Risk Assessment of kINPen Plasma Treatment of Four Human Pancreatic Cancer Cell Lines with Respect to Metastasis
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
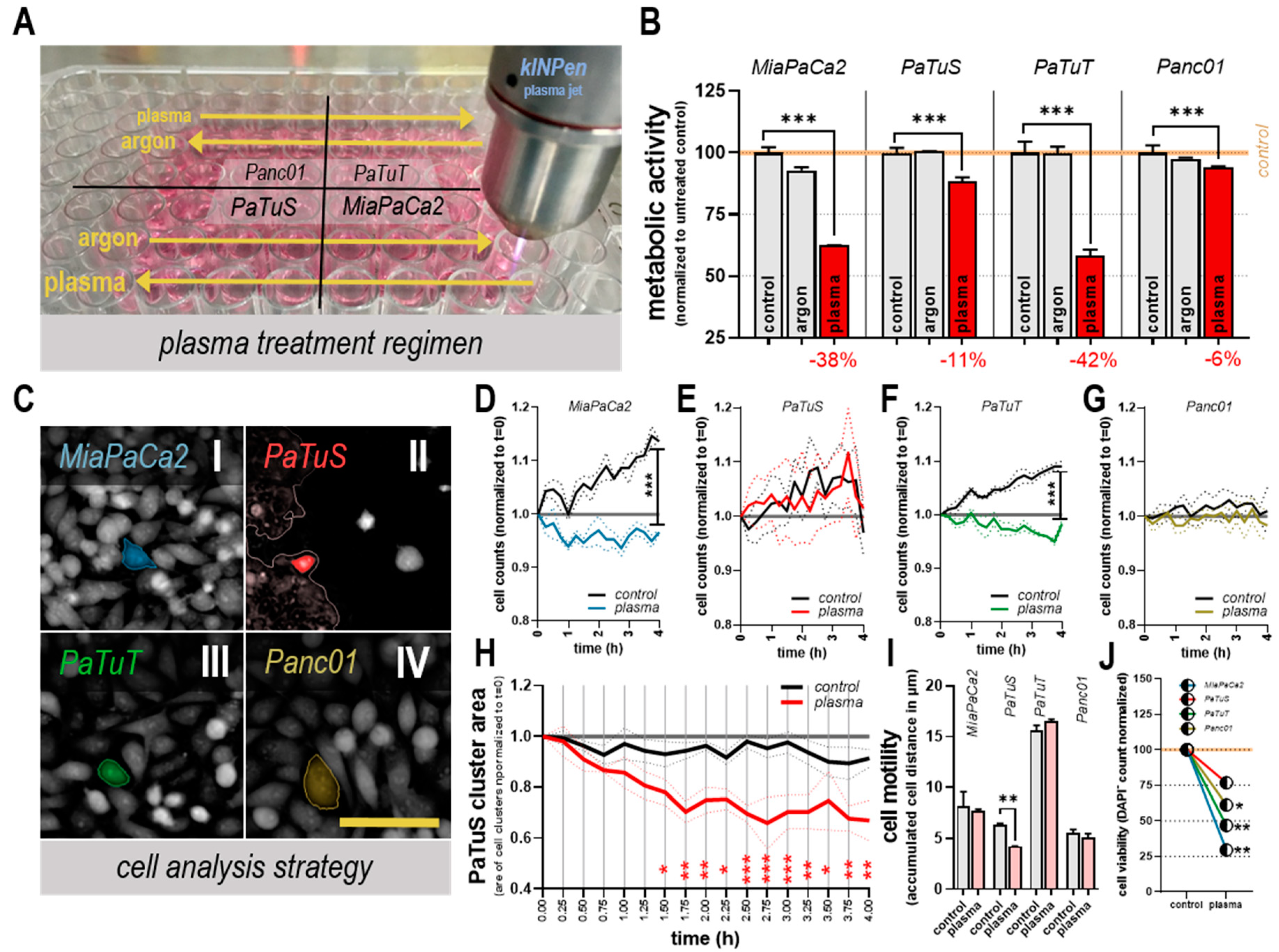
2.2. Cold Physical Plasma and Treatment Regimen
2.3. Quantification of Metabolic Activity
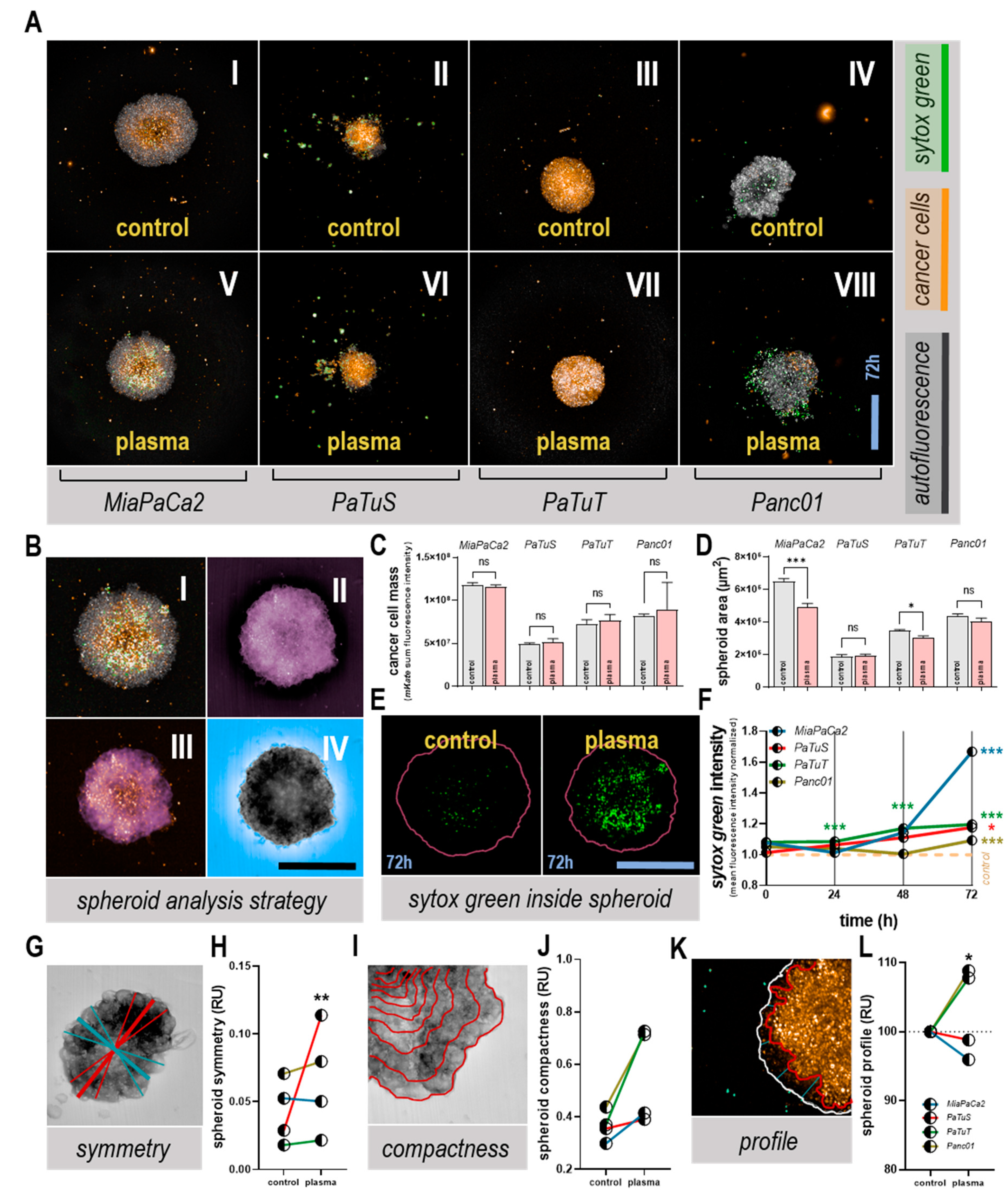
2.4. Culture and Analysis of 3D Tumor Spheroids
2.5. High Content Imaging
2.6. Flow Cytometry
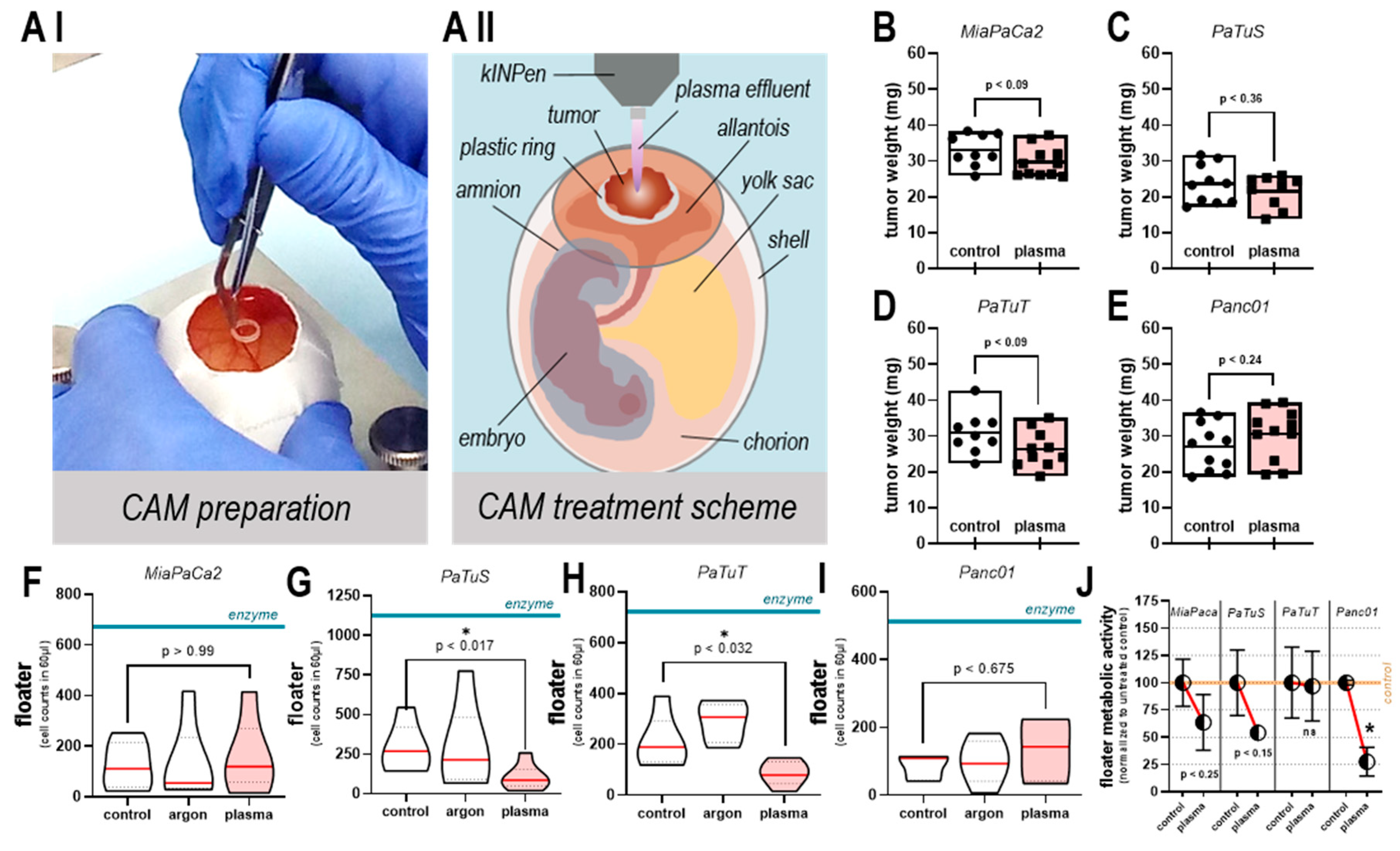
2.7. In ovo Experiments
2.8. Statistical Analysis
3. Results
3.1. Plasma Treatment Reduced Cellular Metabolism, Growth, and Motility in vitro
3.2. Plasma Treatment Modulated the Expression of Cell Adhesion Markers
3.3. Plasma Treatment Altered Tumor Spheroid Morphology, and Induced Toxicity
3.4. Plasma Treatment Unaffected or Decreased Cell Detachment from Tumor Spheroids
3.5. Plasma Treatment did not Increase Tumor Growth or Number of Viable, Detached Cells in ovo
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global Cancer Statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef]
- Abdollahi, A.; Folkman, J. Evading Tumor Evasion: Current Concepts and Perspectives of Anti-angiogenic Cancer Therapy. Drug. Resist. Updat. 2010, 13, 16–28. [Google Scholar] [CrossRef]
- Eckert, F.; Gaipl, U.S.; Niedermann, G.; Hettich, M.; Schilbach, K.; Huber, S.M.; Zips, D. Beyond Checkpoint Inhibition—Immunotherapeutical Strategies in Combination with Radiation. Clin. Transl. Radiat. Oncol. 2017, 2, 29–35. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Booth, L.; Roberts, J.L.; Poklepovic, A.; Kirkwood, J.; Dent, P. HDAC Inhibitors Enhance the Immunotherapy Response of Melanoma Cells. Oncotarget 2017, 8, 83155–83170. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Lee, H.J.; Ko, H.J.; Yoon, B.I.; Choe, J.; Kim, K.C.; Hahn, T.W.; Han, J.A.; Choi, S.S.; Jung, Y.M.; et al. The tlr7 Agonist Imiquimod Induces Anti-cancer Effects Via Autophagic Cell Death and Enhances Anti-tumoral and Systemic Immunity during Radiotherapy for Melanoma. Oncotarget 2017, 8, 24932–24948. [Google Scholar] [CrossRef]
- Duan, X.; Chan, C.; Guo, N.; Han, W.; Weichselbaum, R.R.; Lin, W. Photodynamic Therapy Mediated by Nontoxic Core-shell Nanoparticles Synergizes with Immune Checkpoint Blockade to Elicit Antitumor Immunity and Antimetastatic Effect on Breast Cancer. J. Am. Chem. Soc. 2016, 138, 16686–16695. [Google Scholar] [CrossRef]
- Calvet, C.Y.; Famin, D.; Andre, F.M.; Mir, L.M. Electrochemotherapy with Bleomycin Induces Hallmarks of Immunogenic Cell Death in Murine Colon Cancer Cells. Oncoimmunology 2014, 3, e28131. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Gorbanev, Y.; De Backer, J.; Van Loenhout, J.; Van Boxem, W.; Lemière, F.; Cos, P.; Dewilde, S.; Smits, E.; Bogaerts, A. Non-thermal Plasma as a Unique Delivery System of Short-lived Reactive Oxygen and Nitrogen Species for Immunogenic Cell Death in Melanoma Cells. Adv. Sci. 2019, 6, 1802062. [Google Scholar] [CrossRef]
- Daeschlein, G.; Scholz, S.; Lutze, S.; Arnold, A.; von Podewils, S.; Kiefer, T.; Tueting, T.; Hardt, O.; Haase, H.; Grisk, O.; et al. Comparison between Cold Plasma, Electrochemotherapy and Combined Therapy in a Melanoma Mouse Model. Exp. Dermatol. 2013, 22, 582–586. [Google Scholar] [CrossRef]
- Vandamme, M.; Robert, E.; Lerondel, S.; Sarron, V.; Ries, D.; Dozias, S.; Sobilo, J.; Gosset, D.; Kieda, C.; Legrain, B.; et al. ROS Implication in a New Antitumor Strategy Based on Non-thermal Plasma. Int. J. Cancer 2012, 130, 2185–2194. [Google Scholar] [CrossRef]
- Weltmann, K.D.; von Woedtke, T. Plasma Medicine—Current State of Research and Medical Application. Plasma Phys. Controlled Fusion 2017, 59, 014031. [Google Scholar] [CrossRef]
- Ishaq, M.; Evans, M.M.; Ostrikov, K.K. Effect of Atmospheric Gas Plasmas on Cancer Cell Signaling. Int. J. Cancer 2014, 134, 1517–1528. [Google Scholar] [CrossRef]
- Koritzer, J.; Boxhammer, V.; Schafer, A.; Shimizu, T.; Klampfl, T.G.; Li, Y.F.; Welz, C.; Schwenk-Zieger, S.; Morfill, G.E.; Zimmermann, J.L.; et al. Restoration of Sensitivity in Chemo-resistant Glioma Cells by Cold Atmospheric Plasma. PLoS ONE 2013, 8, e64498. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, N.; Uddin, N.; Sim, G.B.; Hong, Y.J.; Baik, K.Y.; Kim, C.H.; Lee, S.J.; Kaushik, N.K.; Choi, E.H. Responses of Solid Tumor Cells in DMEM to Reactive Oxygen Species Generated by Non-thermal Plasma and Chemically Induced ROS Systems. Sci. Rep. 2015, 5, 8587. [Google Scholar] [CrossRef]
- Binenbaum, Y.; Ben-David, G.; Gil, Z.; Slutsker, Y.Z.; Ryzhkov, M.A.; Felsteiner, J.; Krasik, Y.E.; Cohen, J.T. Cold Atmospheric Plasma, Created at the Tip of an Elongated Flexible Capillary Using Low Electric Current, Can Slow the Progression of Melanoma. PLoS ONE 2017, 12, e0169457. [Google Scholar] [CrossRef] [PubMed]
- Brulle, L.; Vandamme, M.; Ries, D.; Martel, E.; Robert, E.; Lerondel, S.; Trichet, V.; Richard, S.; Pouvesle, J.M.; Le Pape, A. Effects of a Non Thermal Plasma Treatment Alone or in Combination with Gemcitabine in a MIA PaCa2-luc Orthotopic Pancreatic Carcinoma Model. PLoS ONE 2012, 7, e52653. [Google Scholar] [CrossRef]
- Mizuno, K.; Yonetamari, K.; Shirakawa, Y.; Akiyama, T.; Ono, R. Anti-tumor Immune Response Induced by Nanosecond Pulsed Streamer Discharge in Mice. J. Phys. D 2017, 50, 12LT01. [Google Scholar] [CrossRef]
- Metelmann, H.R.; Seebauer, C.; Rutkowski, R.; Schuster, M.; Bekeschus, S.; Metelmann, P. Treating Cancer with Cold Physical Plasma: On the Way to Evidence-based Medicine. Contrib. Plasma Phys. 2018, 58, 415–419. [Google Scholar] [CrossRef]
- Metelmann, H.-R.; Nedrelow, D.S.; Seebauer, C.; Schuster, M.; von Woedtke, T.; Weltmann, K.-D.; Kindler, S.; Metelmann, P.H.; Finkelstein, S.E.; Von Hoff, D.D.; et al. Head and Neck Cancer Treatment and Physical Plasma. Clin. Plas. Med. 2015, 3, 17–23. [Google Scholar] [CrossRef]
- Schuster, M.; Seebauer, C.; Rutkowski, R.; Hauschild, A.; Podmelle, F.; Metelmann, C.; Metelmann, B.; von Woedtke, T.; Hasse, S.; Weltmann, K.D.; et al. Visible Tumor Surface Response to Physical Plasma and Apoptotic Cell Kill in Head and Neck Cancer. J. Craniomaxillofac. Surg. 2016, 44, 1445–1452. [Google Scholar] [CrossRef]
- Metelmann, H.-R.; Seebauer, C.; Miller, V.; Fridman, A.; Bauer, G.; Graves, D.B.; Pouvesle, J.-M.; Rutkowski, R.; Schuster, M.; Bekeschus, S.; et al. Clinical Experience with Cold Plasma in the Treatment of Locally Advanced Head and Neck Cancer. Clin. Plas. Med. 2018, 9, 6–13. [Google Scholar] [CrossRef]
- Bekeschus, S.; Schmidt, A.; Weltmann, K.-D.; von Woedtke, T. The Plasma Jet kINPen—A Powerful Tool for Wound Healing. Clin. Plas. Med. 2016, 4, 19–28. [Google Scholar] [CrossRef]
- Bekeschus, S.; Schmidt, A.; Kramer, A.; Metelmann, H.R.; Adler, F.; von Woedtke, T.; Niessner, F.; Weltmann, K.D.; Wende, K. High Throughput Image Cytometry Micronucleus Assay to Investigate the Presence or Absence of Mutagenic Effects of Cold Physical Plasma. Environ. Mol. Mutagen. 2018, 59, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Kluge, S.; Bekeschus, S.; Bender, C.; Benkhai, H.; Sckell, A.; Below, H.; Stope, M.B.; Kramer, A. Investigating the Mutagenicity of a Cold Argon-plasma Jet in An HET-MN Model. PLoS ONE 2016, 11, e0160667. [Google Scholar] [CrossRef] [PubMed]
- Wende, K.; Bekeschus, S.; Schmidt, A.; Jatsch, L.; Hasse, S.; Weltmann, K.D.; Masur, K.; von Woedtke, T. Risk Assessment of a Cold Argon Plasma jet in Respect to Its Mutagenicity. Mutat. Res. Genet. Toxicol. Environ. Mutagen 2016, 798, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Lademann, J.; Ulrich, C.; Patzelt, A.; Richter, H.; Kluschke, F.; Klebes, M.; Lademann, O.; Kramer, A.; Weltmann, K.D.; Lange-Asschenfeldt, B. Risk Assessment of the Application of Tissue-tolerable Plasma on Human Skin. Clin. Plas. Med. 2013, 1, 5–10. [Google Scholar] [CrossRef]
- Daeschlein, G.; Scholz, S.; Ahmed, R.; Majumdar, A.; von Woedtke, T.; Haase, H.; Niggemeier, M.; Kindel, E.; Brandenburg, R.; Weltmann, K.D.; et al. Cold Plasma is Well-tolerated and Does Not Disturb Skin Barrier or Reduce Skin Moisture. J. Dtsch. Dermatol Ges. 2012, 10, 509–515. [Google Scholar] [CrossRef]
- Metelmann, H.-R.; Vu, T.T.; Do, H.T.; Le, T.N.B.; Hoang, T.H.A.; Phi, T.T.T.; Luong, T.M.L.; Doan, V.T.; Nguyen, T.T.H.; Nguyen, T.H.M. Scar Formation of Laser Skin Lesions after Cold Atmospheric Pressure Plasma (CAP) Treatment: A Clinical Long Term Observation. Clin. Plas. Med. 2013, 1, 30–35. [Google Scholar] [CrossRef]
- Schuster, M.; Rutkowski, R.; Hauschild, A.; Shojaei, R.K.; von Woedtke, T.; Rana, A.; Bauer, G.; Metelmann, P.; Seebauer, C. Side effects in Cold Plasma Treatment of Advanced Oral Cancer—Clinical Data and Biological Interpretation. Clin. Plas. Med. 2018, 10, 9–15. [Google Scholar] [CrossRef]
- Schmidt, A.; von Woedtke, T.; Stenzel, J.; Lindner, T.; Polei, S.; Vollmar, B.; Bekeschus, S. One Year Follow-up Risk Assessment in SKH-1 Mice and Wounds Treated with an Argon Plasma Jet. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef]
- Stathis, A.; Moore, M.J. Advanced pancreatic carcinoma: Current Treatment and Future Challenges. Nat. Rev. Clin. Oncol. 2010, 7, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Flaum, N.; Hubner, R.A.; Valle, J.W.; Amir, E.; McNamara, M.G. Adjuvant Chemotherapy and Outcomes in Patients with Nodal and Resection Margin-negative Pancreatic Ductal Adenocarcinoma: A Systematic Review and Meta-Analysis. J. Surg. Oncol. 2019, 119, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Bekeschus, S.; Kading, A.; Schroder, T.; Wende, K.; Hackbarth, C.; Liedtke, K.R.; van der Linde, J.; von Woedtke, T.; Heidecke, C.D.; Partecke, L.I. Cold Physical Plasma Treated Buffered Saline Solution as Effective Agent Against Pancreatic Cancer Cells. Anticancer Agents Med. Chem. 2018, 18, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Liedtke, K.R.; Diedrich, S.; Pati, O.; Freund, E.; Flieger, R.; Heidecke, C.D.; Partecke, L.I.; Bekeschus, S. Cold Physical Plasma Selectively Elicits Apoptosis in Murine Pancreatic Cancer Cells in Vitro and in Ovo. Anticancer Res. 2018, 38, 5655–5663. [Google Scholar] [CrossRef] [PubMed]
- Masur, K.; von Behr, M.; Bekeschus, S.; Weltmann, K.D.; Hackbarth, C.; Heidecke, C.D.; von Bernstorff, W.; von Woedtke, T.; Partecke, L.I. Synergistic Inhibition of Tumor Cell Proliferation by Cold Plasma and Gemcitabine. Plasma Process. Polym. 2015, 12, 1377–1382. [Google Scholar] [CrossRef]
- Yan, D.; Cui, H.; Zhu, W.; Nourmohammadi, N.; Milberg, J.; Zhang, L.G.; Sherman, J.H.; Keidar, M. The Specific Vulnerabilities of Cancer Cells to the Cold Atmospheric Plasma-stimulated Solutions. Sci. Rep. 2017, 7, 4479. [Google Scholar] [CrossRef]
- Hattori, N.; Yamada, S.; Torii, K.; Takeda, S.; Nakamura, K.; Tanaka, H.; Kajiyama, H.; Kanda, M.; Fujii, T.; Nakayama, G.; et al. Effectiveness of Plasma Treatment on Pancreatic Cancer Cells. Int. J. Oncol. 2015, 47, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Liedtke, K.R.; Bekeschus, S.; Kaeding, A.; Hackbarth, C.; Kuehn, J.P.; Heidecke, C.D.; von Bernstorff, W.; von Woedtke, T.; Partecke, L.I. Non-thermal Plasma-treated Solution Demonstrates Antitumor Activity against Pancreatic Cancer Cells in Vitro and in Vivo. Sci. Rep. 2017, 7, 8319. [Google Scholar] [CrossRef]
- Sato, Y.; Yamada, S.; Takeda, S.; Hattori, N.; Nakamura, K.; Tanaka, H.; Mizuno, M.; Hori, M.; Kodera, Y. Effect of Plasma-activated Lactated Ringer’s Solution on Pancreatic Cancer Cells in Vitro and in Vivo. Ann. Surg. Oncol. 2018, 25, 299–307. [Google Scholar] [CrossRef]
- Partecke, L.I.; Evert, K.; Haugk, J.; Doering, F.; Normann, L.; Diedrich, S.; Weiss, F.U.; Evert, M.; Huebner, N.O.; Guenther, C.; et al. Tissue Tolerable Plasma (TTP) Induces Apoptosis in Pancreatic Cancer Cells in Vitro and in Vivo. BMC Cancer 2012, 12, 473. [Google Scholar] [CrossRef] [PubMed]
- Stoffels, E.; Kieft, I.E.; Sladek, R.E.J.; van den Bedem, L.J.M.; van der Laan, E.P.; Steinbuch, M. Plasma Needle for in Vivo Medical Treatment: Recent Developments and Perspectives. Plasma Sources Sci. Technol. 2006, 15, S169–S180. [Google Scholar] [CrossRef]
- Stoffels, E.; Roks, A.J.M.; Deelmm, L.E. Delayed Effects of Cold Atmospheric Plasma on Vascular Cells. Plasma Process. Polym. 2008, 5, 599–605. [Google Scholar] [CrossRef]
- Stoffels, E.; Sakiyama, Y.; Graves, D.B. Cold Atmospheric Plasma: Charged Species and Their Interactions with Cells and Tissues. IEEE Trans. Plasma Sci. 2008, 36, 1441–1457. [Google Scholar] [CrossRef]
- Khabipov, A.; Kading, A.; Liedtke, K.R.; Freund, E.; Partecke, L.I.; Bekeschus, S. RAW 264.7 Macrophage Polarization by Pancreatic Cancer Cells—A Model for Studying Tumour-promoting Macrophages. Anticancer Res. 2019, 39, 2871–2882. [Google Scholar] [CrossRef] [PubMed]
- Fidler, I.J. Critical Determinants of Metastasis. Semin. Cancer Biol. 2002, 12, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Judee, F.; Fongia, C.; Ducommun, B.; Yousfi, M.; Lobjois, V.; Merbahi, N. Short and Long Time Effects of Low Temperature Plasma Activated Media on 3D Multicellular Tumor Spheroids. Sci. Rep. 2016, 6, 21421. [Google Scholar] [CrossRef]
- Chauvin, J.; Gibot, L.; Griseti, E.; Golzio, M.; Rols, M.P.; Merbahi, N.; Vicendo, P. Elucidation of in Vitro Cellular Steps Induced by Antitumor Treatment with Plasma-activated Medium. Sci. Rep. 2019, 9, 4866. [Google Scholar] [CrossRef]
- Freund, E.; Liedtke, K.R.; van der Linde, J.; Metelmann, H.R.; Heidecke, C.D.; Partecke, L.I.; Bekeschus, S. Physical Plasma-treated Saline Promotes an Immunogenic Phenotype in CT26 Colon Cancer Cells in Vitro and in Vivo. Sci. Rep. 2019, 9, 634. [Google Scholar] [CrossRef]
- Gandhirajan, R.K.; Rodder, K.; Bodnar, Y.; Pasqual-Melo, G.; Emmert, S.; Griguer, C.E.; Weltmann, K.D.; Bekeschus, S. Cytochrome c Oxidase Inhibition and Cold Plasma-derived Oxidants Synergize in Melanoma Cell Death Induction. Sci. Rep. 2018, 8, 12734. [Google Scholar] [CrossRef]
- Sagwal, S.K.; Pasqual-Melo, G.; Bodnar, Y.; Gandhirajan, R.K.; Bekeschus, S. Combination of Chemotherapy and Physical Plasma Elicits Melanoma Cell Death Via Upregulation of SLC22A16. Cell Death Dis. 2018, 9, 1179. [Google Scholar] [CrossRef] [PubMed]
- Privat-Maldonado, A.; Gorbanev, Y.; Dewilde, S.; Smits, E.; Bogaerts, A. Reduction of Human Glioblastoma Spheroids Using Cold Atmospheric Plasma: The Combined Effect of Short- and Long-lived Reactive Species. Cancers 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Naumov, G.N.; MacDonald, I.C.; Weinmeister, P.M.; Kerkvliet, N.; Nadkarni, K.V.; Wilson, S.M.; Morris, V.L.; Groom, A.C.; Chambers, A.F. Persistence of Solitary Mammary Carcinoma Cells in a Secondary Site: A Possible Contributor to Dormancy. Cancer Res. 2002, 62, 2162–2168. [Google Scholar] [PubMed]
- Bekeschus, S.; Rodder, K.; Fregin, B.; Otto, O.; Lippert, M.; Weltmann, K.D.; Wende, K.; Schmidt, A.; Gandhirajan, R.K. Toxicity and Immunogenicity in Murine Melanoma Following Exposure to Physical Plasma-derived Oxidants. Oxid. Med. Cell. Longev. 2017, 2017, 4396467. [Google Scholar] [CrossRef] [PubMed]
- Freund, E.; Liedtke, K.R.; Gebbe, R.; Heidecke, A.K.; Partecke, L.-I.; Bekeschus, S. In Vitro Anticancer Efficacy of Six Different Clinically Approved Types of Liquids Exposed to Physical Plasma. IEEE Trans. Radiation Plasma Med. Sci. 2019. [Google Scholar] [CrossRef]
- Hasse, S.; Muller, M.C.; Schallreuter, K.U.; von Woedtke, T. Stimulation of Melanin Synthesis in Melanoma Cells by Cold Plasma. Biol. Chem. 2018, 400, 101–109. [Google Scholar] [CrossRef]
- Rödder, K.; Moritz, J.; Miller, V.; Weltmann, K.-D.; Metelmann, H.-R.; Gandhirajan, R.; Bekeschus, S. Activation of Murine Immune Cells Upon Co-culture with Plasma-treated B16F10 Melanoma Cells. Applied Sci. 2019, 9, 660. [Google Scholar] [CrossRef]
- Schmidt, A.; Bekeschus, S.; von Woedtke, T.; Hasse, S. Cell Migration and Adhesion of a Human Melanoma Cell Line is Decreased by Cold Plasma Treatment. Clin. Plas. Med. 2015, 3, 24–31. [Google Scholar] [CrossRef]
- Izumi, D.; Ishimoto, T.; Miyake, K.; Sugihara, H.; Eto, K.; Sawayama, H.; Yasuda, T.; Kiyozumi, Y.; Kaida, T.; Kurashige, J.; et al. CXCL12/CXCR4 Activation by Cancer-associated Fibroblasts Promotes Integrin Beta1 Clustering and Invasiveness in Gastric Cancer. Int. J. Cancer 2016, 138, 1207–1219. [Google Scholar] [CrossRef]
- Guan, X. Cancer metastases: Challenges and Opportunities. Acta. Pharm. Sin. B. 2015, 5, 402–418. [Google Scholar] [CrossRef]
- Canel, M.; Serrels, A.; Frame, M.C.; Brunton, V.G. E-cadherin-integrin Crosstalk in Cancer Invasion and Metastasis. J. Cell Sci. 2013, 126, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Menke, A.; Philippi, C.; Vogelmann, R.; Seidel, B.; Lutz, M.P.; Adler, G.; Wedlich, D. Down-regulation of E-cadherin Gene Expression by Collagen Type I and Type III in Pancreatic Cancer Cell Lines. Cancer Res. 2001, 61, 3508–3517. [Google Scholar] [PubMed]
- Ni, J.; Cozzi, P.J.; Duan, W.; Shigdar, S.; Graham, P.H.; John, K.H.; Li, Y. Role of the EpCAM (CD326) in Prostate Cancer Metastasis and Progression. Cancer Metastasis Rev. 2012, 31, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Weinel, R.J.; Neumann, K.; Kisker, O.; Rosendahl, A. Expression and Potential Role of E-cadherin in Pancreatic Carcinoma. Int. J. Pancreatol. 1996, 19, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Ellenrieder, V.; Adler, G.; Gress, T.M. Invasion and Metastasis in Pancreatic Cancer. Ann. Oncol. 1999, 10, S46–S50. [Google Scholar] [CrossRef]
- Grzesiak, J.J.; Ho, J.C.; Moossa, A.R.; Bouvet, M. The Integrin-extracellular Matrix Axis in Pancreatic Cancer. Pancreas 2007, 35, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Schmid, M.C.; Avraamides, C.J.; Foubert, P.; Shaked, Y.; Kang, S.W.; Kerbel, R.S.; Varner, J.A. Combined Blockade of Integrin-alpha4beta1 Plus Cytokines SDF-1alpha or IL-1beta Potently Inhibits Tumor Inflammation and Growth. Cancer Res. 2011, 71, 6965–6975. [Google Scholar] [CrossRef]



| Description of Adhesion Markers Analyzed | Modulation of Adhesion Marker Expression Post Plasma Treatment | ||||||
|---|---|---|---|---|---|---|---|
| CD | Other Names | Ligand | MiaPaCa2 | PaTuS | PaTuT | Panc01 | |
| 49b | VLA-2α, α2 integrin | collagen | = | = | = | = | 4 h |
| − | = | + | = | 24 h | |||
| 49d | VLA-4α, α4 integrin | VCMA-1, MAdCAM-1, fibronectin, CD242 | = | + | + | = | 4 h |
| + | + | + | + | 24 h | |||
| 324 | E-cadherin, CDH1 | CD103, E-cadherin, catenins, internalin | = | = | = | = | 4 h |
| − | − | = | = | 24 h | |||
| 326 | Ep-Cam, TROP1 | LAIR-1, LAIR-2, Ep-CAM | = | = | + | = | 4 h |
| = | − | − | = | 24 h | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bekeschus, S.; Freund, E.; Spadola, C.; Privat-Maldonado, A.; Hackbarth, C.; Bogaerts, A.; Schmidt, A.; Wende, K.; Weltmann, K.-D.; von Woedtke, T.; et al. Risk Assessment of kINPen Plasma Treatment of Four Human Pancreatic Cancer Cell Lines with Respect to Metastasis. Cancers 2019, 11, 1237. https://doi.org/10.3390/cancers11091237
Bekeschus S, Freund E, Spadola C, Privat-Maldonado A, Hackbarth C, Bogaerts A, Schmidt A, Wende K, Weltmann K-D, von Woedtke T, et al. Risk Assessment of kINPen Plasma Treatment of Four Human Pancreatic Cancer Cell Lines with Respect to Metastasis. Cancers. 2019; 11(9):1237. https://doi.org/10.3390/cancers11091237
Chicago/Turabian StyleBekeschus, Sander, Eric Freund, Chiara Spadola, Angela Privat-Maldonado, Christine Hackbarth, Annemie Bogaerts, Anke Schmidt, Kristian Wende, Klaus-Dieter Weltmann, Thomas von Woedtke, and et al. 2019. "Risk Assessment of kINPen Plasma Treatment of Four Human Pancreatic Cancer Cell Lines with Respect to Metastasis" Cancers 11, no. 9: 1237. https://doi.org/10.3390/cancers11091237
APA StyleBekeschus, S., Freund, E., Spadola, C., Privat-Maldonado, A., Hackbarth, C., Bogaerts, A., Schmidt, A., Wende, K., Weltmann, K.-D., von Woedtke, T., Heidecke, C.-D., Partecke, L.-I., & Käding, A. (2019). Risk Assessment of kINPen Plasma Treatment of Four Human Pancreatic Cancer Cell Lines with Respect to Metastasis. Cancers, 11(9), 1237. https://doi.org/10.3390/cancers11091237









