SARS-CoV-2 Infection and Cardioncology: From Cardiometabolic Risk Factors to Outcomes in Cancer Patients
Abstract
:Simple Summary
Abstract
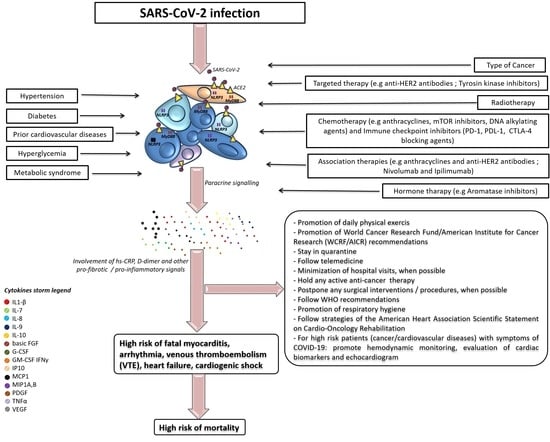
1. Introduction
2. Cardiovascular Outcomes in Non-Cancer Patients with COVID-19
3. Outcomes in Cancer Patients
4. Fatal Myocarditis
5. Venous Thromboembolism
6. Coagulation Dysfunctions: What about the Impact of Anticancer Therapies and Type of Cancer?
7. Angiotensin-Converting Enzyme 2 and Serine Protease TMPRSS2: Role in COVID-19 and Involvement in Cardio-Metabolic Risk Factors
8. Cardiovascular Microenvironment: A Focus on Cytokine Storm
9. Non-Pharmacological Strategies to Reduce Cardiovascular Events in Cancer Patients with COVID-19
- -
- Reduce total body fat, especially visceral fat; pay attention to body weight;
- -
- Do daily exercise, at any time of the day; reduce the time spent on television;
- -
- Limit consumption of energy-dense foods and avoid sugary drinks;
- -
- Eat mostly foods of plant origin and follow a diet rich in whole grains, vegetables (non-starchy), fruit, and legumes;
- -
- Limit the consumption of red meats (beef, pork, sheep), cured meats, and preserved meats;
- -
- Limit alcohol consumption;
- -
- For cancer prevention, do not use supplements. Try to meet nutritional needs through diet alone;
- -
- For mothers: if you have the opportunity to breastfeed, this has benefits for the baby and the mother.
10. Conclusions
Funding
Conflicts of Interest
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Green, M.S. Did the hesitancy in declaring COVID-19 a pandemic reflect a need to redefine the term? Lancet 2020. [Google Scholar] [CrossRef]
- Cárdenas-Conejo, Y.; Liñan-Rico, A.; García-Rodríguez, D.A.; Centeno-Leija, S.; Serrano-Posada, H. An exclusive 42 amino acid signature in pp1ab protein provides insights into the evolutive history of the 2019 novel human-pathogenic coronavirus (SARS-CoV2). J. Med. Virol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Perrella, A.; Carannante, N.; Berretta, M.; Rinaldi, M.; Maturo, N.; Rinaldi, L. Novel Coronavirus 2019 (Sars-CoV2): A global emergency that needs new approaches? Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 2162–2164. [Google Scholar] [CrossRef]
- Ksiazek, T.G.; Erdman, D.; Goldsmith, C.S.; Zaki, S.R.; Peret, T.; Emery, S.; Tong, S.; Urbani, C.; Comer, J.A.; Lim, W. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003, 348, 1953–1966. [Google Scholar] [CrossRef]
- Zaki, A.M.; Van Boheemen, S.; Bestebroer, T.M.; Osterhaus, A.D.; Fouchier, R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012, 367, 1814–1820. [Google Scholar] [CrossRef]
- Ceccarelli, M.; Berretta, M.; Venanzi Rullo, E.; Nunnari, G.; Cacopardo, B. Differences and similarities between Severe Acute Respiratory Syndrome (SARS)-CoronaVirus (CoV) and SARS-CoV-2. Would a rose by another name smell as sweet? Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 2781–2783. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, Y.; Wang, D.W. SARS-CoV-2: A potential novel etiology of fulminant myocarditis. Herz 2020. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.Y.; Ma, Y.T.; Zhang, J.Y.; Xie, X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020. [Google Scholar] [CrossRef] [Green Version]
- Ji, D.; Qin, E.; Xu, J. Non-alcoholic fatty liver diseases in patients with COVID-19: A retrospective study. J. Hepatol. 2020, 73, 451–453. [Google Scholar] [CrossRef]
- Abrams, E.M.; W’t Jong, G.; Yang, C.L. Asthma and COVID-19. CMAJ 2020, 192, E551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.K.; Gupta, R.; Ghosh, A.; Misra, A. Diabetes in COVID-19: Prevalence, pathophysiology, prognosis and practical considerations. Diabetes Metab. Syndr. 2020, 14, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Guo, Y.; Zheng, Y.; Huang, Z.; Sun, S.; Kowal, P.; Shi, Y.; Wu, F. Cardiovascular disease (CVD) and associated risk factors among older adults in six low-and middle-income countries: Results from SAGE Wave 1. BMC Public Health 2018, 18, 778. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, L.A.; Shulman, L.N. Follow-up care of cancer survivors: Challenges and solutions. Lancet Oncol. 2017, 18, e19–e29. [Google Scholar] [CrossRef]
- Zaim, S.; Chong, J.H.; Sankaranarayanan, V.; Harky, A. COVID-19 and Multi-Organ Response. Curr. Probl. Cardiol. 2020, 100618. [Google Scholar] [CrossRef]
- Chen, C.; Chen, C.; Yan, J.T.; Zhou, N.; Zhao, J.P.; Wang, D.W. Analysis of myocardial injury in patients with COVID-19 and association between concomitant cardiovascular diseases and severity of COVID-19. Zhonghua Xin Xue Guan Bing Za Zhi 2020, 48, E008. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Xiong, Y.; Zhao, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020. [Google Scholar] [CrossRef]
- Kang, D.H.; Weaver, M.T.; Park, N.J.; Smith, B.; McArdle, T.; Carpenter, J. Significant impairment in immune recovery after cancer treatment. Nurs. Res. 2009, 58, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Hijano, D.R.; Maron, G.; Hayden, R.T. Respiratory Viral Infections in Patients with Cancer or Undergoing Hematopoietic Cell Transplant. Front. Microbiol. 2018, 9, 3097. [Google Scholar] [CrossRef]
- Available online: https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2020/03/specialty-guide-acute-treatment-cancer-23-march-2020.pdf (accessed on 1 November 2020).
- Liang, W.; Guan, W.; Chen, R. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol. 2020, 21, 335–337. [Google Scholar] [CrossRef]
- Yu, J.; Ouyang, W.; Chua, M.L.K.; Xie, C. SARS-CoV-2 Transmission in Patients with Cancer at a Tertiary Care Hospital in Wuhan, China. JAMA Oncol. 2020, 6, 1108–1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, W.J.; Liang, W.H.; Zhao, Y.; Liang, H.R.; Chen, Z.S.; Li, Y.M.; Liu, X.Q.; Chen, R.C.; Tang, C.L.; Wang, T.; et al. China Medical Treatment Expert Group for COVID-19. Comorbidity and its impact on 1590 patients with COVID-19 in China: A nationwide analysis. Eur. Respir. J. 2020, 55, 2000547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Characteristics of SARS-CoV-2 Patients Dying in Italy Report Based on Available Data on 20 April 2020. Available online: https://www.epicentro.iss.it/en/coronavirus/bollettino/Report-COVID-2019_20_april_2020.pdf (accessed on 20 October 2020).
- Trapani, D.; Marra, A.; Curigliano, G. The experience on coronavirus disease 2019 and cancer from an oncology hub institution in Milan, Lombardy Region. Eur. J. Cancer 2020, 132, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Montopoli, M.; Zumerle, S.; Vettor, R.; Rugge, M.; Zorzi, M.; Catapano, C.V.; Carbone, G.M.; Cavalli, A.; Pagano, F.; Ragazzi, E.; et al. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: A population-based study (n = 4532). Ann. Oncol. 2020. [Google Scholar] [CrossRef]
- Clinckemalie, L.; Spans, L.; Dubois, V.; Laurent, M.; Helsen, C.; Joniau, S.; Claessens, F. Androgen regulation of the TMPRSS2 gene and the effect of a SNP in an androgen response element. Mol. Endocrinol. 2013, 2028–2040. [Google Scholar] [CrossRef]
- de Rojas, T.; Pérez-Martínez, A.; Cela, E.; Baragaño, M.; Galán, V.; Mata, C.; Peretó, A.; Madero, L. COVID-19 infection in children and adolescents with cancer in Madrid. Pediatr. Blood Cancer 2020. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72,314 Cases from the Chinese Center for Disease Control and Prevention. JAMA 2020. [Google Scholar] [CrossRef]
- Onder, G.; Rezza, G.; Brusaferro, S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA 2020, 323, 1775–1776. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, F.; Xie, L. Clinical characteristics of COVID-19-infected cancer patients: A retrospective case study in three hospitals within Wuhan, China. Ann. Oncol. 2020. [Google Scholar] [CrossRef]
- Bonomi, L.; Ghilardi, L.; Arnoldi, E.; Tondini, C.A.; Bettini, A.C. A rapid fatal evolution of Coronavirus Disease-19 (COVID-19) in an advanced lung cancer patient with a long time response to nivolumab. J. Thorac. Oncol. 2020. [Google Scholar] [CrossRef]
- He, W.; Chen, L.; Chen, L.; Yuan, G.; Fang, Y.; Chen, W.; Wu, D.; Liang, B.; Lu, X.; Ma, Y.; et al. COVID-19 in persons with haematological cancers. Leukemia 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, H.; Mikami, T.; Chopra, N. Do Patients with Cancer Have a Poorer Prognosis of COVID-19? An Experience in New York City. Ann. Oncol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Liu, D.; Liu, M. Patients with Cancer Appear More Vulnerable to SARS-COV-2: A Multicenter Study during the COVID-19 Outbreak. Cancer Discov. 2020. [Google Scholar] [CrossRef]
- Deng, G.; Yin, M.; Chen, X.; Zeng, F. Clinical determinants for fatality of 44,672 patients with COVID-19. Crit. Care 2020, 24, 179. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Desai, A.; Sachdeva, S.; Parekh, T.; Desai, R. COVID-19 and Cancer: Lessons from a Pooled Meta-Analysis. JCO Glob. Oncol. 2020, 6, 557–559. [Google Scholar] [CrossRef]
- Emami, A.; Javanmardi, F.; Pirbonyeh, N.; Akbari, A. Prevalence of Underlying Diseases in Hospitalized Patients with COVID-19: A Systematic Review and Meta-Analysis. Arch. Acad. Emerg. Med. 2020, 8, e35. [Google Scholar]
- Wang, B.; Li, R.; Lu, Z.; Huang, Y. Does comorbidity increase the risk of patients with COVID-19: Evidence from meta-analysis. Aging 2020, 12, 6049–6057. [Google Scholar] [CrossRef]
- Ganatra, S.; Hammond, S.P.; Nohria, A. The Novel Coronavirus Disease (COVID-19) Threat for Patients with Cardiovascular Disease and Cancer. JACC CardioOncol. 2020. [Google Scholar] [CrossRef]
- Zordoky, B. Cardiovascular Vulnerability to COVID-19 in Cancer Survivors. Preprints 2020. [Google Scholar] [CrossRef] [Green Version]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Muñoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; ESC Scientific Document Group; et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef] [PubMed]
- Felker, G.M.; Thompson, R.E.; Hare, J.M.; Hruban, R.H.; Clemetson, D.E.; Howard, D.L.; Baughman, K.L.; Kasper, E.K. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N. Engl. J. Med. 2000, 342, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Suter, T.M.; Procter, M.; van Veldhuisen, D.J.; Muscholl, M.; Bergh, J.; Carlomagno, C.; Perren, T.; Passalacqua, R.; Bighin, C.; Klijn, J.G.; et al. Trastuzumab-associated cardiac adverse effects in the herceptin adjuvant trial. J. Clin. Oncol. 2007, 25, 3859–3865. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.X.; Shen, Z.; Tang, L.N.; Yao, Y. Congestive heart failure risk in cancer patients treated with vascular endothelial growth factor tyrosine kinase inhibitors: A systematic review and meta-analysis of 36 clinical trials. Br. J. Clin. Pharmacol. 2014, 78, 748–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maurea, N.; Coppola, C.; Piscopo, G.; Galletta, F.; Riccio, G.; Esposito, E.; De Lorenzo, C.; De Laurentiis, M.; Spallarossa, P.; Mercuro, G. Pathophysiology of cardiotoxicity from target therapy and angiogenesis inhibitors. J. Cardiovasc. Med. 2016, 17 (Suppl. 1), S19–S26. [Google Scholar] [CrossRef]
- Lendvai, N.; Devlin, S.; Patel, M.; Knapp, K.M.; Ekman, D.; Grundberg, I.; Chung, D.J.; Hassoun, H.; Koehne, G.; Lesokhin, A.M.; et al. Biomarkers of cardiotoxicity among multiple myeloma patients subsequently treated with proteasome inhibitor therapy. Blood 2015, 126, 4257. [Google Scholar] [CrossRef]
- Hu, J.R.; Florido, R.; Lipson, E.J.; Naidoo, J.; Ardehali, R.; Tocchetti, C.G.; Lyon, A.R.; Padera, R.F.; Johnson, D.B.; Moslehi, J. Cardiovascular toxicities associated with immune checkpoint inhibitors. Cardiovasc. Res. 2019, 115, 854–868. [Google Scholar] [CrossRef] [Green Version]
- Chen, J. Incidence of heart failure or cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J. Am. Coll. Cardiol. 2012, 60, 2504–2512. [Google Scholar] [CrossRef] [Green Version]
- Vivarelli, S.; Falzone, L.; Grillo, C.M.; Scandurra, G.; Torino, F.; Libra, M. Cancer Management during COVID-19 Pandemic: Is Immune Checkpoint Inhibitors-Based Immunotherapy Harmful or Beneficial? Cancers 2020, 12, 2237. [Google Scholar] [CrossRef]
- Vaddepally, R.K.; Kharel, P.; Pandey, R.; Garje, R.; Chandra, A.B. Review of Indications of FDA-Approved Immune Checkpoint Inhibitors per NCCN Guidelines with the Level of Evidence. Cancers 2020, 12, 738. [Google Scholar] [CrossRef] [Green Version]
- Bersanelli, M. Controversies about COVID-19 and anticancer treatment with immune checkpoint inhibitors. Immunotherapy 2020, 12, 269–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quagliariello, V.; Passariello, M.; Rea, D.; Barbieri, A.; Iovine, M.; Bonelli, A.; Caronna, A.; Botti, G.; De Lorenzo, C.; Maurea, N. Evidences of CTLA-4 and PD-1 Blocking Agents-Induced Cardiotoxicity in Cellular and Preclinical Models. J. Pers. Med. 2020, 10, 179. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-W.; Zhu, Y.-J.; Wang, M.-N.; Xie, Y.; Chen, C.-Y.; Zhang, T.; Xia, F.; Ding, Z.-Y.; Liu, J.-Y. Immune checkpoint inhibitor-associated cardiotoxicity: Current understanding on its mechanism, diagnosis and management. Front. Pharmacol. 2019, 10, 1350. [Google Scholar] [CrossRef] [PubMed]
- Palaskas, N.L.; Lopez-Mattei, J.; Durand, J.B.; Iliescu, C.; Deswal, A. Immune Checkpoint Inhibitor Myocarditis: Pathophysiological Characteristics, Diagnosis, and Treatment. J. Am. Heart Assoc. 2020, 9, e013757. [Google Scholar] [CrossRef]
- Sullivan, R.J.; Johnson, D.B.; Rini, B. COVID-19 and immune checkpoint inhibitors: Initial considerations. J. Immunother. Cancer 2020, 8, e000933. [Google Scholar] [CrossRef]
- Gambichler, T.; Reuther, J.; Scheel, C.H.; Becker, J.C. On the use of immune checkpoint inhibitors in patients with viral infections including COVID-19. J. Immunother. Cancer 2020, 8, e001145. [Google Scholar] [CrossRef]
- Olejniczak, M.; Schwartz, M.; Webber, E.; Shaffer, A.; Perry, T.E. Viral Myocarditis-Incidence, Diagnosis and Management. J. Cardiothorac. Vasc. Anesth. 2020. [Google Scholar] [CrossRef]
- Guillin, O.M.; Vindry, C.; Ohlmann, T.; Chavatte, L. Selenium, Selenoproteins and Viral Infection. Nutrients 2019, 11, 2101. [Google Scholar] [CrossRef] [Green Version]
- Blauwet, L.A.; Cooper, L.T. Myocarditis. Prog. Cardiovasc. Dis. 2010, 52, 274–288. [Google Scholar] [CrossRef] [Green Version]
- Tsatsakis, A.; Calina, D.; Falzone, L.; Petrakis, D.; Mitrut, R.; Siokas, V.; Pennisi, M.; Lanza, G.; Libra, M.; Doukas, S.G.; et al. SARS-CoV-2 pathophysiology and its clinical implications: An integrative overview of the pharmacotherapeutic management of COVID-19. Food Chem. Toxicol. 2020, 146, 111769. [Google Scholar] [CrossRef]
- Driggin, E.; Madhavan, M.V.; Bikdeli, B.; Chuich, T.; Laracy, J.; Biondi-Zoccai, G.; Brown, T.S.; Der Nigoghossian, C.; Zidar, D.A.; Haythe, J. Cardiovascular Considerations for Patients, Health Care Workers, and Health Systems During the COVID-19 Pandemic. J. Am. Coll. Cardiol. 2020, 75, 2352–2371. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Nolan, S.M.; Singh, A.R.; Lovig, L.; Biller, R.; Kamat, A.; Brennan, M.H.; Erb, M.; Rescoe, E.; Tatz, G.; et al. Myocarditis in Multisystem Inflammatory Syndrome in Children Associated with Coronavirus Disease 2019. Cardiol. Rev. 2020, 28, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Ganatra, S.; Neilan, T.G. Immune Checkpoint Inhibitor-Associated Myocarditis. Oncologist 2018, 23, 879–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varricchi, G.; Marone, G.; Mercurio, V.; Galdiero, M.R.; Bonaduce, D.; Tocchetti, C.G. Immune checkpoint inhibitors and cardiac toxicity: An emerging issue. Curr. Med. Chem. 2018, 25, 1327–1339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, S.; Vincelette, N.D.; Mansour, I.; Hariri, D.; Motamed, S. Late onset ipilimumab-induced pericarditis and pericardial effusion: A rare but life threatening complication. Case Rep. Oncol. Med. 2015, 2015, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Salem, J.-E.; Manouchehri, A.; Moey, M.; Lebrun-Vignes, B.; Bastarache, L.; Pariente, A.; Gobert, A.; Spano, J.-P.; Balko, J.M.; Bonaca, M.P.; et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: An observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018, 19, 1579–1589. [Google Scholar] [CrossRef]
- Johnson, D.B.; Balko, J.M.; Compton, M.L.; Chalkias, S.; Gorham, J.; Xu, Y.; Hicks, M.; Puzanov, I.; Alexander, M.R.; Bloomer, T.L.; et al. Fulminant myocarditis with combination immune checkpoint blockade. N. Engl. J. Med. 2016, 375, 1749–1755. [Google Scholar] [CrossRef]
- Gürdoğan, M.; Yalta, K. Myocarditis associated with immune checkpoint inhibitors: Practical considerations in diagnosis and management. Anatol. J. Cardiol. 2020, 24, 68–75. [Google Scholar] [CrossRef]
- Sakai, T.; Yahagi, K.; Hoshino, T.; Yokota, T.; Tanabe, K.; Mori, M.; Ikeda, S. Nivolumab-induced myocardial necrosis in a patient with lung cancer: A case report. Respir. Med. Case Rep. 2019, 27, 100839. [Google Scholar] [CrossRef]
- Tang, N.; Li, D.; Wang, X.; Sun, Z. Abnormal Coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 844–847. [Google Scholar] [CrossRef] [Green Version]
- Klok, F.A.; Kruip, M.J.; van der Meer, N.J.; Arbous, M.S.; Gommers, D.A.; Kant, K.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020, 191, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Farge, D.; Frere, C.; Connors, J.M.; Ay, C.; Khorana, A.A.; Munoz, A.; Brenner, B.; Kakkar, A.; Rafii, H.; Solymoss, S.; et al. International Initiative on Thrombosis and Cancer (ITAC) advisory panel. 2019 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 2019, 20, e566–e581. [Google Scholar] [CrossRef] [Green Version]
- Maurea, N.; Riva, L. Venous thromboembolism and atrial fibrillation in patients with cancer. G. Ital. Cardiol. 2018, 9 (Suppl. 1), 3S–6S. [Google Scholar] [CrossRef]
- Thompson, A.; Morgan, C.; Smith, P.; Jones, C.; Ball, H.; Coulthard, E.J.; Moran, E.; Szewczyk-Krolikowski, K.; Rice, C.M. Cerebral venous sinus thrombosis associated with COVID-19. Pract. Neurol. 2020. [Google Scholar] [CrossRef]
- Rali, P.; O’Corragain, O.; Oresanya, L.; Yu, D.; Sheriff, O.; Weiss, R.; Myers, C.; Desai, P.; Ali, N.; Stack, A.; et al. Temple University COVID-19 Research Group. Incidence of venous thromboembolism in coronavirus disease 2019: An experience from a single large academic center. J. Vasc. Surg. Venous Lymphat. Disord. 2020. [Google Scholar] [CrossRef] [PubMed]
- Mosarla, R.C.; Vaduganathan, M.; Qamar, A.; Moslehi, J.; Piazza, G.; Giugliano, R.P. Anticoagulation Strategies in Patients with Cancer: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 73, 1336–1349. [Google Scholar] [CrossRef]
- Khorana, A.A.; Kuderer, N.M.; Culakova, E.; Lyman, G.H.; Francis, C.W. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008, 111, 4902–4907. [Google Scholar] [CrossRef] [Green Version]
- Aryal, M.R.; Gosain, R.; Donato, A.; Pathak, R.; Bhatt, V.R.; Katel, A.; Kouides, P. Venous Thromboembolism in COVID-19: Towards an Ideal Approach to Thromboprophylaxis, Screening, and Treatment. Curr. Cardiol. Rep. 2020, 22, 52. [Google Scholar] [CrossRef]
- Iba, T.; Levy, J.H.; Levi, M.; Thachil, J. Coagulopathy in COVID-19. J. Thromb. Haemost. 2020. [Google Scholar] [CrossRef]
- Connors, J.M.; Levy, J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020, 135, 2033–2040. [Google Scholar] [CrossRef]
- Al-Samkari, H.; Karp Leaf, R.S.; Dzik, W.H.; Carlson, J.C.T.; Fogerty, A.E.; Waheed, A.; Goodarzi, K.; Bendapudi, P.K.; Bornikova, L.; Leaf, D.; et al. COVID-19 and coagulation: Bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood 2020, 136, 489–500. [Google Scholar] [CrossRef]
- Strongman, H.; Gadd, S.; Matthews, A.; Mansfield, K.E.; Stanway, S.; Lyon, A.R.; Dos-Santos-Silva, I.; Smeeth, L.; Bhaskaran, K. Medium and long-term risks of specific cardiovascular diseases in survivors of 20 adult cancers: A population-based cohort study using multiple linked UK electronic health records databases. Lancet 2019, 394, 1041–1054. [Google Scholar] [CrossRef] [Green Version]
- Kvolik, S.; Jukic, M.; Matijevic, M.; Marjanovic, K.; Glavas-Obrovac, L. An overview of coagulation disorders in cancer patients. Surg. Oncol. 2010, 19, e33–46. [Google Scholar] [CrossRef] [PubMed]
- Rugbjerg, K.; Mellemkjaer, L.; Boice, J.D.; Køber, L.; Ewertz, M.; Olsen, J.H. Cardiovascular disease in survivors of adolescent and young adult cancer: A Danish cohort study, 1943–2009. J. Natl. Cancer Inst. 2014, 106, dju110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnstone, C.; Rich, S.E. Bleeding in cancer patients and its treatment: A review. Ann. Palliat. Med. 2018, 7, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Crist, M.; Hansen, E.; Chablani, L.; Guancial, E. Examining the bleeding incidences associated with targeted therapies used in metastatic renal cell carcinoma. Crit. Rev. Oncol. Hematol. 2017, 120, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Falanga, A.; Marchetti, M.; Vignoli, A. Coagulation and cancer: Biological and clinical aspects. J. Thromb. Haemost. 2013, 11, 223–233. [Google Scholar] [CrossRef]
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural basis for the recognition of the SARS-CoV-2 by full-length human ACE2. Science 2020. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Penninger, J.M.; Li, Y.; Zhong, N.; Slutsky, A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: Molecular mechanisms and potential therapeutic target. Intensiv. Care Med. 2020. [Google Scholar] [CrossRef] [Green Version]
- Zou, X.; Chen, K.; Zou, J.; Han, P.; Hao, J.; Han, Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020. [Google Scholar] [CrossRef] [Green Version]
- Diaz, J.H. Hypothesis: Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers may increase the risk of severe COVID-19. J. Travel Med. 2020, 27, taaa041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurwitz, D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev. Res. 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, G. Bulk and single-cell transcriptomics identify tobacco-use disparity in lung gene expression of ACE2, the receptor of 2019-nCov. medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Shoemaker, R.; Tannock, L.R.; Su, W.; Gong, M.; Gurley, S.B.; Thatcher, S.E.; Yiannikouris, F.; Ensor, C.M.; Cassis, L.A. Adipocyte deficiency of ACE2 increases systolic blood pressures of obese female C57BL/6 mice. Biol. Sex Differ. 2019, 10, 45. [Google Scholar] [CrossRef] [Green Version]
- Simonnet, A.; Chetboun, M.; Poissy, J.; Raverdy, V.; Noulette, J.; Duhamel, A.; Labreuche, J.; Mathieu, D.; Pattou, F.; Jourdain, M.; et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity 2020. [Google Scholar] [CrossRef]
- Fang, L.; Karakiulakis, G.; Roth, M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 2020. [Google Scholar] [CrossRef]
- Yang, M.; Meng, F.; Wang, K.; Gao, M.; Lu, R.; Li, M.; Zhao, F.; Huang, L.; Zhang, Y.; Cheng, G.; et al. Interleukin 17A as a good predictor of the severity of Mycoplasma pneumoniae pneumonia in children. Sci Rep. 2017, 7, 12934. [Google Scholar] [CrossRef] [Green Version]
- Quagliariello, V.; Passariello, M.; Coppola, C.; Rea, D.; Barbieri, A.; Scherillo, M.; Monti, M.G.; Iaffaioli, R.V.; De Laurentiis, M.; Ascierto, P.A.; et al. Cardiotoxicity and pro-inflammatory effects of the immune checkpoint inhibitor Pembrolizumab associated to Trastuzumab. Int. J. Cardiol. 2019, 292, 171–179. [Google Scholar] [CrossRef]
- De Luca, G.; Cavalli, G.; Campochiaro, C.; Tresoldi, M.; Dagna, L. Myocarditis: An Interleukin-1-Mediated Disease? Front. Immunol. 2018, 9, 1335. [Google Scholar] [CrossRef] [Green Version]
- Glowacka, I.; Bertram, S.; Müller, M.A. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J. Virol. 2011, 85, 4122–4134. [Google Scholar] [CrossRef] [Green Version]
- Bertram, S.; Heurich, A.; Lavender, H.; Gierer, S.; Danisch, S.; Perin, P.; Lucas, J.M.; Nelson, P.S.; Pöhlmann, S.; Soilleux, E.J. Influenza and SARS-coronavirus activating proteases TMPRSS2 and HAT are expressed at multiple sites in human respiratory and gastrointestinal tracts. PLoS ONE 2012, 7, e35876. [Google Scholar] [CrossRef] [PubMed]
- Knuuttila, M.; Mehmood, A.; Mäki-Jouppila, J. Intratumoral androgen levels are linked to TMPRSS2-ERG fusion in prostate cancer. Endocr. Relat. Cancer 2018, 25, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Quagliariello, V.; Bonelli, A.; Caronna, A.; Lombari, M.C.; Conforti, G.; Libutti, M.; Iaffaioli, R.V.; Berretta, M.; Botti, G.; Maurea, N. SARS-CoV-2 infection: NLRP3 inflammasome as plausible target to prevent cardiopulmonary complications? Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 9169–9171. [Google Scholar] [CrossRef] [PubMed]
- Quagliariello, V.; Vecchione, R.; Coppola, C.; Di Cicco, C.; De Capua, A.; Piscopo, G.; Paciello, R.; Narciso, V.; Formisano, C.; Taglialatela-Scafati, O.; et al. Cardioprotective Effects of Nanoemulsions Loaded with Anti-Inflammatory Nutraceuticals against Doxorubicin-Induced Cardiotoxicity. Nutrients 2018, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mele, D.; Tocchetti, C.G.; Pagliaro, P.; Madonna, R.; Novo, G.; Pepe, A.; Zito, C.; Maurea, N.; Spallarossa, P. Pathophysiology of anthracycline cardiotoxicity. J. Cardiovasc. Med. 2016, 17 (Suppl. 1), S3–S11. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Ridker, P.M. From C-Reactive Protein to Interleukin-6 to Interleukin-1: Moving Upstream to Identify Novel Targets for Atheroprotection. Circ. Res. 2016, 118, 145–156. [Google Scholar] [CrossRef] [Green Version]
- Pettersson, T.; Söderblom, T.; Nyberg, P.; Riska, H.; Linko, L.; Klockars, M. Pleural fluid soluble interleukin 2 receptor in rheumatoid arthritis and systemic lupus erythematosus. J. Rheumatol. 1994, 21, 1820–1824. [Google Scholar]
- Sakamoto, A.; Ishizaka, N.; Saito, K.; Imai, Y.; Morita, H.; Koike, K.; Kohro, T.; Nagai, R. Serum levels of IgG4 and soluble interleukin-2 receptor in patients with coronary artery disease. Clin. Chim. Acta 2012, 413, 577–581. [Google Scholar] [CrossRef]
- Eisner, R.M.; Husain, A.; Clark, J.I. Case report and brief review: IL-2-induced myocarditis. Cancer Investig. 2004, 22, 401–404. [Google Scholar] [CrossRef]
- Thavendiranathan, P.; Verhaert, D.; Kendra, K.L.; Raman, S.V. Fulminant myocarditis owing to high-dose interleukin-2 therapy for metastatic melanoma. Br. J. Radiol. 2011, 84, e99–e102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.; Paul, A.; Ko, K.W.; Sheldon, M.; Rich, B.E.; Terashima, T.; Dieker, C.; Cormier, S.; Li, L.; Nour, E.A.; et al. Interleukin-7 induces recruitment of monocytes/macrophages to endothelium. Eur. Heart J. 2012, 33, 3114–3123. [Google Scholar] [CrossRef] [PubMed]
- Damås, J.K.; Waehre, T.; Yndestad, A.; Otterdal, K.; Hognestad, A.; Solum, N.O.; Gullestad, L.; Frøland, S.S.; Aukrust, P. Interleukin-7-mediated inflammation in unstable angina: Possible role of chemokines and platelets. Circulation 2003, 107, 2670–2676. [Google Scholar] [CrossRef] [PubMed]
- Kumari, N.; Dwarakanath, B.S.; Das, A.; Bhatt, A.N. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol. 2016, 37, 11553–11572. [Google Scholar] [CrossRef] [PubMed]
- IL6R Genetics Consortium Emerging Risk Factors Collaboration. Interleukin-6 receptor pathways in coronary heart disease: A collaborative meta-analysis of 82 studies. Lancet 2012, 379, 1205–1213. [Google Scholar] [CrossRef] [Green Version]
- Markousis-Mavrogenis, G.; Tromp, J.; Ouwerkerk, W.; Devalaraja, M.; Anker, S.D.; Cleland, J.G.; Dickstein, K.; Filippatos, G.S.; van der Harst, P.; Lang, C.C.; et al. The clinical significance of interleukin-6 in heart failure: Results from the BIOSTAT-CHF study. Eur. J. Heart Fail. 2019, 21, 965–973. [Google Scholar] [CrossRef] [Green Version]
- Ikonomidis, I.; Papadavid, E.; Makavos, G.; Andreadou, I.; Varoudi, M.; Gravanis, K.; Theodoropoulos, K.; Pavlidis, G.; Triantafyllidi, H.; Moutsatsou, P.; et al. Lowering Interleukin-12 Activity Improves Myocardial and Vascular Function Compared with Tumor Necrosis Factor-a Antagonism or Cyclosporine in Psoriasis. Circ. Cardiovasc. Imaging 2017, 10, e006283. [Google Scholar] [CrossRef] [Green Version]
- Katsaros, K.M.; Speidl, W.S.; Demyanets, S.; Kastl, S.P.; Krychtiuk, K.A.; Wonnerth, A.; Zorn, G.; Tentzeris, I.; Farhan, S.; Maurer, G.; et al. G-CSF Predicts Cardiovascular Events in Patients with Stable Coronary Artery Disease. PLoS ONE 2015, 10, e0142532. [Google Scholar] [CrossRef]
- Pourtaji, A.; Jahani, V.; Moallem, S.M.H.; Karimani, A.; Mohammadpour, A.H. Application of G-CSF in Congestive Heart Failure Treatment. Curr. Cardiol. Rev. 2019, 15, 83–90. [Google Scholar] [CrossRef]
- Omura, S.; Kawai, E.; Sato, F.; Martinez, N.E.; Chaitanya, G.V.; Rollyson, P.A.; Cvek, U.; Trutschl, M.; Alexander, J.S.; Tsunoda, I. Bioinformatics multivariate analysis determined a set of phase-specific biomarker candidates in a novel mouse model for viral myocarditis. Circ. Cardiovasc. Genet. 2014, 7, 444–454. [Google Scholar] [CrossRef] [Green Version]
- Niki, T.; Soeki, T.; Yamaguchi, K.; Taketani, Y.; Yagi, S.; Iwase, T.; Yamada, H.; Wakatsuki, T.; Shimabukuro, M.; Sata, M. Elevated Concentration of Interferon-Inducible Protein of 10 kD (IP-10) Is Associated with Coronary Atherosclerosis. Int. Heart J. 2015, 56, 269–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonelli, A.; Fallahi, P.; Ferrari, S.M.; Ghiadoni, L.; Virdis, A.; Mancusi, C.; Centanni, M.; Taddei, S.; Ferrannini, E. High serum levels of CXC (CXCL10) and CC (CCL2) chemokines in untreated essential hypertension. Int. J. Immunopathol. Pharmacol. 2012, 25, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, M.A.; Zhang, Y.; Kelly, R.S.; See, H.; Johnstone, E.K.; McCall, E.A.; Williams, J.H.; Kelly, D.J.; Pfleger, K.D. Functional interaction between angiotensin II receptor type 1 and chemokine (C-C motif) receptor 2 with implications for chronic kidney disease. PLoS ONE 2015, 10, e0119803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Lemos, J.A.; Morrow, D.A.; Sabatine, M.S.; Murphy, S.A.; Gibson, C.M.; Antman, E.M.; McCabe, C.H.; Cannon, C.P.; Braunwald, E. Association between plasma levels of monocyte chemoattractant protein-1 and long-term clinical outcomes in patients with acute coronary syndromes. Circulation 2003, 107, 690–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, R.; Zhao, S.R.; Li, Y.; Liu, F.; Gong, Y.; Xing, J.; Xu, Z.S. Association of tumor necrosis factor-α gene polymorphisms and coronary artery disease susceptibility: A systematic review and meta-analysis. BMC Med. Genet. 2020, 21, 29. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020. [Google Scholar] [CrossRef]
- Alehagen, U.; Dahlström, U.; Lindahl, T.L. Elevated D-dimer level is an independent risk factor for cardiovascular death in out-patients with symptoms compatible with heart failure. Thromb. Haemost. 2004, 92, 1250–1258. [Google Scholar]
- Martínez-Godínez, M.A.; Cruz-Domínguez, M.P.; Jara, L.J. Expression of NLRP3 inflammasome, cytokines and vascular mediators in the skin of systemic sclerosis patients. Isr. Med. Assoc. J. 2015, 17, 5–10. [Google Scholar]
- Grebe, A.; Hoss, F.; Latz, E. NLRP3 Inflammasome and the IL-1 Pathway in Atherosclerosis. Circ. Res. 2018, 122, 1722–1740. [Google Scholar] [CrossRef]
- Ravi Kumar, S.; Paudel, S.; Ghimire, L.; Bergeron, S.; Cai, S.; Zemans, R.L.; Downey, G.P.; Jeyaseelan, S. Emerging Roles of Inflammasomes in Acute Pneumonia. Am. J. Respir. Crit. Care Med. 2018, 197, 160–171. [Google Scholar] [CrossRef]
- Rodriguez, A.E.; Bogart, C.; Gilbert, C.M.; McCullers, J.A.; Smith, A.M. Enhanced IL-1β production is mediated by a TLR2-MYD88-NLRP3 signaling axis during coinfection with influenza A virus and Streptococcus pneumoniae. PLoS ONE 2019, 14, e0212236. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Fernández, A.; Skouras, D.B.; Dinarello, C.A.; López-Vales, R. OLT1177 (Dapansutrile), a Selective NLRP3 Inflammasome Inhibitor, Ameliorates Experimental Autoimmune Encephalomyelitis Pathogenesis. Front. Immunol. 2019, 10, 2578. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, C.; Swartzwelter, B.; Koenders, M.I. NLRP3 inflammasome inhibitor OLT1177 suppresses joint inflammation in murine models of acute arthritis. Arthritis Res. Ther. 2018, 20, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toldo, S.; Abbate, A. The NLRP3 inflammasome in acute myocardial infarction. Nat. Rev. Cardiol. 2018, 15, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Liu, Y.; Qiu, L.; Liu, X.; Liu, D.; Li, J. Tocilizumab treatment in COVID-19: A single center experience. J. Med. Virol. 2020. [Google Scholar] [CrossRef]
- Xu, X.; Han, M.; Li, T. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. USA 2020, 117, 10970–10975. [Google Scholar] [CrossRef]
- Jia, H.; Liu, J.; Han, B. Reviews of Interleukin-37: Functions, Receptors, and Roles in Diseases. Biomed. Res. Int. 2018, 2018, 3058640. [Google Scholar] [CrossRef] [Green Version]
- Zhan, Q.; Zeng, Q.; Song, R.; Zhai, Y.; Xu, D.; Fullerton, D.A.; Dinarello, C.A.; Meng, X. IL-37 suppresses MyD88-mediated inflammatory responses in human aortic valve interstitial cells. Mol. Med. 2017, 23, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Allam, G.; Gaber, A.M.; Othman, S.I.; Abdel-Moneim, A. The potential role of interleukin-37 in infectious diseases. Int. Rev. Immunol. 2020, 39, 3–10. [Google Scholar] [CrossRef]
- An, B.; Liu, X.; Li, G.; Yuan, H. Interleukin-37 Ameliorates Coxsackievirus B3-induced Viral Myocarditis by Modulating the Th17/Regulatory T cell Immune Response. J. Cardiovasc. Pharmacol. 2017, 69, 305–313. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. A Trial of Remdesivir in Adults With Severe COVID-19. Available online: https://clinicaltrials.gov/ct2/show/NCT04257656 (accessed on 17 March 2020).
- Sakabe, M.; Yoshioka, R.; Fujiki, A. Sick sinus syndrome induced by interferon and ribavirin therapy in a patient with chronic hepatitis C. J. Cardiol. Cases 2013, 8, 173–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yarza, R.; Bover, M.; Paredes, D.; López-López, F.; Jara-Casas, D.; Castelo-Loureiro, A.; Baena, J.; Mazarico, J.M.; Folgueira, M.D.; Meléndez-Carmona, M.Á.; et al. SARS-CoV-2 infection in cancer patients undergoing active treatment: Analysis of clinical features and predictive factors for severe respiratory failure and death. Eur. J. Cancer 2020, 135, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; Swaminathan, S.; Angarone, M.; Blouin, G.; Camins, B.C.; Casper, C.; Cooper, B.; Dubberke, E.R.; Engemann, A.M.; Freifeld, A.G.; et al. Prevention and Treatment of Cancer-Related Infections, Version 2.2016, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2016, 14, 882–913. [Google Scholar] [CrossRef] [PubMed]
- López-Sendón, J.; Álvarez-Ortega, C.; Zamora Auñon, P.; Buño Soto, A.; Lyon, A.R.; Farmakis, D.; Cardinale, D.; Canales Albendea, M.; Feliu Batlle, J.; Rodríguez Rodríguez, I.; et al. Classification, prevalence, and outcomes of anticancer therapy-induced cardiotoxicity: The CARDIOTOX registry. Eur Heart J. 2020. [Google Scholar] [CrossRef]
- Li, T.; Wei, S.; Shi, Y.; Pang, S.; Qin, Q.; Yin, J.; Deng, Y.; Chen, Q.; Wei, S.; Nie, S.; et al. The dose-response effect of physical activity on cancer mortality: Findings from 71 prospective cohort studies. Br. J. Sports Med. 2016, 50, 339–345. [Google Scholar] [CrossRef]
- Scott, J.M.; Nilsen, T.S.; Gupta, D.; Jones, L.W. Exercise Therapy and Cardiovascular Toxicity in Cancer. Circulation 2018, 137, 1176–1191. [Google Scholar] [CrossRef]
- World Cancer Research Fund/American Institute for Cancer. Research Second Expert Report—Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective WCRF/AICR; World Cancer Research Fund/American Institute for Cancer: Washington, DC, USA, 2007. [Google Scholar]
- Solans, M.; Chan, D.S.M.; Mitrou, P.; Norat, T.; Romaguera, D. A systematic review and meta-analysis of the 2007 WCRF/AICR score in relation to cancer-related health outcomes. Ann. Oncol. 2020, 31, 352–368. [Google Scholar] [CrossRef] [Green Version]
- Lohse, T.; Faeh, D.; Bopp, M.; Rohrmann, S. Swiss National Cohort Study Group.Adherence to the cancer prevention recommendations of the World Cancer Research Fund/American Institute for Cancer Research and mortality: A census-linked cohort. Am. J. Clin. Nutr. 2016, 104, 678–685. [Google Scholar] [CrossRef] [Green Version]
- Inoue-Choi, M.; Robien, K.; Lazovich, D. Adherence to the WCRF/AICR guidelines for cancer prevention is associated with lower mortality among older female cancer survivors. Cancer Epidemiol. Biomark. Prev. 2013, 22, 792–802. [Google Scholar] [CrossRef] [Green Version]
- Grafetstätter, M.; Pletsch-Borba, L.; Sookthai, D.; Karavasiloglou, N.; Johnson, T.; Katzke, V.A.; Hoffmeister, M.; Bugert, P.; Kaaks, R.; Kühn, T. Thrombomodulin and Thrombopoietin, Two Biomarkers of Hemostasis, Are Positively Associated with Adherence to the World Cancer Research Fund/American Institute for Cancer Research Recommendations for Cancer Prevention in a Population-Based Cross-Sectional Study. Nutrients 2019, 11, 2067. [Google Scholar] [CrossRef] [Green Version]
- Park, Y.; Subar, A.F.; Hollenbeck, A.; Schatzkin, A. Dietary fiber intake and mortality in the NIH-AARP diet and health study. Arch. Intern. Med. 2011, 171, 1061–1068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolk, A.; Manson, J.E.; Stampfer, M.J.; Colditz, G.A.; Hu, F.B.; Speizer, F.E.; Hennekens, C.H.; Willett, W.C. Long-term intake of dietary fiber and decreased risk of coronary heart disease among women. JAMA 1999, 281, 1998–2004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilchrist, S.C.; Barac, A.; Ades, P.A.; Alfano, C.M.; Franklin, B.A.; Jones, L.W.; La Gerche, A.; Ligibel, J.A.; Lopez, G.; Madan, K.; et al. American Heart Association Exercise, Cardiac Rehabilitation, and Secondary Prevention Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; and Council on Peripheral Vascular Disease. Cardio-Oncology Rehabilitation to Manage Cardiovascular Outcomes in Cancer Patients and Survivors: A scientific statement from the American Heart Association. Circulation 2019, 139, e997–e1012. [Google Scholar] [PubMed]
- Ceriello, A. Hyperglycemia and the worse prognosis of COVID-19. Why a fast blood glucose control should be mandatory. Diabetes Res. Clin. Pract. 2020, 163, 108186. [Google Scholar] [CrossRef] [PubMed]




| Study | Date of Publication | Total Patients (n) | Type of Population | Outcomes in Cancer Patients | Reference |
|---|---|---|---|---|---|
| Liang et al. | 14 February 2020 | 1580 | Chinese | 1% (95% CI 0.61–1.65) of COVID-19 cases had a history of cancer. Cancer patients have a high probability of severe events than non-cancer patients (hazard ratio 3.56, 95% CI 1.65–7.69). Risk of clinically severe events in cancer patients who underwent chemotherapy or surgery in the past month is higher than other cancer patients (odds ratio 5.34, 95% CI 1.80–16.18; p = 0.0026). | [21] |
| Jing Yu et al. | 25 March 2020 | 1524 | Chinese | Patients with cancer had a higher risk of SARS-CoV-2 infection (OR, 2.31; 95% CI, 1.89–3.02) compared with other people. | [22] |
| Guan et al. | 26 March 2020 | 1590 | Chinese | Cancer patients had a hazard ratio of 3.50 (95% CI, 1.60–7.64) for admission to intensive care unit, or invasive ventilation, or death, compared to non-cancer patients. | [23] |
| Istituto Superiore di Sanità | 2 April 2020 | 909 | Italian | Among 909 patients who died, more than 50% had three or more comorbidities such as ischemic heart disease (27.4%), atrial fibrillation (23%), heart failure (16.4%), stroke (12%), hypertension (73.5%), diabetes mellitus (31.5%), and active cancer in the past 5 years (16.5%) | [24] |
| Trapani et al. | 28 April 2020 | 9 | Italian | All cancer patients with COVID-19 had a smoking history, being either former smokers (n = 4) or current smokers (n = 5). Comorbidities were arterial hypertension (n = 4), diabetes mellitus type II (n = 2), and chronic kidney diseases. | [25] |
| Montopoli et al. | 29 April 2020 | 9280 | Italian | Cancer patients have an increased risk of SARS-CoV-2 infections than non-cancer patients. 8.5% had a diagnosis of cancer and 1.3% had prostate cancer. Comparing the total number of SARS-CoV-2 positive cases, patients with prostate cancer receiving androgen-deprivation therapy had a significantly lower risk of SARS-CoV-2 infections compared to patients who did not receive androgen-deprivation therapy (OR 4.05; 95% CI 1.55–10.59). | [26] |
| de Rojas et al. | 8 May 2020 | 15 | Spanish | The prevalence of COVID-19 infection among children with cancer in Madrid is 1.3% vs. 0.8% of the general pediatric population. | [28] |
| Wu et al. | 24 February 2020 | 72314 | Chinese | Case fatality report was higher among patients with pre-existing comorbidities: 10.5% for cardiovascular disease, 7.3% for diabetes, 6.3% for chronic respiratory disease, 6.0% for hypertension, and 5.6% for cancer. | [29] |
| Onder et al. | 23 March 2020 | 1625 | Italian | In a subsample of 355 COVID-19 patients who died in Italy, 30% had ischemic heart disease, 35.5% had diabetes, 20.3% had active cancer, 24.5% had atrial fibrillation, 6.8% had dementia, and 9.6% had a history of stroke. | [30] |
| Zhang et al. | 26 March 2020 | 28 | Chinese | The mortality rate of cancer patients was 28.6% (more than ten times higher than that reported in all COVID-19 patients in China). The recent use of anticancer therapies within 14 days of infection (including chemotherapy, immunotherapy, and radiation) was an independent predictor of death or other severe events with a hazard ratio > 4. | [31] |
| Bonomi et al. | 31 March 2020 | Single report | Italian | A 65-year-old patient with metastatic non-small-cell lung cancer in treatment from 6 years with anti PD-1 antibody (nivolumab). After COVID-19 diagnosis and hospitalization, the patient presented a rapid evolution of respiratory failure and was not treated with more invasive procedures, probably due to his cancer and emphysema history. The authors discuss the need for a multidisciplinary approach for the management of COVID-19-infected cancer patients. | [32] |
| He et al. | 1 April 2020 | 128 | Chinese | There was no significant difference in the proportion of patients with hematological cancers vs. healthy subjects (10% vs. 7%, p = 0.322), but a significant difference was seen in fatality rate (62% vs. 0%, p = 0.002). Among cancer patients, 39% had acute myeloid leukemia, 20% had acute lymphoblastic leukemia, 59% had received chemotherapy in the last 7–19 days. | [33] |
| Miyashita et al. | 21 April 2020 | 334 | American | Patients with cancer were significantly more likely to require intubation (RR 1.89, 95% CI 1.37–2.61) than non-cancer patients. Cancer patients younger than 50 years of age had a significantly higher rate of mortality than the other (RR 5.01, 95% CI 1.55–16.2). | [34] |
| Dai et al. | 28 April 2020 | 641 | Chinese | Patients with cancer had a higher mortality rate than those without (odds ratio 2.34, 95% CI 1.15–4.77, p = 0.03. The death rate was highest in those with hematological cancer (33.33%) and lung cancer (18.18%). Patients with metastatic cancer had a higher risk of death (OR 5.58, 95% CI 1.71–18.23, p = 0.01). Patients with cancer were significantly more likely to require ICU admission (OR 2.84, 95% CI 1.59–5.08, p < 0.01) and have higher rates of severe/critical symptoms (OR 2.79, 95% CI 1.74–4.41, p < 0.01). | [35] |
| Deng et al. | 28 April 2020 | 44672 | Chinese | Patients with cancer had a significantly higher risk of death than those without (RR 2.93, 95% CI 1.34–6.41, p = 0.006) | [36] |
| Mehta et al. | 1 May 2020 | 218 | American | The mortality rate in cancer patients with COVID-19 was 25% for solid tumors and 37% for hematological malignancies. | [37] |
| Cytokine | Involvement in Pathogenesis of COVID-19 and Cardiovascular Diseases | References |
|---|---|---|
| Interleukin-1β (IL-1β) | Interleukin-1β is significantly increased in COVID-19 patients vs. healthy subjects, and it is one of the most important and studied cardiovascular risk factor involved in the NLRP3 inflammasome activation; it has been studied for many years in cardio-oncology; in fact, its expression is enhanced during doxorubicin-induced cardiotoxicity. Some IL-1-blocking antibodies are currently under study and used in clinical trials with great improvements of cardiovascular outcomes. Moreover, IL-1 promotes the expression of other pro-inflammatory cytokines, including interleukin-6 and cardiovascular risk factors such as hs-CRP. | [105,106,107,108,109] |
| Interleukin-2 (IL-2) | Interleukin-2, overexpressed in both ICU-COVID-19 and non-ICU COVID-19 patients, indicating a Th1 immune reaction to SARS-CoV-2, is a T-cell growth factor of key importance in immune-reactive processes. High levels of IL-2 receptor are associated to rheumatoid arthritis multiple sclerosis and coronary artery diseases; two case reports described myocarditis induced by an overexpression of myocardial IL-2. | [110,111,112,113] |
| Interleukin-7 (IL-7) | Interleukin-7 (IL-7) is a key regulator of T-cell growth; IL-7 recruits monocytes and macrophages to the endothelium and plays a crucial role in the pathogenesis of atherosclerosis through PI3K-AKT and NF-kB pathways; in another study, IL-7 promoted clinical instability in patients with coronary artery disease. | [114,115] |
| Interleukin 6 (IL-6) | Interleukin 6, which is significantly increased in COVID-19 patients compared to healthy subjects (but without differences compared to ICU-patients), is another key regulator of immune-related reaction. IL-6 is studied in oncology and cardio-oncology because it is a key promoter of cancer survival, chemoresistance, anticancer-induced cardiotoxicity, and cancer progression. Meta-analysis confirmed the association between IL-6 receptor and coronary heart diseases, and in a recent study in 2329 patients with heart failure, high plasma levels of IL-6 (seen in 50% of patients) were associated to atrial fibrillation, a reduction of Left Ventricular Ejection Fraction (LVEF), and a worse prognosis. | [116,117,118] |
| Interleukin-12 (IL-12) | Interleukin-12 acts as a protective cytokine in anticancer-induced myocardial injuries, although some authors discussed on its possible association to heart failure or atherosclerosis; however, the role of IL-12 remains controversial and needs further observational and interventional studies. | [119] |
| Granulocyte-colony-stimulating-factor (G-CSF) | Granulocyte colony-stimulating-factor (G-CSF) is produced by leukocytes and fibroblasts; it is well associated to a higher risk of MACE (death, myocardial infarction, re-hospitalization) in patients with stable coronary artery disease, although other authors discussed its cardioprotective role through the induction of tissue repair after myocardial infarction. | [120,121] |
| C-X-C motif chemokine 10 (CXCL10 or Induced protein 10) | C-X-C motif chemokine 10 (CXCL10 or induced protein 10) is a chemoattractant chemokine of Th1 and cytotoxic T cells. It is overexpressed in viral myocarditis, coronary atherosclerosis, hypertension, and left ventricular dysfunction. | [122,123,124,125] |
| CCL2 (Monocyte Chemoattractant Protein (MCP-1) | CCL2 (Monocyte Chemoattractant Protein, called also MCP-1) in another well-known cytokine in cardiology, strictly related to several cardiovascular events such as atherosclerosis, myocardial injury, hypertension, angiotensin-2 homeostasis (through functional interaction with angiotensin2 type 1 receptor), and other diseases. | [126] |
| Tumour Necrosis Factor-α (TNF-α) | TNF-α, significantly associated to severe cases of COVID-19, has been studied as a driver of vascular dysfunction, atherosclerosis, and heart failure. | [127] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quagliariello, V.; Bonelli, A.; Caronna, A.; Conforti, G.; Iovine, M.; Carbone, A.; Berretta, M.; Botti, G.; Maurea, N. SARS-CoV-2 Infection and Cardioncology: From Cardiometabolic Risk Factors to Outcomes in Cancer Patients. Cancers 2020, 12, 3316. https://doi.org/10.3390/cancers12113316
Quagliariello V, Bonelli A, Caronna A, Conforti G, Iovine M, Carbone A, Berretta M, Botti G, Maurea N. SARS-CoV-2 Infection and Cardioncology: From Cardiometabolic Risk Factors to Outcomes in Cancer Patients. Cancers. 2020; 12(11):3316. https://doi.org/10.3390/cancers12113316
Chicago/Turabian StyleQuagliariello, Vincenzo, Annamaria Bonelli, Antonietta Caronna, Gabriele Conforti, Martina Iovine, Andreina Carbone, Massimiliano Berretta, Gerardo Botti, and Nicola Maurea. 2020. "SARS-CoV-2 Infection and Cardioncology: From Cardiometabolic Risk Factors to Outcomes in Cancer Patients" Cancers 12, no. 11: 3316. https://doi.org/10.3390/cancers12113316