima, Y.; Iwata, H. Interaction of poly(ethylene glycol)-conjugated phospholipids with supported lipid membranes and their influence on protein adsorption. Sci. Technol. Adv. Mater. 2016, 17, 677–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, L.; Dong, D.; Yu, Z.-L.; Zhao, Y.-F.; Pang, D.-W.; Zhang, Z.-L. Folate-engineered microvesicles for enhanced target and synergistic therapy toward breast cancer. ACS Appl. Mater. Interfaces 2017, 9, 5100–5108. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhu, J.-Y.; Zhang, Z.-L.; Zhang, W.; Ren, J.-G.; Wu, M.; Hong, Z.-Y.; Lv, C.; Pang, D.-W.; Zhao, Y.-F. Transformation of cell-derived microparticles into quantum-dot-labeled nanovectors for antitumor siRNA delivery. Angew. Chem. Int. Ed. 2015, 54, 1036–1040. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, W.; Zhang, L.; Ban, L.; Chen, P.; Du, W.; Feng, X.; Liu, B.-F. Chemically edited exosomes with dual ligand purified by microfluidic device for active targeted drug delivery to tumor cells. ACS Appl. Mater. Interfaces 2017, 9, 27441–27452. [Google Scholar] [CrossRef]
- Wang, J.; Dong, Y.; Li, Y.; Li, W.; Cheng, K.; Qian, Y.; Xu, G.; Zhang, X.; Hu, L.; Chen, P.; et al. Designer exosomes for active targeted chemo-photothermal synergistic tumor therapy. Adv. Funct. Mater. 2018, 28, 1707360. [Google Scholar] [CrossRef]
- Suetsugu, A.; Honma, K.; Saji, S.; Moriwaki, H.; Ochiya, T.; Hoffman, R.M. Imaging exosome transfer from breast cancer cells to stroma at metastatic sites in orthotopic nude-mouse models. Adv. Drug Deliv. Rev. 2013, 65, 383–390. [Google Scholar] [CrossRef]
- Lai, C.P.; Kim, E.Y.; Badr, C.E.; Weissleder, R.; Mempel, T.R.; Tannous, B.A.; Breakefield, X.O. Visualization and tracking of tumour extracellular vesicle delivery and RNA translation using multiplexed reporters. Nat. Commun. 2015, 6, 7029. [Google Scholar] [CrossRef]
- Zeelenberg, I.S.; Ostrowski, M.; Krumeich, S.; Bobrie, A.; Jancic, C.; Boissonnas, A.; Delcayre, A.; Le Pecq, J.-B.; Combadière, B.; Amigorena, S.; et al. Targeting tumor antigens to secreted membrane vesicles in vivo induces efficient antitumor immune responses. Cancer Res. 2008, 68, 1228. [Google Scholar] [CrossRef] [Green Version]
- Zeelenberg, I.S.; van Maren, W.W.C.; Boissonnas, A.; Van Hout-Kuijer, M.A.; Den Brok, M.H.M.G.M.; Wagenaars, J.A.L.; van der Schaaf, A.; Jansen, E.J.R.; Amigorena, S.; Théry, C.; et al. Antigen localization controls T cell-mediated tumor immunity. J. Immunol. 2011, 187, 1281. [Google Scholar] [CrossRef]
- Delcayre, A.; Estelles, A.; Sperinde, J.; Roulon, T.; Paz, P.; Aguilar, B.; Villanueva, J.; Khine, S.; Le Pecq, J.-B. Exosome display technology: Applications to the development of new diagnostics and therapeutics. Blood Cells Mol. Dis. 2005, 35, 158–168. [Google Scholar] [CrossRef]
- Ohno, S.-I.; Takanashi, M.; Sudo, K.; Ueda, S.; Ishikawa, A.; Matsuyama, N.; Fujita, K.; Mizutani, T.; Ohgi, T.; Ochiya, T.; et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol. Ther. 2013, 21, 185–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alhasan, A.H.; Patel, P.C.; Choi, C.H.J.; Mirkin, C.A. Exosome encased spherical nucleic acid gold nanoparticle conjugates as potent microRNA regulation agents. Small 2014, 10, 186–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, S.-C.; Yuan, T.; Zhang, Y.-L.; Yin, W.-J.; Guo, S.-C.; Zhang, C.-Q. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics 2017, 7, 180–195. [Google Scholar] [CrossRef]
- Cho, J.-A.; Yeo, D.-J.; Son, H.-Y.; Kim, H.-W.; Jung, D.-S.; Ko, J.-K.; Koh, J.S.; Kim, Y.-N.; Kim, C.-W. Exosomes: A new delivery system for tumor antigens in cancer immunotherapy. Int. J. Cancer 2005, 114, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Kolluri, K.K.; Gowers, K.H.C.; Janes, S.M. TRAIL delivery by MSC-derived extracellular vesicles is an effective anticancer therapy. J. Extracell. Vesicles 2017, 6, 1265291. [Google Scholar] [CrossRef] [PubMed]
- Rivoltini, L.; Chiodoni, C.; Squarcina, P.; Tortoreto, M.; Villa, A.; Vergani, B.; Bürdek, M.; Botti, L.; Arioli, I.; Cova, A.; et al. TNF-related apoptosis-inducing ligand (TRAIL)-armed exosomes deliver proapoptotic signals to tumor site. Clin. Cancer Res. 2016, 22, 3499. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Hong, Y.; Cho, E.; Kim, G.B.; Kim, I.-S. Extracellular vesicles as a platform for membrane-associated therapeutic protein delivery. J. Extracell. Vesicles 2018, 7, 1440131. [Google Scholar] [CrossRef] [Green Version]
- Di Bonito, P.; Chiozzini, C.; Arenaccio, C.; Anticoli, S.; Manfredi, F.; Olivetta, E.; Ferrantelli, F.; Falcone, E.; Ruggieri, A.; Federico, M. Antitumor HPV E7-specific CTL activity elicited by in vivo engineered exosomes produced through DNA inoculation. Int. J. Nanomed. 2017, 12, 4579–4591. [Google Scholar] [CrossRef] [Green Version]
- Meyer, C.; Losacco, J.; Stickney, Z.; Li, L.; Marriott, G.; Lu, B. Pseudotyping exosomes for enhanced protein delivery in mammalian cells. Int. J. Nanomed. 2017, 12, 3153–3170. [Google Scholar] [CrossRef] [Green Version]
- Kochenderfer, J.N.; Rosenberg, S.A. Treating B-cell cancer with T cells expressing anti-CD19 chimeric antigen receptors. Nat. Rev. Clin. Oncol. 2013, 10, 267–276. [Google Scholar] [CrossRef]
- Yim, N.; Ryu, S.-W.; Choi, K.; Lee, K.R.; Lee, S.; Choi, H.; Kim, J.; Shaker, M.R.; Sun, W.; Park, J.-H.; et al. Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein–protein interaction module. Nat. Commun. 2016, 7, 12277. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, H.; Bai, M.; Ning, T.; Ge, S.; Deng, T.; Liu, R.; Zhang, L.; Ying, G.; Ba, Y. Exosomes serve as nanoparticles to deliver anti-miR-214 to reverse chemoresistance to cisplatin in gastric cancer. Mol. Ther. 2018, 26, 774–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, K.; Lowry, M.C.; Corcoran, C.; Martinez, V.G.; Daly, M.; Rani, S.; Gallagher, W.M.; Radomski, M.W.; MacLeod, R.A.F.; O’Driscoll, L. MiR-134 in extracellular vesicles reduces triple-negative breast cancer aggression and increases drug sensitivity. Oncotarget 2015, 6, 32774–32789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lou, G.; Song, X.; Yang, F.; Wu, S.; Wang, J.; Chen, Z.; Liu, Y. Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J. Hematol. Oncol. 2015, 8, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katakowski, M.; Buller, B.; Zheng, X.; Lu, Y.; Rogers, T.; Osobamiro, O.; Shu, W.; Jiang, F.; Chopp, M. Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett. 2013, 335, 201–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimbo, K.; Miyaki, S.; Ishitobi, H.; Kato, Y.; Kubo, T.; Shimose, S.; Ochi, M. Exosome-formed synthetic microRNA-143 is transferred to osteosarcoma cells and inhibits their migration. Biochem. Biophys. Res. Commun. 2014, 445, 381–387. [Google Scholar] [CrossRef]
- Munoz, J.L.; Bliss, S.A.; Greco, S.J.; Ramkissoon, S.H.; Ligon, K.L.; Rameshwar, P. Delivery of functional anti-miR-9 by mesenchymal stem cell-derived exosomes to glioblastoma multiforme cells conferred chemosensitivity. Mol. Ther. Nucleic Acids 2013, 2, e126. [Google Scholar] [CrossRef]
- Mizrak, A.; Bolukbasi, M.F.; Ozdener, G.B.; Brenner, G.J.; Madlener, S.; Erkan, E.P.; Ströbel, T.; Breakefield, X.O.; Saydam, O. Genetically engineered microvesicles carrying suicide mRNA/protein inhibit schwannoma tumor growth. Mol. Ther. 2013, 21, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Wang, Y.; Bai, M.; Wang, J.; Zhu, K.; Liu, R.; Ge, S.; Li, J.; Ning, T.; Deng, T.; et al. Exosomes serve as nanoparticles to suppress tumor growth and angiogenesis in gastric cancer by delivering hepatocyte growth factor siRNA. Cancer Sci. 2018, 109, 629–641. [Google Scholar] [CrossRef] [Green Version]
- Shtam, T.A.; Kovalev, R.A.; Varfolomeeva, E.Y.; Makarov, E.M.; Kil, Y.V.; Filatov, M.V. Exosomes are natural carriers of exogenous siRNA to human cells in vitro. Cell Commun. Signal. 2013, 11, 88. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Gu, N.; Zhang, X.-E.; Wang, D.-B. Light-inducible exosome-based vehicle for endogenous RNA loading and delivery to leukemia cells. Adv. Funct. Mater. 2019, 29, 1807189. [Google Scholar] [CrossRef]
- Aspe, J.R.; Diaz Osterman, C.J.; Jutzy, J.M.S.; Deshields, S.; Whang, S.; Wall, N.R. Enhancement of Gemcitabine sensitivity in pancreatic adenocarcinoma by novel exosome-mediated delivery of the Survivin-T34A mutant. J. Extracell. Vesicles 2014, 3. [Google Scholar] [CrossRef]
- Tang, K.; Zhang, Y.; Zhang, H.; Xu, P.; Liu, J.; Ma, J.; Lv, M.; Li, D.; Katirai, F.; Shen, G.-X.; et al. Delivery of chemotherapeutic drugs in tumour cell-derived microparticles. Nat. Commun. 2012, 3, 1282. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.K.A.; Kolosnjaj-Tabi, J.; Bonneau, S.; Marangon, I.; Boggetto, N.; Aubertin, K.; Clément, O.; Bureau, M.F.; Luciani, N.; Gazeau, F.; et al. Magnetic and photoresponsive theranosomes: Translating cell-released vesicles into smart nanovectors for cancer therapy. ACS Nano 2013, 7, 4954–4966. [Google Scholar] [CrossRef]
- Silva, A.K.A.; Luciani, N.; Gazeau, F.; Aubertin, K.; Bonneau, S.; Chauvierre, C.; Letourneur, D.; Wilhelm, C. Combining magnetic nanoparticles with cell derived microvesicles for drug loading and targeting. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 645–655. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Kim, J.; Jeong, M.; Lee, H.; Goh, U.; Kim, H.; Kim, B.; Park, J.-H. Liposome-based engineering of cells to package hydrophobic compounds in membrane vesicles for tumor penetration. Nano Lett. 2015, 15, 2938–2944. [Google Scholar] [CrossRef]
- Lee, J.; Lee, H.; Goh, U.; Kim, J.; Jeong, M.; Lee, J.; Park, J.-H. Cellular engineering with membrane fusogenic liposomes to produce functionalized extracellular vesicles. ACS Appl. Mater. Interfaces 2016, 8, 6790–6795. [Google Scholar] [CrossRef]
- Sancho-Albero, M.; Navascués, N.; Mendoza, G.; Sebastián, V.; Arruebo, M.; Martín-Duque, P.; Santamaría, J. Exosome origin determines cell targeting and the transfer of therapeutic nanoparticles towards target cells. J. Nanobiotechnol. 2019, 17, 16. [Google Scholar] [CrossRef]
- Yong, T.; Zhang, X.; Bie, N.; Zhang, H.; Zhang, X.; Li, F.; Hakeem, A.; Hu, J.; Gan, L.; Santos, H.A.; et al. Tumor exosome-based nanoparticles are efficient drug carriers for chemotherapy. Nat. Commun. 2019, 10, 3838. [Google Scholar] [CrossRef] [Green Version]
- Mulens-Arias, V.; Nicolas-Boluda, A.; Brun, A.; Gazeau, F. Theranostic iron oxide nanoparticle cargo defines extracellular vesicle-dependent modulation of macrophage activation and migratory behavior. Adv. Biosyst. 2018, 2. [Google Scholar] [CrossRef]
- Burdakov, V.S.; Kovalev, R.A.; Pantina, R.A.; Varfolomeeva, E.Y.; Makarov, E.M.; Filatov, M.V. Exosomes transfer p53 between cells and can suppress growth and proliferation of p53-negative cells. Cell Tissue Biol. 2018, 12, 20–26. [Google Scholar] [CrossRef] [Green Version]
- Maguire, C.A.; Balaj, L.; Sivaraman, S.; Crommentuijn, M.H.W.; Ericsson, M.; Mincheva-Nilsson, L.; Baranov, V.; Gianni, D.; Tannous, B.A.; Sena-Esteves, M.; et al. Microvesicle-associated AAV vector as a novel gene delivery system. Mol. Ther. 2012, 20, 960–971. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Zhou, X.; Wei, M.; Gao, X.; Zhao, L.; Shi, R.; Sun, W.; Duan, Y.; Yang, G.; Yuan, L. In vitro and in vivo RNA inhibition by CD9-HuR functionalized exosomes encapsulated with miRNA or CRISPR/dCas9. Nano Lett. 2019, 19, 19–28. [Google Scholar] [CrossRef]


| EVs Type | Nanotechnological Modification | Application | Reference |
|---|---|---|---|
| Exosomes from 4T1, MCF-7 and PC3 cells | Labeling with DiR a | In vivo fluorescence imaging of tumor-derived exosomes | [47] |
| Exosomes from 4T1 cells | Surface conjugation with azide-fluor545 by click chemistry | In vitro fluorescence imaging | [50] |
| Exosomes from PC12 cells | Labeling of exosomes proteins with TAMRA-NHS b | In vitro fluorescence imaging | [49] |
| Exosomes from fetal bovine serum | PEGylation by post-insertion of DSPE-PEG-mannose or chemical conjugation of NHS-PEG | Stealth and targeted Exosomes for elevated uptake in DCs | [54] |
| Exosomes from embryonic stem cells | DSPE-PEG-c(RGDyK) | Targeting glioblastoma, lung cancer and prostate cancer cells | [73] |
| Exosomes from RAW 264.7 cells | Post-insertion of DSPE-PEG-AA | Stealth and targeted exosomes for the in vitro and in vivo treatment of lung cancer | [55] |
| EVs from Neuro2A cells | Post-insertion of DMPE-PEG-EGa1 nanobody | Stealth and targeted EVs for the in vitro and in vivo treatment of cancer cells | [52] |
| EVs from HEK 293T cells | Post-insertion of cholesterolTEG-pRNA3WJ-targeting ligands | Targeted EVs for the in vivo treatment of breast, prostate and colorectal cancer | [57] |
| Exosomes from MSCs | Chemical functionalization with cationized pullulan | Targeted exosomes for the in vitro and in vivo treatment of liver injury | [74] |
| EVs from MLP29 cells | Modification of EVs surface glycosylation by neuraminidase | Modification of EVs glycosylation for altered in vivo biodistribution | [67] |
| EVs from U87 and GBM8 cells | Enzymatic modification of EVs surface glycosylation and insertion of targeting ligand to DC-SIGN | Modification of EVs glycosylation and insertion of targeting ligand for improved uptake in DCs | [68] |
| Exosomes from Hela cells | Hexadecaarginine (R16) peptide, an arginine-rich cell-penetrating peptide | Activation of the macropinocytosis pathway, affecting cellular uptake of EVs | [75] |
| Exosomes from HeLa cells | Modification with LTX and GALA peptide via electrostatic interactions | Charge modified exosomes for enhanced cellular uptake and in vitro cytosolic release | [59] |
| Exosomes from transfected HEK 293T | Glycosylation of targeting-peptide-Lamp2b fusion proteins | Stabilization of targeting peptide-Lamp2b fusion protein with glycosylation motif | [70] |
| Exosomes from human colorectal carcinoma | Fe3O4 Superparamagnetic nanoparticles with high density A33 antibody | Antiproliferative effect in colorectal cancer | [71] |
| Extracellular vesicles from fibroblasts | Apoptotic peptide Lys-Leu-Ala (KLA) or LDL | Extravasation across BBB and target glioblastoma multiforme | [72] |
| Exosomes from bovine milk | Folic acid | Human lung and breast cancer reduction | [41] |
| Plasma membrane vesicles | Bond of the EGF ligand to the transmembrane domain of transferrin receptor | Target EGFR-expressing cancers | [76] |
| Exosomes-like nanoparticles from grapefruit | Inflammatory related receptor enriched membranes of activated leukocytes | Target inflammatory tumor tissues | [77] |
| EVs Type | Nanotechnological Modification | Application | Loading Method | Reference |
|---|---|---|---|---|
| Exosomes from mesenchymal stem cells | Glucose-coated gold nanoparticles (NPs) | In vivo neuroimaging | Co-incubation | [78] |
| Exosome from lung cancer or fibroblasts | Gold NPs and doxorubicin | Lung cancer treatment | Co-incubation | [118] |
| EVs from breast adenocarcinoma | MOF NPs. NPs matrix contained gelonin | Inhibit adenocarcinoma growth | Sonication and extrusion | [119] |
| Exosomes from Hela cells | MOF NPs | Hela cells | Co-incubation | [82] |
| EVs from KB cells | ZnO NPs | Cytotoxic effect against KB cells | Co-incubation | [122] |
| EVs from endothelial, cancer and stem cell lines | Porphyrins | To improve photodynamic therapy | Electroporation, extrusion, saponin-assisted and dialysis | [97] |
| Exosomes from embryonic stem cells | Paclitaxel | Glioma therapy | Co-incubation | [73] |
| Milk-derived exosomes | To reduce paclitaxel’s side effects | Co-incubation | [102] | |
| Exosomes from macrophages | To overcome MDR in cancer cells | Co-incubation, electroporation and sonication | [56] | |
| Exosomes from brain cell lines | To treat brain tumor | Co-incubation | [81] | |
| EVs from prostatic cancer | Cytotoxic effect against prostate cancer | Co-incubation | [83] | |
| Exosomes from human colorectal carcinoma | Doxorubicin | Antiproliferative effect in colorectal cancer | Dialysis | [71] |
| Exosomes from breast cancer | To treat breast and ovarian cancer | Electroporation | [106] | |
| Exosomes from breast cancer | To reduce cardiotoxicity of doxorubicin | Electroporation | [107] | |
| Exosomes from 4T1, MCF-7, and PC3 cell line | Breast cancer | Co-incubation | [47] | |
| Exosomes from mouse immature dendritic cells | For targeted delivery of chemotherapeutic | Electroporation | [79] | |
| Milk-derived exosomes | Curcumin | Cervical cancer | Co-incubation | [109] |
| Exosomes from lymphoma cells | Activate myeloid cells in vivo | Co-incubation | [123] | |
| Plant exosomes | Colon cancer | NCT01294072 | ||
| Milk-derived exosomes | Paclitaxel, Docetaxel, Withaferin A and curcumin | Targeting and therapy of lung cancer cells | Co-incubation | [41] |
| Milk-derived exosomes | Celastrol | Inhibition of Hsp90 and NF-κB a activation pathways in lung cancer | Co-incubation | [124] |
| EVs from lung cancer | Oncolytic adenovirus and paclitaxel | Enhance immunogenicity in lung cancer | Co-incubation | [125] |
| Exosomes from HEK 293 cells | siRNA | Efficient delivery of siRNA in cancer cells | Electroporation | [126] |
| Exosomes from HEK 293 cells | Polo-like kinase 1 (PLK-1) siRNA | Silencing PLK-1 gene in bladder cancer cells | Electroporation | [127] |
| Exosomes from HEK 293 and MCF-7 cells | siRNA, miRNA and ssDNA b | Oncogene knockdown | Sonication | [95] |
| Plasma-derived EVs | miRNA cel-39 | Promote apoptosis of hepatocellular carcinoma | Electroporation | [128] |
| EVs Type | Nanotechnological Modification | Application | Reference |
|---|---|---|---|
| Exosomes from breast cancer | Ac4ManNAz labeled with ADIBO-fluorescent dyes | Breast cancer imaging | [133] |
| Exosomes from melanoma | Gaussian Luciferase | Biodistribution and tumor targeting | [137] |
| Exosomes from HEK 293T | [134,135] | ||
| Exosomes from melanoma | [136] | ||
| Exosomes from HEK 293T | Alexa Fluor 680-Streptavidin | Biodistribution and tumor targeting | [135] |
| Exosomes from different cell lines | GFP | Monitoring and tracking of exosomes uptake in different types of cancer | [130,131,132,149] |
| EVs from HEK 293T | Palmitoylation signal genetically fused in-frame to the N terminus of enhanced green fluorescence protein (EGFP) and tandem dimer Tomato (tdTomato) | Monitoring the uptake by cancer cells | [150] |
| Exosomes from macrophage | Arginyl–glycyl–aspartic acid (RGD)-functionalized DSPE-PEG (DSPE-PEG-RGD), sulfhydryl-functionalized DSPE-PEG (DSPE-PEG-SH) and folic acid | Targeting Hela cells | [148] |
| EVs from squamous cell carcinoma | DSPE-PEG-Biotin and folate | Targeting breast cancer for diagnosis and therapy | [145] |
| Exosomes from HUVEC | DSPE-PEG-biotin | Targeting hepatocellular carcinoma | [147] |
| EVs from macrophage | DSPE-PEG-Biotin and folate | Targeting Hela cells | [86] |
| EVs from HUVEC | DSPE-PEG-Biotin | Targeting melanoma | [146] |
| Exosomes from HEK 293T | DARPins | Targeting HER-2 over-expressing cancer cells (breast, ovarian and gastric cancers) | [139] |
| Exosomes from HEK 293 | Anti-HER2 scFv antibody (ML39) | Inhibit the growth of HER2 positive breast cancer | [140] |
| Exosomes from murine melanoma | Streptavidin-lactadherin fusion protein linked with biotinylated pH-sensitive fusogenic GALA peptide | Cancer immunotherapy | [138] |
| Extracellular vesicles from murine neural stem cells | Anti-EGFR fused to GPI anchor signal peptides | Targeting of Hela cells | [143] |
| Exosomes from dendritic cells | Lamp2b fused with iRGD (CRGDKGPDC) targeting peptide for αv integrin | Targeting breast cancer | [79] |
| Exosomes from dendritic cells | Carcinoembryonic antigen or HER2 linked to the C1C2 domain of lactadherin | Targeting breast cancer | [141] |
| Exosomes from HEK 293F cells | Prostate-specific antigen, and prostatic acid phosphatase linked to the C1C2 domain of lactadherin | Targeting prostate cancer | [142] |
| Exosomes from fibrosarcoma cells | Chicken egg ovalbumin by fusing it to the C1C2 domain of the lactadherin | In vivo fibrosarcoma. More efficient antitumor immune response | [151,152] |
| Exosomes from dendritic cell line | C1C2 domain of lactadherin is fused to soluble proteins or extracellular domain of membrane proteins | Generate antibodies against tumor biomarkers | [153] |
| Transmembrane protein HLA-A2 a |
| EVs Type | Nanotechnological Modification | Application | Reference |
|---|---|---|---|
| Human placental mesenchymal stem cells | Hollow gold NPs | Hyperthermia therapy against different type of cancer | [181] |
| Exosomes from hepatocellular carcinoma | Porous silicon NPs loaded with doxorubicin | Decreased the expression of multidrug-resistant protein P-glycoprotein | [182] |
| EVs from mesenchymal stem cells | SPIONs | Therapy against leukemia | [183] |
| EVs from HUVEC | Iron oxide NPs and clinical photosensitizer (Foscan) | Phototoxicity against prostate adenocarcinoma cells | [120] |
| Extracellular vesicles from human macrophages | Iron oxide nanoparticles and m-THPC photosensitizer | Theranostic approach against cervical and prostate cancer | [177] |
| Microvesicles from different cancer cell lines | A hydrophobic photosensitizer zinc phthalocyanine encapsulated in liposomes | Photodynamic therapy for different cancer cell lines | [179] |
| Microvesicles from human macrophages | Doxorubicin, tissue-plasminogen activator and two photosensitizers | Targeting and therapy of ovarian and prostate cancers | [178] |
| Exosomes from mesenchyme stromal cells | Paclitaxel | Treatment of pancreatic cancer | [101] |
| Exosomes from melanoma cell line | Survivin-T34A and Gemcitabine | Treatment of pancreatic adenocarcinoma | [175] |
| Apoptotic bodies from tumoral cells | Doxorubicin or Metotrexate | Tumor cells killing with reduce side effects | [176] NCT01854866 |
| Exosomes from breast cancer | Curcumin | Reverse inhibition of NK cell tumor cytotoxicity in breast cancer | [110] |
| Exosomes from HEK 293 | P53 gene | Transfer p53 gene to p53-deficient cells | [184] |
| Exosomes from HEK 293T | miR-21 sponges | Therapy for leukemia cells | [174] |
| Extracellular vesicles from breast cancer | Anti-miR-21 | Theranostic method for breast cancer | [121] |
| Exosomes from HEK 293T | Inhibitor of miR-214 | Reverse chemoresistance to cisplatin in gastric cancer | [165] |
| Exosomes from prostate cancer cells | Anti-miR-21 spherical nucleic acid | Prostate cancer | [155] |
| Exosomes from mammary carcinomas | miR-134 | Increase sensitivity of breast cancers to chemotherapeutic drugs | [166] |
| Exosomes from mesenchyme stem cells | miR-122 | Increase sensitivity of hepatocellular carcinoma to chemotherapeutic drugs | [167] |
| Exosomes from mesenchymal stem cells | miR-143 | Inhibit migration of osteosarcoma cells | [169] |
| Exosomes from mesenchymal stem cells | anti-miR-9 | Increase sensitivity of glioblastoma multiforme to chemotherapeutic drugs | [170] |
| Exosomes from mesenchyme stem cells | miR-146b | Inhibit glioma growth | [168] |
| Microvesicles from HEK 293T | Suicide gene mRNA and protein-cytosine deaminase fused to uracil phosphoribosyltransferase | Inhibit schwannoma tumor growth | [171] |
| Exosomes from HEK 293T | HGF siRNA | Inhibition of tumor growth and angiogenesis in gastric cancer | [172] |
| Exosomes from breast cancer cells | RAD51 and RAD52 siRNA | Gene therapy against breast cancer | [173] |
| Extracellular vesicles from mesenchymal stem cells | TNF-related apoptosis-inducing ligand (TRAIL) | Lung, breast, kidney cancer, pleural mesothelioma and neuroblastoma | [158] |
| Exosomes from chronic myelogenous leukemia cells | TNF-related apoptosis-inducing ligand (TRAIL) | Enhance apoptosis in lymphoma | [159] |
| Exosomes from HEK 293T | VSVG | Glioblastoma and liver cancer cells | [162] |
| Exosomes from lymphoblast | Nef-E7 fusion protein | T lymphocytes immune response | [161] |
| Exosomes from two mouse cell lines | Human MUC1 tumor antigen | Generate immune response against tumor | [157] |
| Microvesicles from different cancer cell lines | DiD, carboxyfluorescein, paclitaxel, tirapazamine encapsulated in fusogenic liposomes | The same cancer cell lines used to produce microvesicles | [180] |
| EVs Type | Nanotechnological Modification | Application | Reference |
|---|---|---|---|
| Exosomes from HEK 293T | Functionalization: CD9-HuR Load: miR-155 or CRISPR/Cas9 | Targeting and therapy of liver cancer | [186] |
| Exosomes from HEK 293T | Functionalization: Lamp2b, fused to a fragment of IL-3 Load: Imatinib or BCR-ABL siRNA | Inhibition of chronic myeloid leukemia growth | [84] |
| Exosomes from HEK 293T | Functionalization: GE 11 peptide Load: let-7a miRNA | Targeting and therapy of EGFR-expressing cancer tissues | [154] |
| Exosomes from HEK 293T cells | Functionalization: BAP-TM receptor and biotin ligase BirA Load: viral capside | Gene therapy against glioma | [185] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Susa, F.; Limongi, T.; Dumontel, B.; Vighetto, V.; Cauda, V. Engineered Extracellular Vesicles as a Reliable Tool in Cancer Nanomedicine. Cancers 2019, 11, 1979. https://doi.org/10.3390/cancers11121979
Susa F, Limongi T, Dumontel B, Vighetto V, Cauda V. Engineered Extracellular Vesicles as a Reliable Tool in Cancer Nanomedicine. Cancers. 2019; 11(12):1979. https://doi.org/10.3390/cancers11121979
Chicago/Turabian StyleSusa, Francesca, Tania Limongi, Bianca Dumontel, Veronica Vighetto, and Valentina Cauda. 2019. "Engineered Extracellular Vesicles as a Reliable Tool in Cancer Nanomedicine" Cancers 11, no. 12: 1979. https://doi.org/10.3390/cancers11121979