Anthracyclines Suppress Both NADPH Oxidase- Dependent and -Independent NETosis in Human Neutrophils
Abstract
:1. Introduction
2. Results
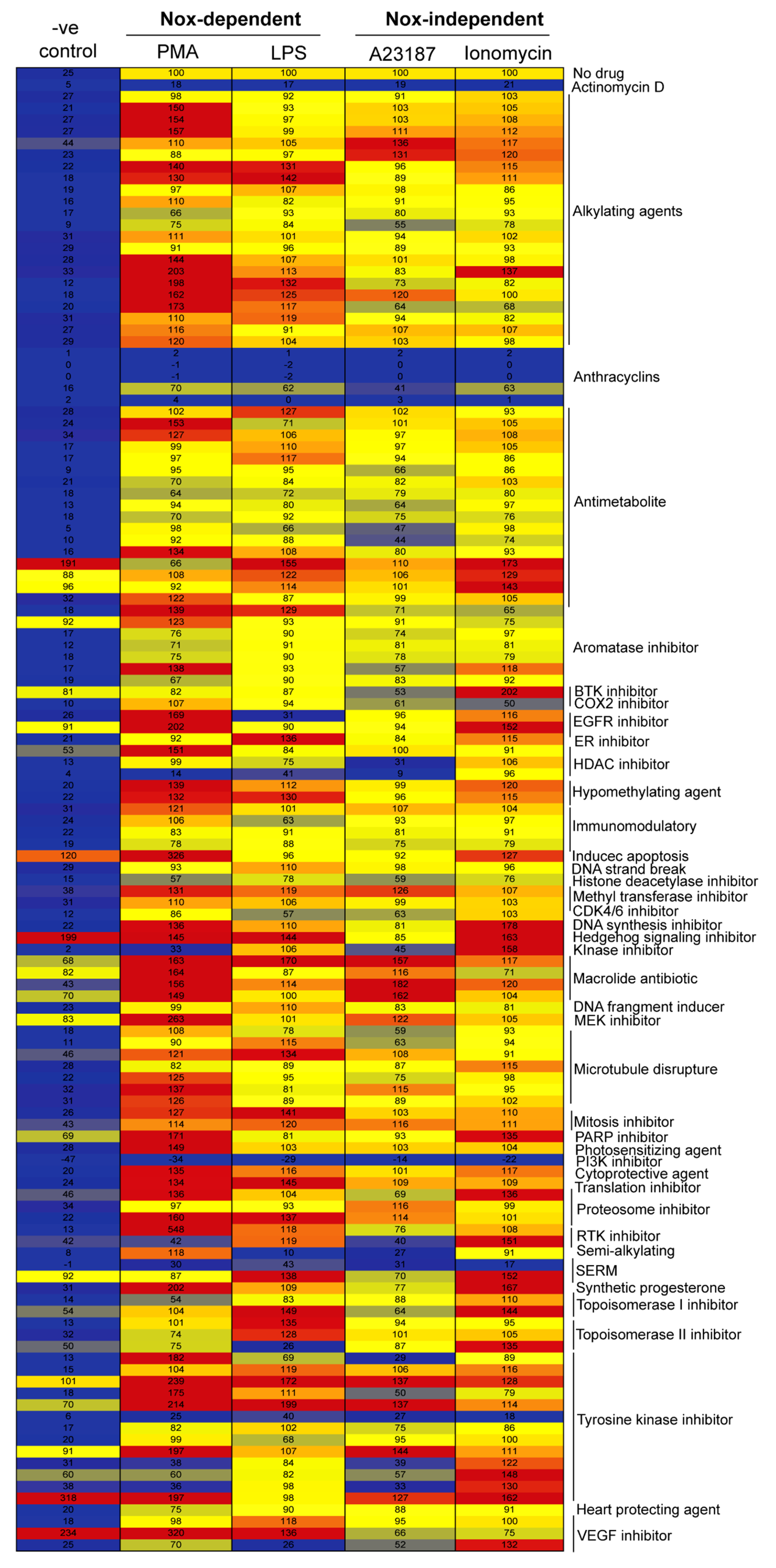
2.1. Drug Screening Shows That Anthracyclines Drastically Suppress NETosis
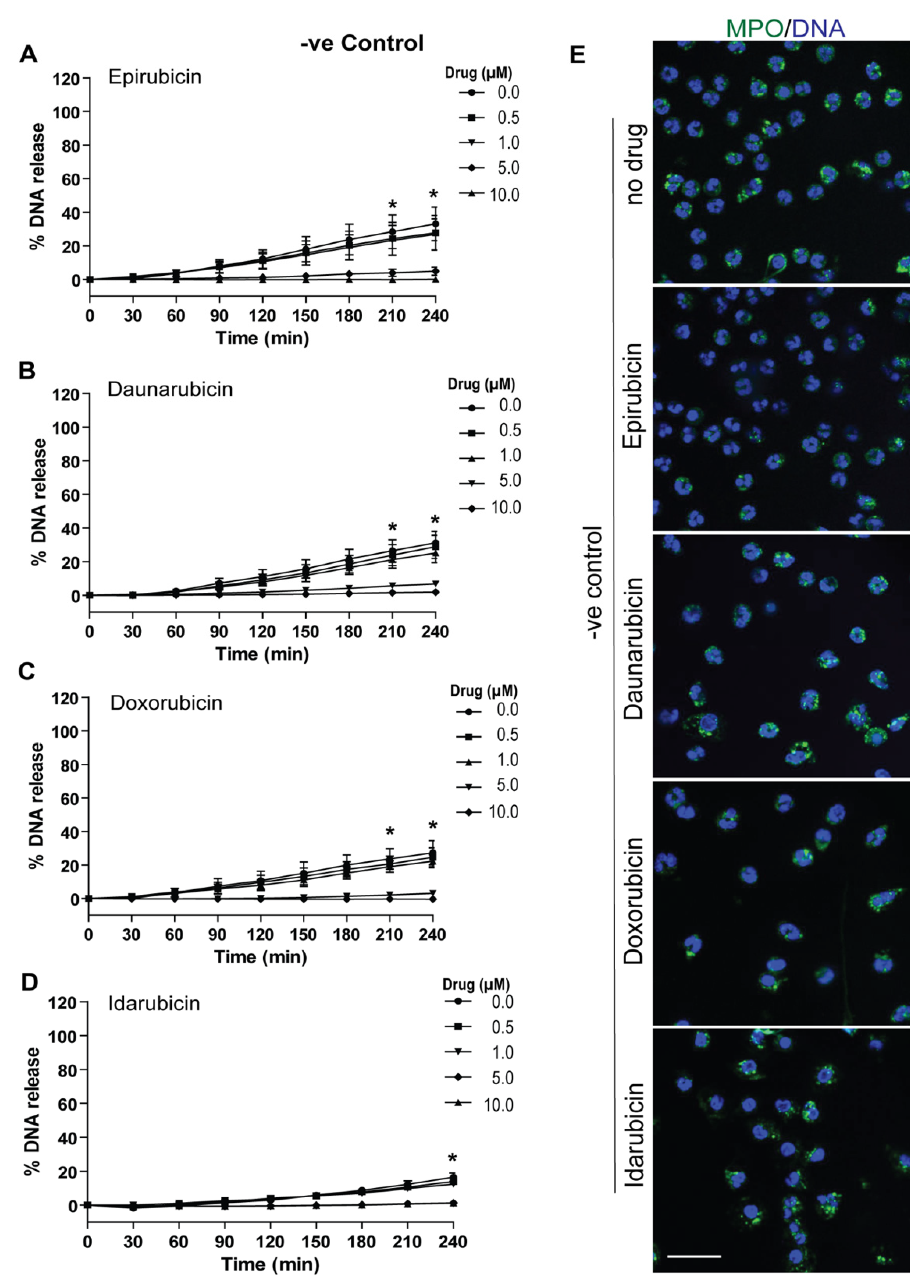
2.2. Anthracyclines Dose-Dependently Suppress Baseline NETosis
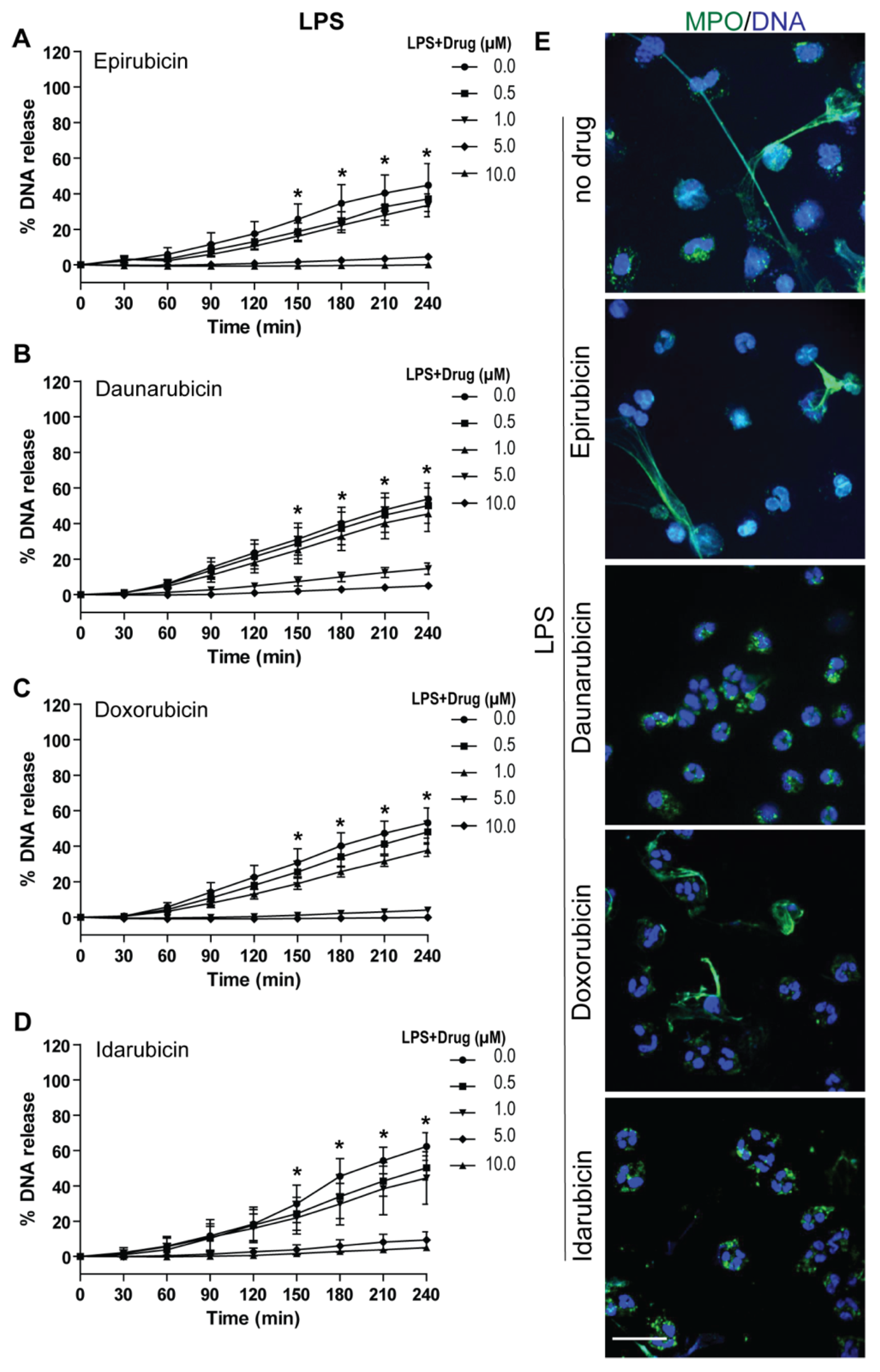
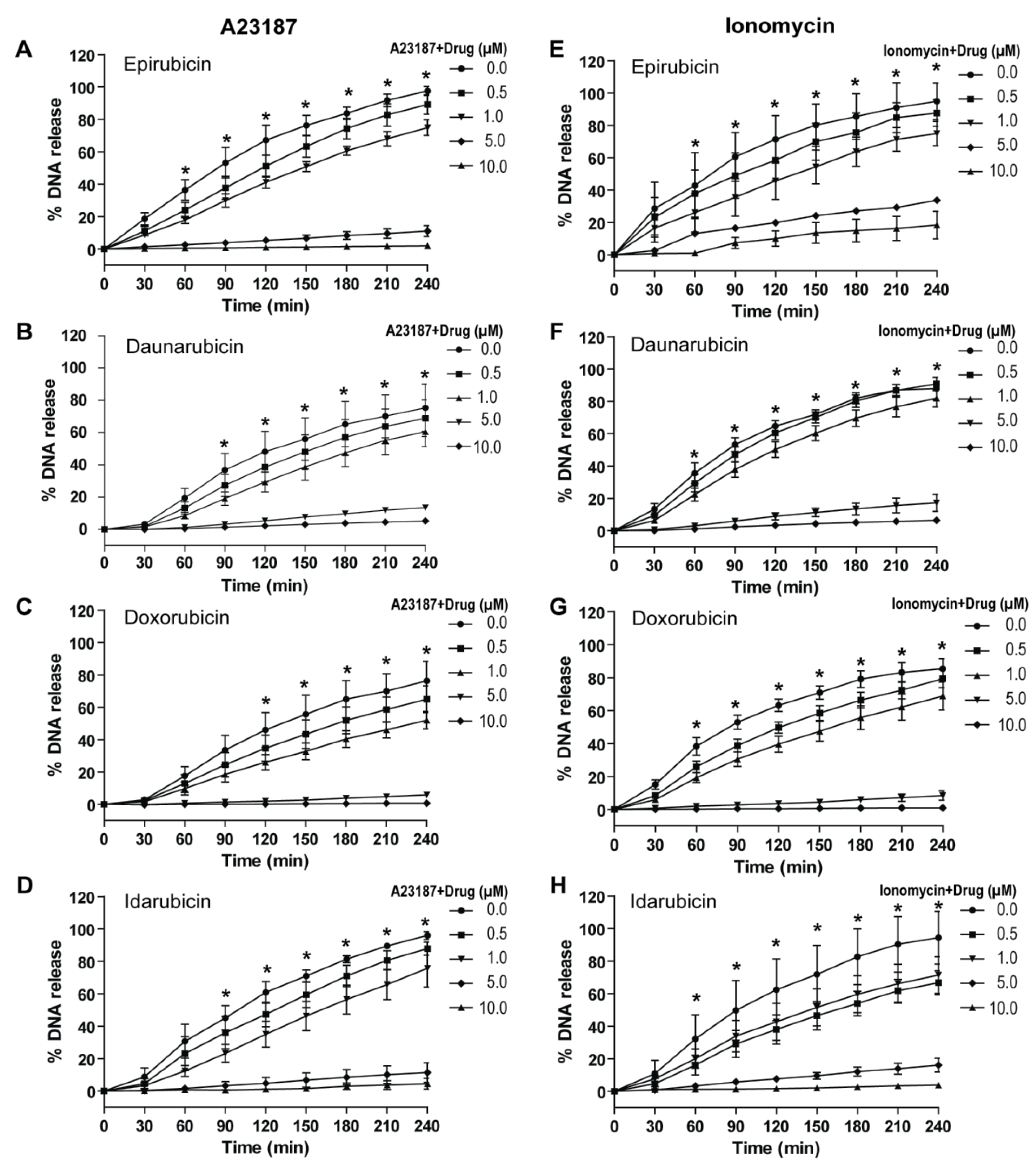
2.3. Anthracyclines Dose-Dependently Suppress Nox-Dependent NETosis without Affecting ROS Production
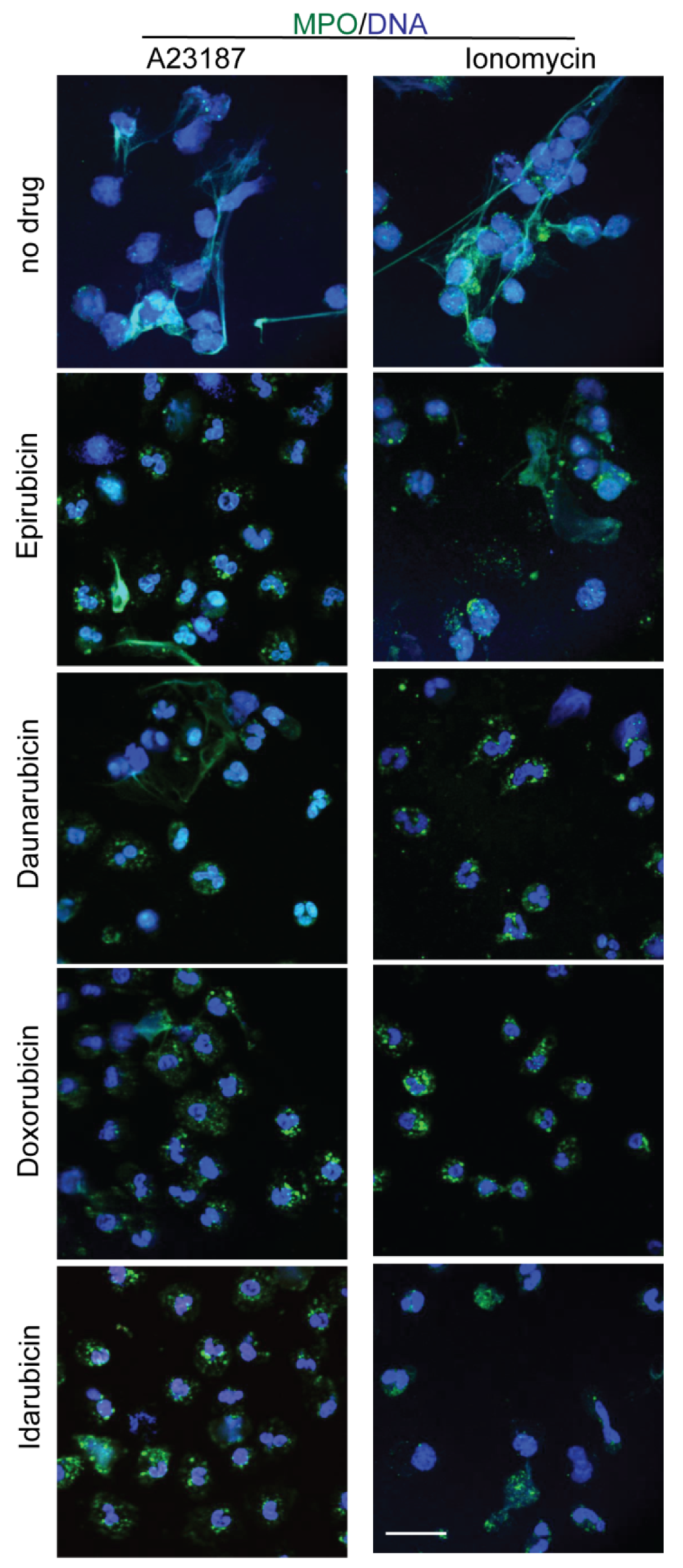
2.4. Anthracyclines Dose-Dependently Suppress Nox-Independent NETosis
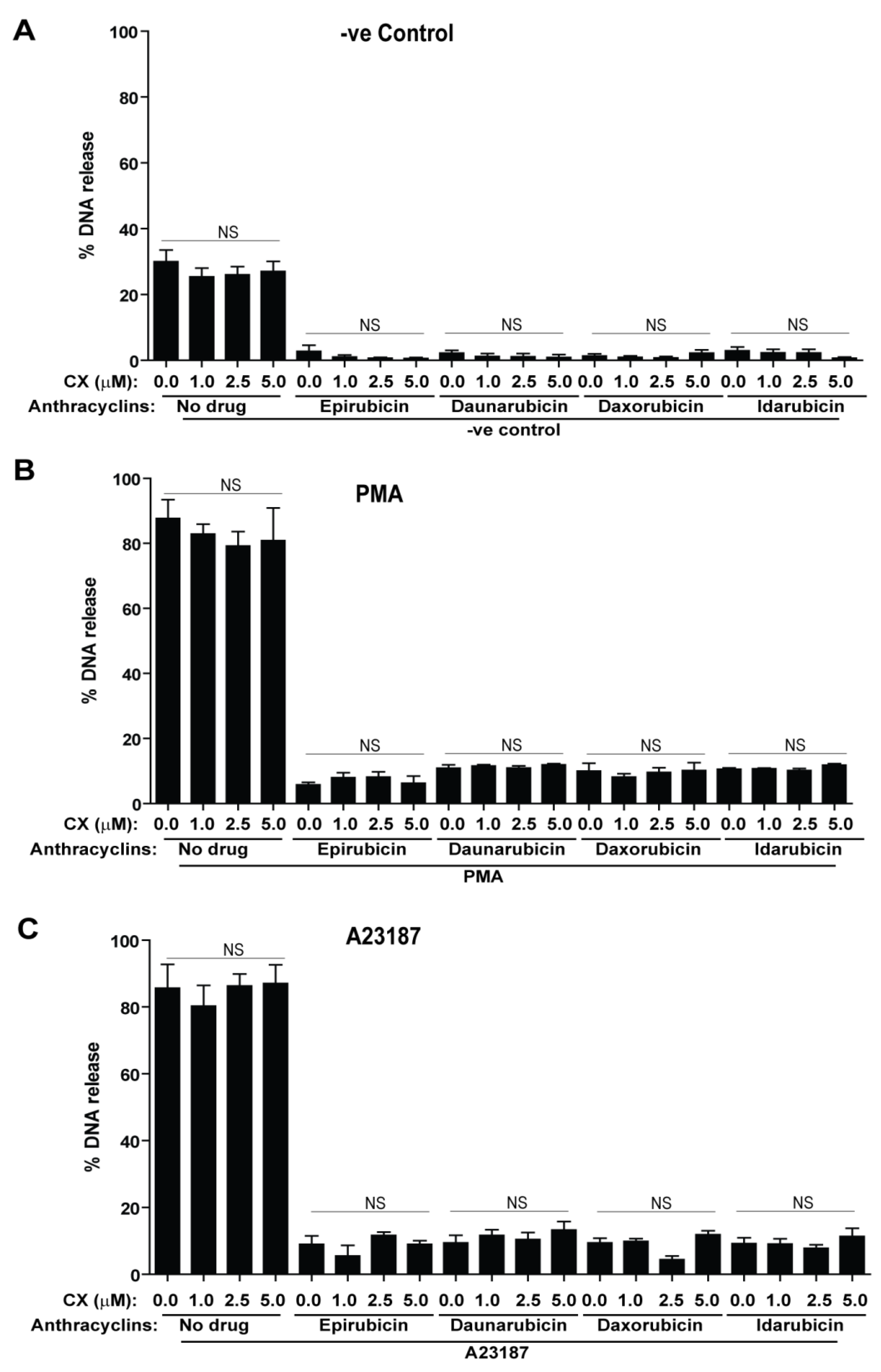
2.5. Dexrazoxane, a Cardioprotective Agent, Does Not Alter the Suppressive Effect of Anthracyclines on NETosis
2.6. Doses of Anthracyclines That Fully Suppress NETosis Do Not Induce Apoptosis
3. Discussion
4. Materials and Methods
4.1. Research Ethics Board Approval
4.2. Reagents
4.3. Initial Screening Dosage Selection
4.4. Neutrophil Isolation
4.5. Sytox Green- NETosis Assay
4.6. DHR123- ROS Assay
4.7. Immunofluorescence Confocal Microscopy
4.8. Confocal Imaging of Apoptosis
4.9. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cheng, O.Z.; Palaniyar, N. NET balancing: A problem in inflammatory lung diseases. Front. Immunol. 2013, 4, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Takei, H.; Araki, A.; Watanabe, H.; Ichinose, A.; Sendo, F. Rapid killing of human neutrophils by the potent activator phorbol 12-myristate 13-acetate (PMA) accompanied by changes different from typical apoptosis or necrosis. J. Leukoc. Biol. 1996, 59, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Philip, L.M.; Cheung, G.; Vadakepeedika, S.; Grasemann, H.; Sweezey, N.; Palaniyar, N. Regulating NETosis: Increasing pH Promotes NADPH Oxidase-Dependent NETosis. Front. Med. 2018, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Azzouz, L.; Cherry, A.; Riedl, M.; Khan, M.; Pluthero, F.G.; Kahr, W.H.A.; Palaniyar, N.; Licht, C. Relative antibacterial functions of complement and NETs: NETs trap and complement effectively kills bacteria. Mol. Immunol. 2018, 97, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, C.; Palaniyar, N.; Otulakowski, G.; Khan, M.A.; Post, M.; Kuebler, W.M.; Tanswell, K.; Belcastro, R.; Masood, A.; Engelberts, D.; et al. Mechanical ventilation induces neutrophil extracellular trap formation. Anesthesiology 2015, 122, 864–875. [Google Scholar] [CrossRef]
- Brinkmann, V.; Zychlinsky, A. Neutrophil extracellular traps: Is immunity the second function of chromatin? J. Cell. Biol. 2012, 198, 773–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil Extracellular Traps Kill Bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Urban, C.F.; Reichard, U.; Brinkmann, V.; Zychlinsky, A. Neutrophil extracellular traps capture and kill Candida albicans and hyphal forms. Cell. Microbiol. 2006, 8, 668–676. [Google Scholar] [CrossRef]
- Urban, C.F.; Ermert, D.; Schmid, M.; Abu-Abed, U.; Goosmann, C.; Nacken, W.; Brinkmann, V.; Jungblut, P.R.; Zychlinsky, A. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009, 5. [Google Scholar] [CrossRef]
- Averhoff, P.; Kolbe, M.; Zychlinsky, A.; Weinrauch, Y. Single Residue Determines the Specificity of Neutrophil Elastase for Shigella Virulence Factors. J. Mol. Biol. 2008, 377, 1053–1066. [Google Scholar] [CrossRef]
- Megens, R.T.A.; Vijayan, S.; Lievens, D.; Döring, Y.; van Zandvoort, M.A.M.J.; Grommes, J.; Weber, C.; Soehnlein, O. Presence of luminal neutrophil extracellular traps in atherosclerosis. Thromb. Haemost. 2012, 107, 597–598. [Google Scholar] [CrossRef] [PubMed]
- Friday, S.; Fox, D.A.; Carmona-Rivera, C.; Subramanian, V.; Kaplan, M.J.; Thompson, P.; Pennathur, S.; Knight, J.S.; Chen, P.; Khandpur, R.; et al. NETs Are a Source of Citrullinated Autoantigens and Stimulate Inflammatory Responses in Rheumatoid Arthritis. Sci. Transl. Med. 2013, 5, 178ra40. [Google Scholar] [CrossRef]
- Kessenbrock, K.; Krumbholz, M.; Schönermarck, U.; Back, W.; Gross, W.L.; Werb, Z.; Gröne, H.J.; Brinkmann, V.; Jenne, D.E. Netting neutrophils in autoimmune small-vessel vasculitis. Nat. Med. 2009, 15, 623–625. [Google Scholar] [CrossRef] [PubMed]
- Cools-Lartigue, J.; Spicer, J.; Najmeh, S.; Ferri, L. Neutrophil extracellular traps in cancer progression. Cell. Mol. Life Sci. 2014, 71, 4179–4194. [Google Scholar] [CrossRef] [PubMed]
- Demers, M.; Wagner, D.D. NETosis: A New Factor in Tumor Progression and Cancer-Associated Thrombosis. Semin. Thromb. Hemost. 2014, 40, 277–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaplan, J.M. Neutrophil extracelullar traps (NETs): Double-edged swords of innate immunity 1. J. Immunol. 2013, 189, 2689–2695. [Google Scholar] [CrossRef] [PubMed]
- Remijsen, Q.; Kuijpers, T.W.; Wirawan, E.; Lippens, S.; Vandenabeele, P.; Vanden Berghe, T. Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell Death Differ. 2011, 18, 581–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douda, D.N.; Khan, M.A.; Grasemann, H.; Palaniyar, N. SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx. Proc. Natl. Acad. Sci. USA 2015, 112, 2817–2822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoiber, W.; Obermayer, A.; Steinbacher, P.; Krautgartner, W.-D. The Role of Reactive Oxygen Species (ROS) in the Formation of Extracellular Traps (ETs) in Humans. Biomolecules 2015, 5, 702–723. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.A.; Farahvash, A.; Douda, D.N.; Licht, J.C.; Grasemann, H.; Sweezey, N.; Palaniyar, N. JNK Activation Turns on LPS-And Gram-Negative Bacteria-Induced NADPH Oxidase-Dependent Suicidal NETosis. Sci. Rep. 2017, 7, 1–16. [Google Scholar] [CrossRef]
- Douda, D.N.; Yip, L.; Khan, M.A.; Grasemann, H.; Palaniyar, N. To the editor: Akt is essential to induce NADPH-dependent NETosis and to switch the neutrophil death to apoptosis. Blood 2014, 123, 597–600. [Google Scholar] [CrossRef] [PubMed]
- Keshari, R.S.; Verma, A.; Barthwal, M.K.; Dikshit, M. Reactive oxygen species-induced activation of ERK and p38 MAPK mediates PMA-induced NETs release from human neutrophils. J. Cell. Biochem. 2013, 114, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Papayannopoulos, V.; Metzler, K.D.; Hakkim, A.; Zychlinsky, A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell. Biol. 2010, 191, 677–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neeli, I.; Dwivedi, N.; Khan, S.; Radic, M. Regulation of extracellular chromatin release from neutrophils. J. Innate Immun. 2009, 1, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Chicca, I.J.; Milward, M.R.; Chapple, I.L.C.; Griffiths, G.; Benson, R.; Dietrich, T.; Cooper, P.R. Development and application of high-content biological screening for modulators of NET production. Front. Immunol. 2018, 9, 337. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.S.; Renshaw, S.A. Using in vivo zebrafish models to understand the biochemical basis of neutrophilic respiratory disease. Biochem. Soc. Trans. 2009, 37, 830–837. [Google Scholar] [CrossRef]
- Balis, F.M. Evolution of anticancer drug discovery and the role of cell-based screening. J. Natl. Cancer Inst. 2002, 94, 78–79. [Google Scholar] [CrossRef]
- Kong, G.; Anyarambhatla, G.; Petros, W.P.; Braun, R.D.; Colvin, O.M.; Needham, D.; Dewhirst, M.W. Efficacy of liposomes and hyperthermia in a human tumor xenograft model: Importance of triggered drug release. Cancer Res. 2000, 6950–6957. [Google Scholar] [CrossRef]
- Nitiss, J.L. Targeting DNA topoisomerase II in cancer chemotherapy. Nat. Rev. Cancer 2009, 9, 338–350. [Google Scholar] [CrossRef] [Green Version]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin pathways: Pharmacodynamics and adverse effects. Pharmacogenet. Genom. 2011, 21, 440–446. [Google Scholar] [CrossRef]
- Liesse, K.; Harris, J.; Chan, M.; Schmidt, M.L.; Chiu, B. Dexrazoxane significantly reduces anthracycline-induced cardiotoxicity in pediatric solid tumor patients: A systematic review. J. Pediatr. Hematol. Oncol. 2018, 40, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Remijsen, Q.; Berghe, T.V.; Wirawan, E.; Asselbergh, B.; Parthoens, E.; De Rycke, R.; Noppen, S.; Delforge, M.; Willems, J.; Vandenabeele, P. Neutrophil extracellular trap cell death requires both autophagy and superoxide generation. Cell Res. 2011, 21, 290–304. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.E. What is nanomechanics of bone and why is it important? J. Musculoskelet. Neuronal Interact. 2008, 8, 327–328. [Google Scholar] [CrossRef] [PubMed]
- Cedervall, J.; Olsson, A. NETosis in cancer. Oncoscience 2015, 2, 900–901. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, M.; Khan, M.A.; Palaniyar, N. Neutrophil Extracellular Trap Formation: Physiology, Pathology, and Pharmacology. Biomolecules 2019, 9, E365. [Google Scholar] [CrossRef]
- Beretta, G.L.; Zunino, F. Molecular mechanisms of anthracycline activity. Top Curr. Chem. 2008, 283, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Palaniyar, N. Transcriptional firing helps to drive NETosis. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Šimůnek, T.; Štěrba, M.; Popelová, O.; Adamcová, M.; Hrdina, R.; Gerši, V. Anthracycline-induced cardiotoxicity: Overview of studies examining the roles of oxidative stress and free cellular iron. Pharmacol. Rep. 2009, 61, 154–171. [Google Scholar] [CrossRef]
- Swystun, L.L.; Mukherjee, S.; Liaw, P.C. Breast cancer chemotherapy induces the release of cell-free DNA, a novel procoagulant stimulus. J. Thromb. Haemost. 2011, 9, 2313–2321. [Google Scholar] [CrossRef]
- Kenny, E.F.; Herzig, A.; Krüger, R.; Muth, A.; Mondal, S.; Thompson, P.R.; Brinkmann, V.; von Bernuth, H.; Zychlinsky, A. Diverse stimuli engage different neutrophil extracellular trap pathways. eLife 2017, 6. [Google Scholar] [CrossRef]
- Ducore, J.; Lawrence, J.B.; Simpson, M.; Boggio, L.; Bellon, A.; Burggraaf, J.; Stevens, J.; Moerland, M.; Frieling, J.; Reijers, J.; et al. Safety and dose-dependency of eptacog beta (activated) in a dose escalation study of non-bleeding congenital haemophilia A or B patients, with or without inhibitors. Haemophilia 2017, 23, 844–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douda, D.N.; Jackson, R.; Grasemann, H.; Palaniyar, N. Innate Immune Collectin Surfactant Protein D Simultaneously Binds Both Neutrophil Extracellular Traps and Carbohydrate Ligands and Promotes Bacterial Trapping. J. Immunol. 2011, 187, 1856–1865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, M.B.H.; Kuiper, J.W.P.; Katchky, A.; Goldberg, H.; Glogauer, M. Rac2 is required for the formation of neutrophil extracellular traps. J. Leukoc. Biol. 2011, 90, 771–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akong-Moore, K.; Chow, O.A.; von Köckritz-Blickwede, M.; Nizet, V. Influences of chloride and hypochlorite on neutrophil extracellular trap formation. PLoS ONE 2012, 7, e42984. [Google Scholar] [CrossRef] [PubMed]
- de Souza, C.N.; Breda, L.C.D.; Khan, M.A.; de Almeida, S.R.; Câmara, N.O.S.; Sweezey, N.; Palaniyar, N. Alkaline pH promotes NADPH oxidase-independent neutrophil extracellular trap formation: A matter of mitochondrial reactive oxygen species generation and citrullination and cleavage of histone. Front. Immunol. 2018, 8, 1849. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Giaglis, S.; Hasler, P.; Hahn, S. Efficient neutrophil extracellular trap induction requires mobilization of both intracellular and extracellular calcium pools and is modulated by cyclosporine A. PLoS ONE 2014, 9, e97088. [Google Scholar] [CrossRef] [PubMed]
- Singel, K.L.; Segal, B.H. NOX2-dependent regulation of inflammation. Clin. Sci. 2016, 130, 479–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.-T.; Kelsey, S.M.; Newland, A.C.; Jia, L. Generation of reactive oxygen species is not involved in idarubicin-induced apoptosis in human leukaemic cells. Br. J. Haematol. 2001, 115, 817–825. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, D.; Zhao, Y.; O’Neill, K.M.; Edgar, K.S.; Dunne, P.D.; Kearney, A.M.; Grieve, D.J.; McDermott, B.J. Signalling mechanisms underlying doxorubicin and Nox2 NADPH oxidase-induced cardiomyopathy: Involvement of mitofusin-2. Br. J. Pharmacol. 2017, 174, 3677–3695. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Teves, S.S.; Kemp, C.J.; Henikoff, S. Doxorubicin, DNA torsion, and chromatin dynamics. Biochim. Biophys. Acta 2014, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Azzouz, D.; Khan, M.A.; Sweezey, N.; Palaniyar, N. Two-in-one: UV radiation simultaneously induces apoptosis and NETosis. Cell Death Discov. 2018, 4, 51. [Google Scholar] [CrossRef] [PubMed]
- Nitiss, J.L. DNA topoisomerase II and its growing repertoire of biological functions. Nat. Rev. Cancer 2009, 9, 327–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, A.H.; Ughetto, G.; Quigley, G.J.; Rich, A. Interactions between an Anthracycline Antibiotic and DNA: Molecular Structure of Daunomycin Complexed to d(CpGpTpApCpG) at 1.2-Å Resolution. Biochemistry 1987, 26, 1152–1163. [Google Scholar] [CrossRef] [PubMed]
- Marin, S.; Mansilla, S.; Garcia-Reyero, N.; Rojas, M.; Portugal, J.; Pina, B. Promoter-Specific Inhibition of Transcription by Daunorubicin in Saccharomyces Cerevisiae. Biochem. J. 2002, 368, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Logan, K.; Zhang, J.; Davis, E.A.; Ackerman, S. Drug inhibitors of RNA polymerase II transcription. DNA 1989, 8, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Pommier, Y.; Leo, E.; Zhang, H.; Marchand, C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem. Biol. 2010, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, F.; Turriziani, M.; Tortorelli, G.; Avvisati, G.; Torino, F.; De Vecchis, L. Triazene compounds: Mechanism of action and related DNA repair systems. Pharmacol. Res. 2007, 56, 275–287. [Google Scholar] [CrossRef] [PubMed]
- White, P.C.; Chicca, I.J.; Cooper, P.R.; Milward, M.R.; Chapple, I.L.C. Neutrophil Extracellular Traps in Periodontitis: A Web of Intrigue. J. Dent. Res. 2016, 95, 26–34. [Google Scholar] [CrossRef]
- Matthews, J.B.; Wright, H.J.; Roberts, A.; Cooper, P.R.; Chapple, I.L.C. Hyperactivity and reactivity of peripheral blood neutrophils in chronic periodontitis. Clin. Exp. Immunol. 2007, 147, 255–264. [Google Scholar] [CrossRef]
- Wolter, J.; Seeney, S.; Bell, S.; Bowler, S.; Masel, P.; McCormack, J. Effect of long term treatment with azithromycin on disease parameters in cystic fibrosis: A randomised trial. Thorax 2002, 57, 212–216. [Google Scholar] [CrossRef]
- Cedervall, J.; Zhang, Y.; Olsson, A.K. Tumor-induced NETosis as a risk factor for metastasis and organ failure. Cancer Res. 2016, 76, 4311–4315. [Google Scholar] [CrossRef] [PubMed]
- Aapro, M.; Crawford, J.; Kamioner, D. Prophylaxis of chemotherapy-induced febrile neutropenia with granulocyte colony-stimulating factors: Where are we now? Support. Care Cancer 2010, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Alemán, S.R.; Campos-García, L.; Palma-Nicolas, J.P.; Hernández-Bello, R.; González, G.M.; Sánchez-González, A. Understanding the Entanglement: Neutrophil Extracellular Traps (NETs) in Cystic Fibrosis. Front. Cell. Infect. Microbiol. 2017, 7, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueiredo, N.; Chora, A.; Raquel, H.; Pejanovic, N.; Pereira, P.; Hartleben, B.; Neves-Costa, A.; Moita, C.; Pedroso, D.; Pinto, A.; et al. Anthracyclines induce DNA damage response-mediated protection against severe sepsis. Immunity 2013, 39, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Studzian, K.; Kik, K.; Lukawska, M.; Oszczapowicz, I.; Strek, M.; Szmigiero, L. Subcellular localization of anthracyclines in cultured rat cardiomyoblasts as possible predictors of cardiotoxicity. Invest. New Drugs 2015, 33, 1032–1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eloisi, C.L.; Shieh, J.-H.; Srikanth, A.; Su, T.-L.; Fabian, C.; Elizabeth, P.; Malcolm, A.S.M. Novel Alkylating Agent, Ureidomustine Exhibit Pre-Clinical Efficacy in B-Cell Lymphoma with Minimal Dose-Limiting Myelotoxicity. Blood 2015, 126, 1556. [Google Scholar]
- Hughes, J.P.; Rees, S.; Kalindjian, S.B.; Philpott, K.L. Principles of early drug discovery. Br. J. Pharmacol. 2011, 162, 1239–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunne, A.; Jowett, M.; Rees, S. Use of primary human cells in high-throughput screens. Methods Mol. Biol. 2009, 565, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Djiadeu, P.; Azzouz, D.; Khan, M.A.; Kotra, L.P.; Sweezey, N.; Palaniyar, N. Ultraviolet irradiation increases green fluorescence of dihydrorhodamine (DHR) 123: False-positive results for reactive oxygen species generation. Pharmacol. Res. Perspect. 2017, 5, e00303. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.A.; D’Ovidio, A.; Tran, H.; Palaniyar, N. Anthracyclines Suppress Both NADPH Oxidase- Dependent and -Independent NETosis in Human Neutrophils. Cancers 2019, 11, 1328. https://doi.org/10.3390/cancers11091328
Khan MA, D’Ovidio A, Tran H, Palaniyar N. Anthracyclines Suppress Both NADPH Oxidase- Dependent and -Independent NETosis in Human Neutrophils. Cancers. 2019; 11(9):1328. https://doi.org/10.3390/cancers11091328
Chicago/Turabian StyleKhan, Meraj A., Adam D’Ovidio, Harvard Tran, and Nades Palaniyar. 2019. "Anthracyclines Suppress Both NADPH Oxidase- Dependent and -Independent NETosis in Human Neutrophils" Cancers 11, no. 9: 1328. https://doi.org/10.3390/cancers11091328
APA StyleKhan, M. A., D’Ovidio, A., Tran, H., & Palaniyar, N. (2019). Anthracyclines Suppress Both NADPH Oxidase- Dependent and -Independent NETosis in Human Neutrophils. Cancers, 11(9), 1328. https://doi.org/10.3390/cancers11091328





