Review: Targeting the Transforming Growth Factor-Beta Pathway in Ovarian Cancer
Abstract
:1. Introduction
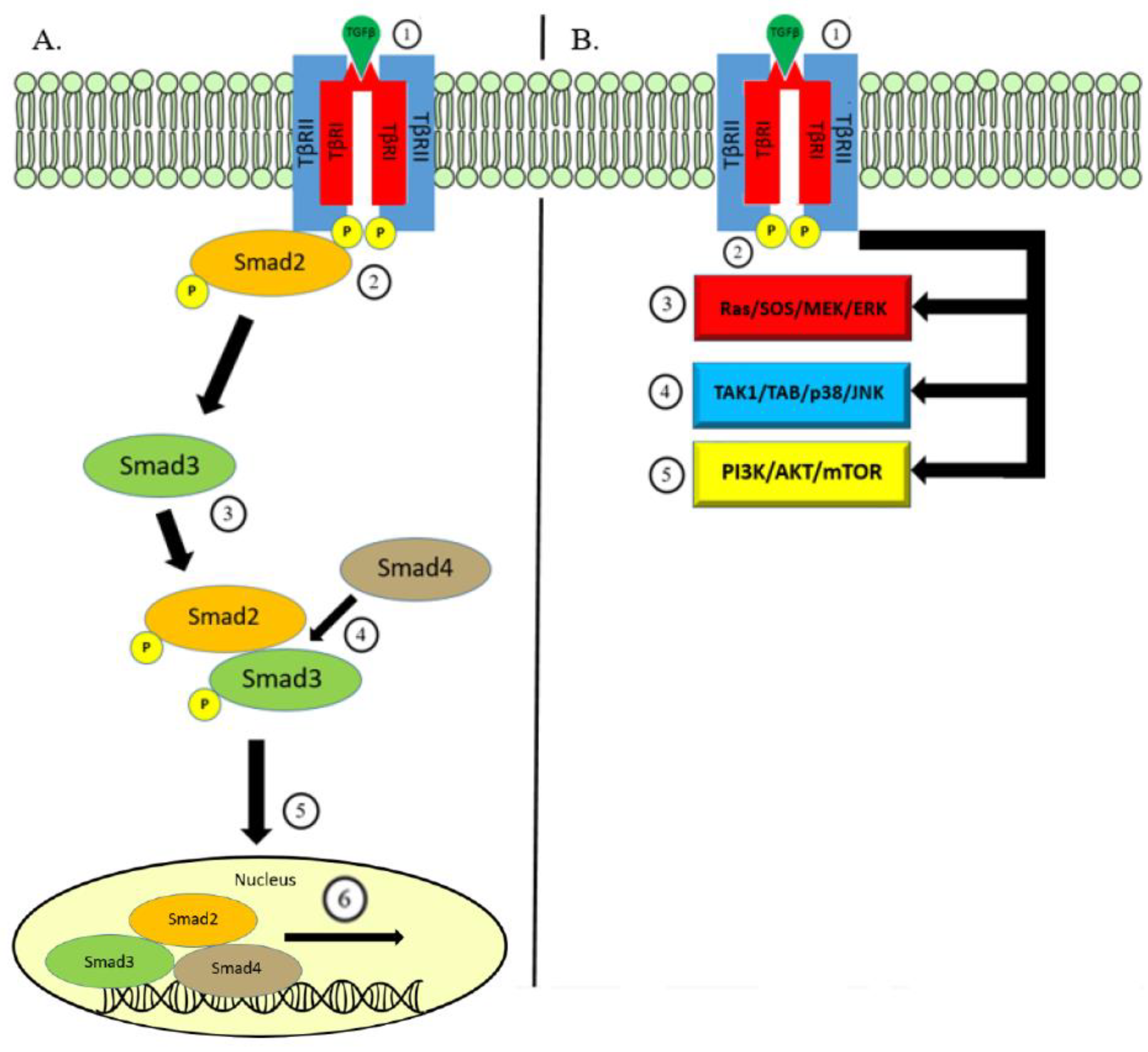
2. The TGF-β Signaling Pathway
3. TGF-β Signaling in Ovarian Cancer
4. TGF-β Regulated Immune Evasion
5. Targeting TGF-β in Ovarian Cancer
6. Conclusion
Funding
Conflicts of Interest
References
- American Cancer Society. Key Statistics for Ovarian Cancer. 2018. Available online: https://www.cancer.org/cancer/ovarian-cancer/about/key-statistics.html (accessed on 29 December 2018).
- Barakat, R.; Berchuck, A.; Markman, M.; Randall, M.E. Principles and Practice of Gynecologic Oncology, 6th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Heintz, A.; Odicino, F.; Maisonneuve, P.; Quinn, M.; Benedet, J.; Creasman, W.; Ngan, H.; Pecorelli, S.; Beller, U. Carcinoma of the Ovary. Int. J. Gynecol. Obstet. 2006, 95, S161–S192. [Google Scholar] [CrossRef]
- Monk, B.J.; Coleman, R.L. Changing the Paradigm in the Treatment of Platinum-Sensitive Recurrent Ovarian Cancer: From Platinum Doublets to Nonplatinum Doublets and Adding Antiangiogenesis Compounds. Int. J. Gynecol. Cancer 2009, 19, S63–S67. [Google Scholar] [CrossRef]
- Cheon, D.J.; Tong, Y.; Sim, M.S.; Dering, J.; Berel, D.; Cui, X.; Lester, J.; Beach, J.A.; Tighiouart, M.; Walts, A.E.; et al. A collagen-remodeling gene signature regulated by TGF-beta signaling is associated with metastasis and poor survival in serous ovarian cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2014, 20, 711–723. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Wakefield, L.M.; Hill, C.S. Beyond TGFβ: Roles of other TGFβ superfamily members in cancer. Nat. Rev. Cancer 2013, 13, 328. [Google Scholar] [CrossRef]
- Feng, X.-H.; Derynck, R. Specificity and versatility in TGF-β signaling through Smads. Annu. Rev. Cell Dev. Biol. 2005, 21, 659–693. [Google Scholar] [CrossRef]
- Wu, M.Y.; Hill, C.S. TGF-β; Superfamily Signaling in Embryonic Development and Homeostasis. Dev. Cell 2009, 16, 329–343. [Google Scholar] [CrossRef]
- Heldin, C.H.; Landstrom, M.; Moustakas, A. Mechanism of TGF-beta signaling to growth arrest, apoptosis, and epithelial-mesenchymal transition. Curr. Opin. Cell Biol. 2009, 21, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Pang, Y.; Moses, H.L. TGF-beta and immune cells: An important regulatory axis in the tumor microenvironment and progression. Trends Immunol. 2010, 31, 220–227. [Google Scholar] [CrossRef]
- Gordon, K.J.; Blobe, G.C. Role of transforming growth factor-beta superfamily signaling pathways in human disease. Biochim. Biophys. Acta 2008, 1782, 197–228. [Google Scholar] [CrossRef] [PubMed]
- Neuzillet, C.; Tijeras-Raballand, A.; Cohen, R.; Cros, J.; Faivre, S.; Raymond, E.; de Gramont, A. Targeting the TGFbeta pathway for cancer therapy. Pharmacol. Ther. 2015, 147, 22–31. [Google Scholar] [CrossRef]
- Mishra, L.; Derynck, R.; Mishra, B. Transforming growth factor-beta signaling in stem cells and cancer. Science 2005, 310, 68–71. [Google Scholar] [CrossRef]
- Padua, D.; Massague, J. Roles of TGFbeta in metastasis. Cell Res. 2009, 19, 89–102. [Google Scholar] [CrossRef]
- Levy, L.; Hill, C.S. Alterations in components of the TGF-beta superfamily signaling pathways in human cancer. Cytokine Growth Factor Rev. 2006, 17, 41–58. [Google Scholar] [CrossRef]
- Jakowlew, S.B. Transforming growth factor-β in cancer metastasis. Cancer Metastasis Rev. 2006, 25, 435–457. [Google Scholar] [CrossRef]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef]
- Tian, M.; Neil, J.R.; Schiemann, W.P. Transforming growth factor-beta and the hallmarks of cancer. Cell. Signal. 2011, 23, 951–962. [Google Scholar] [CrossRef]
- Yamada, S.D.; Baldwin, R.L.; Karlan, B.Y. Ovarian carcinoma cell cultures are resistant to TGF-beta1-mediated growth inhibition despite expression of functional receptors. Gynecol. Oncol. 1999, 75, 72–77. [Google Scholar] [CrossRef]
- Baldwin, R.L.; Tran, H.; Karlan, B.Y. Loss of c-myc Repression Coincides with Ovarian Cancer Resistance to Transforming Growth Factor β Growth Arrest Independent of Transforming Growth Factor β/Smad Signaling. Cancer Res. 2003, 63, 1413–1419. [Google Scholar]
- Alsina-Sanchis, E.; Figueras, A.; Lahiguera, A.; Vidal, A.; Casanovas, O.; Graupera, M.; Villanueva, A.; Vinals, F. The TGFbeta pathway stimulates ovarian cancer cell proliferation by increasing IGF1R levels. Int. J. Cancer 2016, 139, 1894–1903. [Google Scholar] [CrossRef]
- Chen, X.; Du, Y.; Lin, X.; Qian, Y.; Zhou, T.; Huang, Z. CD4+CD25+ regulatory T cells in tumor immunity. Int. Immunopharmacol. 2016, 34, 244–249. [Google Scholar] [CrossRef]
- Garib, F.Y.; Rizopulu, A.P. T-Regulatory Cells as Part of Strategy of Immune Evasion by Pathogens. Biochem. Biokhimiia 2015, 80, 957–971. [Google Scholar] [CrossRef] [PubMed]
- Ellenrieder, V.; Buck, A.; Gress, T.M. TGFβ-regulated transcriptional mechanisms in cancer. Int. J. Gastrointest. Cancer 2002, 31, 61–69. [Google Scholar] [CrossRef]
- Sheen, Y.Y.; Kim, M.J.; Park, S.A.; Park, S.Y.; Nam, J.S. Targeting the Transforming Growth Factor-beta Signaling in Cancer Therapy. Biomol. Ther. 2013, 21, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Massagué, J. TGF-β Signal Transduction. Annu. Rev. 1998, 67, 753–791. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.; Morris, J.C. Transforming growth factor-beta: A therapeutic target for cancer. Hum. Vaccines Immunother. 2017, 13, 1741–1750. [Google Scholar] [CrossRef]
- Travis, M.A.; Sheppard, D. TGF-beta activation and function in immunity. Annu. Rev. Immunol. 2014, 32, 51–82. [Google Scholar] [CrossRef] [PubMed]
- Cottrez, F.; Groux, H. Regulation of TGF-beta response during T cell activation is modulated by IL-10. J. Immunol. 2001, 167, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Schmierer, B.; Hill, C.S. Kinetic analysis of Smad nucleocytoplasmic shuttling reveals a mechanism for transforming growth factor beta-dependent nuclear accumulation of Smads. Mol. Cell. Biol. 2005, 25, 9845–9858. [Google Scholar] [CrossRef] [PubMed]
- Siegel, P.M.; Massague, J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat. Rev. Cancer 2003, 3, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Ayyaz, A.; Attisano, L.; Wrana, J. Recent advances in understanding contextual TGF signaling. F1000Research 2017, 6. [Google Scholar] [CrossRef]
- Bakin, A.V.; Rinehart, C.; Tomlinson, A.K.; Arteaga, C.L. p38 mitogen-activated protein kinase is required for TGFbeta-mediated fibroblastic transdifferentiation and cell migration. J. Cell Sci. 2002, 115, 3193–3206. [Google Scholar]
- Bakin, A.V.; Tomlinson, A.K.; Bhowmick, N.A.; Moses, H.L.; Arteaga, C.L. Phosphatidylinositol 3-kinase function is required for transforming growth factor beta-mediated epithelial to mesenchymal transition and cell migration. J. Biol. Chem. 2000, 275, 36803–36810. [Google Scholar] [CrossRef]
- Bristow, R.E.; Baldwin, R.L.; Yamada, S.D.; Korc, M.; Karlan, B.Y. Altered expression of transforming growth factor-beta ligands and receptors in primary and recurrent ovarian carcinoma. Cancer 1999, 85, 658–668. [Google Scholar] [CrossRef]
- Ito, M.; Yasui, W.; Kyo, E.; Yokozaki, H.; Nakayam, H.; Ito, H.; Tahara, E. Growth Inhibition of Transforming Growth Factor Beta on Human Gastric Carcinoma Cells: Receptor and Postreceptor Signaling. Cancer Res. 1992, 52, 295–300. [Google Scholar]
- Marth, C.; Lang, T.; Koza, A.; Mayer, I.; Daxenbichler, G. Transforming growth factor- beta and ovarian carcinoma cells: Regulation of proliferation and surface antigen expression. Cancer Lett. 1990, 51, 221–225. [Google Scholar] [CrossRef]
- Zhou, L.; Leung, B.S. Growth regulation of ovarian cancer cells by epidermal growth factor and transforming growth factors alpha and beta 1. Biochimica et Biophysica Acta 1992, 1180, 130–136. [Google Scholar] [CrossRef]
- Landen, C.N., Jr.; Birrer, M.J.; Sood, A.K. Early events in the pathogenesis of epithelial ovarian cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 995–1005. [Google Scholar] [CrossRef]
- Chen, T.; Triplett, J.; Dehner, B.; Hurst, B.; Colligan, B.; Pemberton, J.; Graff, J.R.; Carter, J.H. Transforming Growth Factor-β Receptor Type I Gene Is Frequently Mutated in Ovarian Carcinomas. Cancer Res. 2001, 61, 4679–4682. [Google Scholar]
- Lynch, M.A.; Nakashima, R.; Song, H.; DeGroff, V.L.; Wang, D.; Enomoto, T.; Weghorst, C.M. Mutational analysis of the transforming growth factor beta receptor type II gene in human ovarian carcinoma. Cancer Res. 1998, 58, 4227–4232. [Google Scholar]
- Alvi, A.J.; Rader, J.S.; Broggini, M.; Latif, F.; Maher, E.R. Microsatellite instability and mutational analysis of transforming growth factor beta receptor type II gene (TGFBR2) in sporadic ovarian cancer. Mol. Pathol. 2001, 54, 240–243. [Google Scholar] [CrossRef] [Green Version]
- Manderson, E.N.; Mes-Masson, A.M.; Provencher, D.; Tonin, P.N. Mutations in a 10-bp polyadenine repeat of transforming growth factor beta-receptor type II gene is an infrequent event in human epithelial ovarian cancer. Clin. Genet. 2000, 57, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Spillman, M.A.; Schildkraut, J.M.; Halabi, S.; Moorman, P.; Calingaert, B.; Bentley, R.C.; Marks, J.R.; Murphy, S.; Berchuck, A. Transforming growth factor beta receptor I polyalanine repeat polymorphism does not increase ovarian cancer risk. Gynecol. Oncol. 2005, 97, 543–549. [Google Scholar] [CrossRef]
- Schutte, M.; Hruban, R.H.; Hedrick, L.; Cho, K.R.; Nadasdy, G.M.; Weinstein, C.L.; Bova, G.S.; Isaacs, W.B.; Cairns, P.; Nawroz, H.; et al. DPC4 gene in various tumor types. Cancer Res. 1996, 56, 2527–2530. [Google Scholar]
- Wang, D.; Kanuma, T.; Mizunuma, H.; Takama, F.; Ibuki, Y.; Wake, N.; Mogi, A.; Shitara, Y.; Takenoshita, S. Analysis of specific gene mutations in the transforming growth factor-beta signal transduction pathway in human ovarian cancer. Cancer Res. 2000, 60, 4507–4512. [Google Scholar] [PubMed]
- Antony, M.L.; Nair, R.; Sebastian, P.; Karunagaran, D. Changes in expression, and/or mutations in TGF-beta receptors (TGF-beta RI and TGF-beta RII) and Smad 4 in human ovarian tumors. J. Cancer Res. Clin. Oncol. 2010, 136, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Xi, L.; Hu, W.; Meng, L.; Zhou, J.; Lu, Y.; Wang, C.; Ma, D. Dysregulation of the TGF- β postreceptor signaling pathway in cell lines derived from primary or metastatic ovarian cancer. J. Huazhong Univ. Sci. Technol. 2004, 24, 62–65. [Google Scholar] [CrossRef]
- Levy, L.; Hill, C.S. Smad4 dependency defines two classes of transforming growth factor-beta target genes and distinguishes TGF–induced epithelial-mesenchymal transition from its antiproliferative and migratory responses. Mol. Cell. Biol. 2005, 25, 8108–8125. [Google Scholar] [CrossRef]
- Dunfield, L.D.; Dwyer, E.J.; Nachtigal, M.W. TGF beta-induced Smad signaling remains intact in primary human ovarian cancer cells. Endocrinology 2002, 143, 1174–1181. [Google Scholar] [CrossRef]
- Hurteau, J.; Rodriguez, G.C.; Whitaker, R.S.; Shah, S.; Mills, G.; Bast, R.C.; Berchuck, A. Transforming growth factor-beta inhibits proliferation of human ovarian cancer cells obtained from ascites. Cancer 1994, 74, 93–99. [Google Scholar] [CrossRef] [Green Version]
- Kurman, R.H.; Shih, I.M. The origin and pathogenesis of epithelial ovarian cancer: A proposed unifying theory. Am. J. Surg. Pathol. 2010, 34, 433–443. [Google Scholar] [CrossRef]
- Middlebrook, B.S.; Eldin, K.; Li, X.; Shivasankaran, S.; Pangas, S.A. Smad1-Smad5 ovarian conditional knockout mice develop a disease profile similar to the juvenile form of human granulosa cell tumors. Endocrinology 2009, 150, 5208–5217. [Google Scholar] [CrossRef]
- Anttonen, M.; Pihlajoki, M.; Andersson, N.; Georges, A.; L’hote, D.; Vattulainen, S.; Farkkila, A.; Unikla-Kallio, L.; Veitia, R.A.; Heikinheimo, M. FOXL2, GATA4, and SMAD3 co-operatively modulate gene expression, cell viability and apoptosis in ovarian granulosa cell tumor cells. PLoS ONE 2014, 9, e85545. [Google Scholar] [CrossRef]
- Norris, D.; McTavish, K.J.; Shimasaki, S. Essential but differential role of FOXL2wt and FOXL2C134W in GDF-9 stimulation of follistatin transcription in co-operation with Smad3 in the human granulosa cell line COV434. Mol. Cell Endocrinol. 2014, 372, 42–48. [Google Scholar] [CrossRef]
- Shah, S.P.; Köbel, M.; Senz, J.; Morin, R.D.; Clarke, B.A.; Wiegand, K.C.; Leung, G.; Zayed, A.; Mehl, E.; Kalloger, S.E.; et al. Mutation of FOXL2 in granulosa-cell tumors of the ovary. N. Engl. J. Med. 2009, 360, 2719–2729. [Google Scholar] [CrossRef]
- Losi, L.; Fonda, S.; Saponaro, S.; Chelbi, S.T.; Lancellotti, C.; Gozzi, G.; Alberti, L.; Fabbiani, L.; Botticelli, L.; Benhattar, J. Distinct DNA Methylation Profiles in Ovarian Tumors: Opportunities for Novel Biomarkers. Int. J. Mol. Sci. 2018, 19, 1559. [Google Scholar] [CrossRef]
- Kurokawa, M.; Mitani, K.; Yamagata, T.; Takahashi, T.; Izutsu, K.; Ogawa, S.; Moriguchi, T.; Nishida, E.; Yazaki, Y.; Hirai, H. The evi-1 oncoprotein inhibits c-Jun N-terminal kinase and prevents stress-induced cell death. EMBO J. 2000, 19, 2958–2968. [Google Scholar] [CrossRef]
- Izutsu, K.; Kurokawa, M.; Imai, Y.; Maki, K.; Mitani, K.; Hirai, H. The corepressor CtBP interacts with Evi-1 to repress transforming growth factor beta signaling. Blood 2001, 97, 2815–2822. [Google Scholar] [CrossRef] [Green Version]
- Sunde, J.S.; Donninger, H.; Wu, K.; Johnson, M.E.; Pestell, R.G.; Rose, G.S.; Mok, S.C.; Brady, J.; Bonome, T.; Birrer, M.J. Expression profiling identifies altered expression of genes that contribute to the inhibition of transforming growth factor-beta signaling in ovarian cancer. Cancer Res. 2006, 66, 8404–8412. [Google Scholar] [CrossRef]
- Miyazono, K. TGF-beta signaling by Smad proteins. Cytokine Growth Factor Rev. 2000, 11, 15–22. [Google Scholar] [CrossRef]
- Alexandrow, M.G.; Moses, H.L. Transforming growth factor beta and cell cycle regulation. Cancer Res. 1995, 55, 1452–1457. [Google Scholar] [PubMed]
- Warner, B.J.; Blain, S.W.; Seoane, J.; Massague, J. Myc downregulation by transforming growth factor beta required for activation of the p15(Ink4b) G(1) arrest pathway. Mol. Cell. Biol. 1999, 19, 5913–5922. [Google Scholar] [CrossRef]
- Mu, Y.; Sundar, R.; Thakur, N.; Ekman, M.; Gudey, S.K.; Yakymovych, M.; Hermansson, A.; Dimitriou, H.; Bengoechea-Alonso, M.T.; Ericsson, J.; et al. TRAF6 ubiquitinates TGFβ type I receptor to promote its cleavage and nuclear translocation in cancer. Nat. Commun. 2011, 2. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, F.; Drabsch, Y.; Gao, R.; Snaar-Jagalska, B.E.; Mickanin, C.; Huang, H.; Sheppard, K.A.; Porter, J.A.; Lu, C.X.; et al. USP4 is regulated by AKT phosphorylation and directly deubiquitylates TGF-β type I receptor. Nat. Cell Biol. 2012, 14, 717–726. [Google Scholar] [CrossRef]
- Vergara, D.; Merlot, B.; Lucot, J.P.; Collinet, P.; Vinatier, D.; Fournier, I.; Salzet, M. Epithelial-mesenchymal transition in ovarian cancer. Cancer Lett. 2010, 291, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Drabsch, Y.; ten Dijke, P. TGF-beta signalling and its role in cancer progression and metastasis. Cancer Metastasis Rev. 2012, 31, 553–568. [Google Scholar] [CrossRef]
- Yeung, T.L.; Leung, C.S.; Wong, K.K.; Samimi, G.; Thompson, M.S.; Liu, J.; Zaid, T.M.; Ghosh, S.; Birrer, M.J.; Mok, S.C. TGF-beta modulates ovarian cancer invasion by upregulating CAF-derived versican in the tumor microenvironment. Cancer Res. 2013, 73, 5016–5028. [Google Scholar] [CrossRef]
- Riester, M.; Wei, W.; Waldron, L.; Culhane, A.C.; Trippa, L.; Oliva, E.; Kim, S.-h.; Michor, F.; Huttenhower, C.; Parmigiani, G.; et al. Risk Prediction for Late-Stage Ovarian Cancer by Meta-analysis of 1525 Patient Samples. J. Natl. Cancer Inst. 2014, 106, 48. [Google Scholar] [CrossRef]
- Perez, R.P.; Godwin, A.K.; Hamilton, T.C.; Ozols, R.F. Ovarian cancer biology. Semin. Oncol. 1991, 18, 186–204. [Google Scholar]
- Nagy, J.A.; Masse, E.M.; Herzberg, K.T.; Meyers, M.S.; Yeo, K.T.; Yeo, T.K.; Sioussat, T.M.; Dvorak, H.F. Pathogenesis of ascites tumor growth: Vascular permeability factor, vascular hyperpermeability, and ascites fluid accumulation. Cancer Res. 1995, 55, 360–368. [Google Scholar]
- Nakanishi, Y.; Kodama, J.; Yoshinouchi, M.; Tokumo, K.; Kamimura, S.; Okuda, H.; Kudo, T. The expression of vascular endothelial growth factor and transforming growth factor-beta associates with angiogenesis in epithelial ovarian cancer. Int. J. Gynecol. Pathol. Off. J. Int. Soc. Gynecol. Pathol. 1997, 16, 256–262. [Google Scholar]
- Liao, S.; Liu, J.; Lin, P.; Shi, T.; Jain, R.K.; Xu, L. TGF-beta blockade controls ascites by preventing abnormalization of lymphatic vessels in orthotopic human ovarian carcinoma models. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011, 17, 1415–1424. [Google Scholar] [CrossRef]
- Baert, T.; Timmerman, D.; Vergote, I.; Coosemans, A. Immunological parameters as a new lead in the diagnosis of ovarian cancer. Facts Views Vis. ObGyn 2015, 7, 67–72. [Google Scholar] [PubMed]
- Kao, J.Y.; Gong, Y.; Chen, C.M.; Zheng, Q.D.; Chen, J.J. Tumor-derived TGF-beta reduces the efficacy of dendritic cell/tumor fusion vaccine. J. Immunol. 2003, 170, 3806–3811. [Google Scholar] [CrossRef] [PubMed]
- Flavell, R.A.; Sanjabi, S.; Wrzesinski, S.H.; Licona-Limon, P. The polarization of immune cells in the tumour environment by TGFbeta. Nat. Rev. Immunol. 2010, 10, 554–567. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wahl, S.M. TGF-beta: The missing link in CD4+CD25+ regulatory T cell-mediated immunosuppression. Cytokine Growth Factor Rev. 2003, 14, 85–89. [Google Scholar] [CrossRef]
- Ghiringhelli, F.; Menard, C.; Terme, M.; Flament, C.; Taieb, J.; Chaput, N.; Puig, P.E.; Novault, S.; Escudier, B.; Vivier, E.; et al. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J. Exp. Med. 2005, 202, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Kobie, J.J.; Wu, R.S.; Kurt, R.A.; Lou, S.; Adelman, M.K.; Whitesell, L.J.; Ramanathapuram, L.V.; Arteaga, C.L.; Akporiaye, E.T. Transforming growth factor beta inhibits the antigen-presenting functions and antitumor activity of dendritic cell vaccines. Cancer Res. 2003, 63, 1860–1864. [Google Scholar]
- Yigit, R.; Massuger, L.F.; Figdor, C.G.; Torensma, R. Ovarian cancer creates a suppressive microenvironment to escape immune elimination. Gynecol. Oncol. 2010, 117, 366–372. [Google Scholar] [CrossRef]
- Mule, J.J.; Schwarz, S.L.; Roberts, A.B.; Sporn, M.B.; Rosenberg, S.A. Transforming growth factor-beta inhibits the in vitro generation of lymphokine-activated killer cells and cytotoxic T cells. Cancer Immunol. Immunother. 1988, 26, 95–100. [Google Scholar] [CrossRef]
- Curiel, T.J.; Coukos, G.; Zou, L.; Alvarez, X.; Cheng, P.; Mottram, P.; Evdemon-Hogan, M.; Conejo-Garcia, J.R.; Zhang, L.; Burow, M.; et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 2004, 10, 942–949. [Google Scholar] [CrossRef]
- Wolf, D.; Wolf, A.M.; Rumpold, H.; Fiegl, H.; Zeimet, A.G.; Muller-Holzner, E.; Deibl, M.; Gastl, G.; Gunsilius, E.; Marth, C. The expression of the regulatory T cell-specific forkhead box transcription factor FoxP3 is associated with poor prognosis in ovarian cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2005, 11, 8326–8331. [Google Scholar] [CrossRef]
- Napoletano, C.; Bellati, F.; Landi, R.; Pauselli, S.; Marchetti, C.; Visconti, V.; Sale, P.; Liberati, M.; Rughetti, A.; Frati, L.; et al. Ovarian cancer cytoreduction induces changes in T cell population subsets reducing immunosuppression. J. Cell. Mol. Med. 2010, 14, 2748–2759. [Google Scholar] [CrossRef]
- Leffers, N.; Gooden, M.J.; de Jong, R.A.; Hoogeboom, B.N.; ten Hoor, K.A.; Hollema, H.; Boezen, H.M.; van der Zee, A.G.; Daemen, T.; Nijman, H.W. Prognostic significance of tumor-infiltrating T-lymphocytes in primary and metastatic lesions of advanced stage ovarian cancer. Cancer Immunol. Immunother. 2009, 58, 449–459. [Google Scholar] [CrossRef]
- Fabregat, I.; Fernando, J.; Mainez, J.; Sancho, P. TGF-beta signaling in cancer treatment. Curr. Pharm. Des. 2014, 20, 2934–2947. [Google Scholar] [CrossRef]
- Grunewald, T.; Ledermann, J.A. Targeted Therapies for Ovarian Cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 41, 139–152. [Google Scholar] [CrossRef] [Green Version]
- Dunn, G.P.; Bruce, A.T.; Ikeda, H.; Old, L.J.; Schreiber, R.D. Cancer immunoediting: From immunosurveillance to tumor escape. Nat. Immunol. 2002, 3, 991–998. [Google Scholar] [CrossRef]
- Zou, W.; Chen, L. Inhibitory B7-family molecules in the tumour microenvironment. Nat. Rev. Immunol. 2008, 8, 467–477. [Google Scholar] [CrossRef]
- Keir, M.E.; Butte, M.J.; Freeman, G.J.; Sharpe, A.H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008, 26, 677–704. [Google Scholar] [CrossRef]
- Hamanishi, J.; Mandai, M.; Konishi, I. Immune checkpoint inhibition in ovarian cancer. Int. Immunol. 2016, 28, 339–348. [Google Scholar] [CrossRef] [Green Version]
- Tauriello, D.V.F.; Palomo-Ponce, S.; Stork, D.; Berenguer-Llergo, A.; Badia-Ramentol, J.; Iglesias, M.; Sevillano, M.; Ibiza, S.; Canellas, A.; Hernando-Momblona, X.; et al. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 2018, 554, 538–543. [Google Scholar] [CrossRef]
- Holmgaard, R.B.; Schaer, D.A.; Li, Y.; Castaneda, S.P.; Murphy, M.Y.; Xu, X.; Inigo, I.; Dobkin, J.; Manro, J.R.; Iversen, P.W.; et al. Targeting the TGFβ pathway with galunisertib, a TGFβRI small molecule inhibitor, promotes anti-tumor immunity leading to durable, complete responses, as monotherapy and in combination with checkpoint blockade. J. Immunother. Cancer 2018, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Mariathasan, S.; Turley, S.J.; Nickles, D.; Castiglioni, A.; Yuen, K.; Wang, Y.; Kadel, E.E., III; Koeppen, H.; Astarita, J.L.; Cubas, R.; et al. TGFbeta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018, 554, 544–548. [Google Scholar] [CrossRef]
- Lan, Y.; Zhang, D.; Xu, C.; Hance, K.W.; Marelli, B.; Qi, J.; Yu, H.; Qin, G.; Sircar, A.; Hernández, V.M.; et al. Enhanced preclinical antitumor activity of M7824, a bifunctional fusion protein simultaneously targeting PD-L1 and TGF-β. Sci. Transl. Med. 2018, 10, eaan5488. [Google Scholar] [CrossRef]
- Ravi, R.; Noonan, K.A.; Pham, V.; Bedi, R.; Zhavoronkov, A.; Ozerov, I.V.; Makarev, E.; Artemov, A.V.; Wysocki, P.T.; Mehra, R.; et al. Bifunctional immune checkpoint-targeted antibody-ligand traps that simultaneously disable TGFβ enhance the efficacy of cancer immunotherapy. Nat. Commun. 2018, 9, 741. [Google Scholar] [CrossRef] [PubMed]
- Garrison, K.; Hahn, T.; Lee, W.C.; Ling, L.E.; Weinberg, A.D.; Akporiaye, E.T. The small molecule TGF-β signaling inhibitor SM16 synergizes with agonistic OX40 antibody to suppress established mammary tumors and reduce spontaneous metastasis. Cancer Immunol. Immunother. 2012, 61, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Wrzesinski, S.H.; Stern, E.; Look, M.; Criscione, J.; Ragheb, R.; Jay, S.M.; Demento, S.L.; Agawu, A.; Licona Limon, P.; et al. Combination delivery of TGF-β inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy. Nat. Mater. 2012, 11, 895–905. [Google Scholar] [CrossRef] [Green Version]
- Vanpouille-Box, C.; Diamond, J.M.; Pilones, K.A.; Zavadil, J.; Babb, J.S.; Formenti, S.C.; Barcellos-Hoff, M.H.; Demaria, S. TGFβ Is a Master Regulator of Radiation Therapy-Induced Antitumor Immunity. Cancer Res. 2015, 75, 2232–2242. [Google Scholar] [CrossRef] [PubMed]
- Hutzen, B.; Chen, C.; Wang, P.; Sprague, L.; Swain, H.M.; Love, J.; Conner, J.; Boon, L.; Cripe, T.P. TGF-β Inhibition Improves Oncolytic Herpes Viroimmunotherapy in Murine Models of Rhabdomyosarcoma. Mol. Oncolytics 2017, 7, 17–26. [Google Scholar] [CrossRef]
- Bogdahn, U.; Hau, P.; Stockhammer, G.; Venkataramana, N.K.; Mahapatra, A.K.; Suri, A.; Balasubramaniam, A.; Nair, S.; Oliushine, V.; Parfenov, V.; et al. Targeted therapy for high-grade glioma with the TGF-beta2 inhibitor trabedersen: Results of a randomized and controlled phase IIb study. Neuro-Oncology 2011, 13, 132–142. [Google Scholar] [CrossRef]
- Hwang, L.; Ng, K.; Wang, W.; Trieu, V.N. OT-101: An anti-TGF-beta-2 antisense- primed tumors to subsequent chemotherapies. J. Clin. Oncol. 2016, 34, e15727. [Google Scholar] [CrossRef]
- D’Cruz, O.; Lee, C.; Trieu, V.; Hwang, L. 1662PSynergistic antitumor effects of OT-101 (trabedersen), a transforming growth factor-beta 2 (TGF-β2) antisense oligonucleotide (ASO) and chemotherapy in preclinical tumor models. Ann. Oncol. 2017, 28, mdx390.034. [Google Scholar] [CrossRef]
- Gao, Y.; Shan, N.; Zhao, C.; Wang, Y.; Xu, F.; Li, J.; Yu, X.; Gao, L.; Yi, Z. LY2109761 enhances cisplatin antitumor activity in ovarian cancer cells. Int. J. Clin. Exp. Pathol. 2015, 8, 4923–4932. [Google Scholar]
- Connolly, E.C.; Saunier, E.F.; Quigley, D.; Luu, M.T.; De Sapio, A.; Hann, B.; Yingling, J.M.; Akhurst, R.J. Outgrowth of drug-resistant carcinomas expressing markers of tumor aggression after long-term TbetaRI/II kinase inhibition with LY2109761. Cancer Res. 2011, 71, 2339–2349. [Google Scholar] [CrossRef] [PubMed]
- A Study of Galunisertib (LY2157299) in Combination with Nivolumab in Advanced Refractory Solid Tumors and in Recurrent or Refractory NSCLC, or Hepatocellular Carcinoma. Available online: https://ClinicalTrials.gov/show/NCT02423343 (accessed on 7 January 2019).
- Zhang, Q.; Hou, X.; Evans, B.J.; VanBlaricom, J.L.; Weroha, S.J.; Cliby, W.A. LY2157299 Monohydrate, a TGF-betaR1 Inhibitor, Suppresses Tumor Growth and Ascites Development in Ovarian Cancer. Cancers 2018, 10, 260. [Google Scholar] [CrossRef]
- Yamamura, S.; Matsumura, N.; Mandai, M.; Huang, Z.; Oura, T.; Baba, T.; Hamanishi, J.; Yamaguchi, K.; Kang, H.S.; Okamoto, T.; et al. The activated transforming growth factor-beta signaling pathway in peritoneal metastases is a potential therapeutic target in ovarian cancer. Int. J. Cancer 2012, 130, 20–28. [Google Scholar] [CrossRef]
- Olivares, J.; Kumar, P.; Yu, Y.; Maples, P.B.; Senzer, N.; Bedell, C.; Barve, M.; Tong, A.; Pappen, B.O.; Kuhn, J.; et al. Phase I trial of TGF-beta 2 antisense GM-CSF gene-modified autologous tumor cell (TAG) vaccine. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011, 17, 183–192. [Google Scholar] [CrossRef]
- Nemunaitis, J.; Barve, M.; Orr, D.; Kuhn, J.; Magee, M.; Lamont, J.; Bedell, C.; Wallraven, G.; Pappen, B.O.; Roth, A.; et al. Summary of bi-shRNA/GM-CSF augmented autologous tumor cell immunotherapy (FANG) in advanced cancer of the liver. Oncology 2014, 87, 21–29. [Google Scholar] [CrossRef]
- Rocconi, R.P.; Scalici, J.M.; Barve, M.; Manning, L.; Wallraven, G.; Senzer, N.; Nemunaitis, J. Phase I trial of Vigil® personalized engineered autologous tumor cells (EATC) in ovarian cancer. Gynecol. Oncol. 2018, 149, 40–41. [Google Scholar] [CrossRef]
- Phase 2 Trial of Maintenance Vigil for High Risk Stage IIIb-IV Ovarian Cancer. Available online: https://ClinicalTrials.gov/show/NCT02346747 (accessed on 6 January 2019).
- Terabe, M.; Ambrosino, E.; Takaku, S.; O’Konek, J.J.; Venzon, D.; Lonning, S.; McPherson, J.M.; Berzofsky, J.A. Synergistic enhancement of CD8+ T cell-mediated tumor vaccine efficacy by an anti-transforming growth factor-beta monoclonal antibody. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009, 15, 6560–6569. [Google Scholar] [CrossRef]
- Newsted, D.; Banerjee, S.; Watt, K.; Nersesian, S.; Truesdell, P.; Blazer, L.L.; Cardarelli, L.; Adams, J.J.; Sidhu, S.S.; Craig, A.W. Blockade of TGF-β signaling with novel synthetic antibodies limits immune exclusion and improves chemotherapy response in metastatic ovarian cancer models. Oncoimmunology 2018, 20, e1539613. [Google Scholar] [CrossRef]
- Ganapathy, V.; Ge, R.; Grazioli, A.; Xie, W.; Banach-Petrosky, W.; Kang, Y.; Lonning, S.; McPherson, J.; Yingling, J.M.; Biswas, S.; et al. Targeting the Transforming Growth Factor-beta pathway inhibits human basal-like breast cancer metastasis. Mol. Cancer 2010, 9, 122. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.S.; Terabe, M.; Mamura, M.; Kang, M.J.; Chae, H.; Stuelten, C.; Kohn, E.; Tang, B.; Sabzevari, H.; Anver, M.R.; et al. An anti-transforming growth factor beta antibody suppresses metastasis via cooperative effects on multiple cell compartments. Cancer Res. 2008, 68, 3835–3843. [Google Scholar] [CrossRef]
- Arteaga, C.L.; Hurd, S.D.; Winnier, A.R.; Johnson, M.D.; Fendly, B.M.; Forbes, J.T. Anti-transforming growth factor (TGF)-beta antibodies inhibit breast cancer cell tumorigenicity and increase mouse spleen natural killer cell activity. Implications for a possible role of tumor cell/host TGF-beta interactions in human breast cancer progression. J. Clin. Investig. 1993, 92, 2569–2576. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Carroll, K.D.; Policarpio, D.; Osborn, C.; Gregory, M.; Bassi, R.; Jimenez, X.; Prewett, M.; Liebisch, G.; Persaud, K.; et al. Anti-transforming growth factor beta receptor II antibody has therapeutic efficacy against primary tumor growth and metastasis through multieffects on cancer, stroma, and immune cells. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2010, 16, 1191–1205. [Google Scholar] [CrossRef]
- Part 2 of Phase 1 Study of GC1008 to Treat Advanced Melanoma (Part 2 Will Only Accept and Treat Patients with Advanced Malignant Melanoma). Available online: https://ClinicalTrials.gov/show/NCT00923169 (accessed on 6 January 2019).
- Anti-TGF Monoclonal Antibody (GC1008) in Relapsed Malignant Pleural Mesothelioma. Available online: https://ClinicalTrials.gov/show/NCT01112293 (accessed on 6 January 2019).
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roane, B.M.; Arend, R.C.; Birrer, M.J. Review: Targeting the Transforming Growth Factor-Beta Pathway in Ovarian Cancer. Cancers 2019, 11, 668. https://doi.org/10.3390/cancers11050668
Roane BM, Arend RC, Birrer MJ. Review: Targeting the Transforming Growth Factor-Beta Pathway in Ovarian Cancer. Cancers. 2019; 11(5):668. https://doi.org/10.3390/cancers11050668
Chicago/Turabian StyleRoane, Brandon M., Rebecca C. Arend, and Michael J. Birrer. 2019. "Review: Targeting the Transforming Growth Factor-Beta Pathway in Ovarian Cancer" Cancers 11, no. 5: 668. https://doi.org/10.3390/cancers11050668



