Effect of COVID-19 on Lungs: Focusing on Prospective Malignant Phenotypes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Cancer-Related Viruses
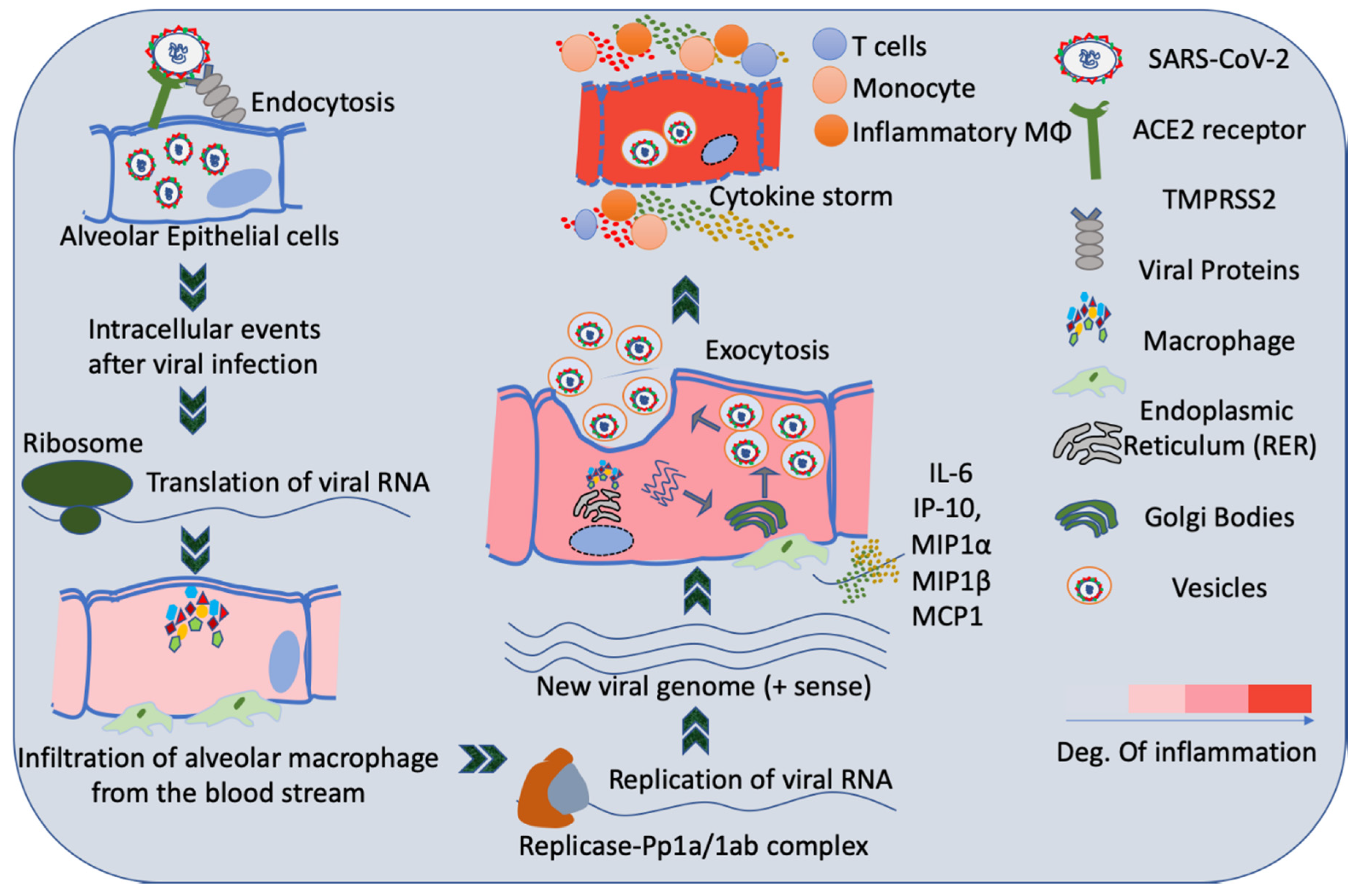
3. Infection of Lungs by SARS-CoV2
4. Host Immune System and SARS-CoV2 Infection
5. COVID-19 and Cancer
6. Ground Glass Opacity (GGO) of Lung Determined by CT in COVID-19 Patients
7. Involvement of Inflammatory Cascades in Carcinogenesis and COVID-19 Disease
8. Summary and Future Perspectives
Funding
Conflicts of Interest
References
- Ahn, D.-G.; Shin, H.-J.; Kim, M.-H.; Lee, S.; Kim, H.-S.; Myoung, J.; Kim, B.-T.; Kim, S.-J. Current Status of Epidemiology, Diagnosis, Therapeutics, and Vaccines for Novel Coronavirus Disease 2019 (COVID-19). J. Microbiol. Biotechnol. 2020, 30, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Chen, X.; Kost, J.; Sulovari, A.; Wong, N.; Liang, W.S.; Cao, J.; Li, D. A virome-wide clonal integration analysis platform for discovering cancer viral etiology. Genome Res. 2019, 29, 819–830. [Google Scholar] [CrossRef] [Green Version]
- Cerwenka, A.; Lanier, L.L. Natural killer cell memory in infection, inflammation and cancer. Nat. Rev. Immunol. 2016, 16, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Siddique, R.; Shereen, M.A.; Ali, A.; Liu, J.; Bai, Q.; Bashir, N.; Xue, M. Emergence of a Novel Coronavirus, Severe Acute Respiratory Syndrome Coronavirus 2: Biology and Therapeutic Options. J. Clin. Microbiol. 2020, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Y.; Yang, H.; Ji, W.; Wu, W.; Chen, S.; Zhang, W.; Duan, G. Virology, Epidemiology, Pathogenesis, and Control of COVID-19. Viruses 2020, 12, 372. [Google Scholar] [CrossRef] [Green Version]
- Mousavizadeh, L.; Ghasemi, S. Genotype and phenotype of COVID-19: Their roles in pathogenesis. J. Microbiol. Immunol. Infect. 2020. [Google Scholar] [CrossRef]
- Satarker, S.; Nampoothiri, M. Structural Proteins in Severe Acute Respiratory Syndrome Coronavirus-2. Arch. Med. Res. 2020, 51, 482–491. [Google Scholar] [CrossRef]
- Shereen, M.A.; Khan, S.; Kazmi, A.; Bashir, N.; Siddique, R. COVID-19 infection: Origin, transmission, and characteristics of human coronaviruses. J. Adv. Res. 2020, 24, 91–98. [Google Scholar] [CrossRef]
- Luo, L.; Luo, Z.; Jia, Y.; Zhou, C.; He, J.; Lyu, J.; Shen, X. CT differential diagnosis of COVID-19 and non-COVID-19 in symptomatic suspects: A practical scoring method. BMC Pulm. Med. 2020, 20, 1–9. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, Y.; Zhu, T.; Xia, L. CT Features of Coronavirus Disease 2019 (COVID-19) Pneumonia in 62 Patients in Wuhan, China. Am. J. Roentgenol. 2020, 214, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.S.Y.; Chan, J.W.Y.; Lau, R.W.H.; Ng, C.S.H. Screening-detected pure ground-glass opacities: Malignant potential beyond conventional belief? Transl. Lung Cancer Res. 2020, 9, 816–818. [Google Scholar] [CrossRef] [PubMed]
- Farrell, P.J. Epstein–Barr virus and cancer. Annu. Rev. Pathol. Mech. Dis. 2019, 14, 29–53. [Google Scholar] [CrossRef]
- Plummer, M.; De Martel, C.; Vignat, J.; Ferlay, J.; Bray, F.; Franceschi, S. Global burden of cancers attributable to infections in 2012: A synthetic analysis. Lancet Glob. Health 2016, 4, e609–e616. [Google Scholar] [CrossRef] [Green Version]
- De Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef] [Green Version]
- Bodily, J.; Laimins, L. Persistence of human papillomavirus infection: Keys to malignant progression. Trends Microbiol. 2011, 19, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Ouyang, W.; Huang, C. Inflammation, a Key Event in Cancer Development. Mol. Cancer Res. 2006, 4, 221–233. [Google Scholar] [CrossRef] [Green Version]
- Di Bisceglie, A.M. Hepatitis B and hepatocellular carcinoma. Hepatology 2009, 49, S56–S60. [Google Scholar] [CrossRef] [Green Version]
- O’Rourke, J.M.; Sagar, V.M.; Shah, T.; Shetty, S. Carcinogenesis on the background of liver fibrosis: Implications for the management of hepatocellular cancer. World J. Gastroenterol. 2018, 24, 4436–4447. [Google Scholar] [CrossRef]
- Kutok, J.; Wang, F. SPECTRUM OF EPSTEIN-BARR VIRUS–ASSOCIATED DISEASES. Annu. Rev. Pathol. Mech. Dis. 2006, 1, 375–404. [Google Scholar] [CrossRef]
- Akram, N.; Imran, M.; Noreen, M.; Ahmed, F.; Atif, M.; Fatima, Z.; Waqar, A.B. Oncogenic Role of Tumor Viruses in Humans. Viral Immunol. 2017, 30, 20–27. [Google Scholar] [CrossRef]
- Shuda, M.; Kwun, H.J.; Feng, H.; Chang, Y.; Moore, P.S. Human Merkel cell polyomavirus small T antigen is an oncoprotein targeting the 4E-BP1 translation regulator. J. Clin. Investig. 2011, 121, 3623–3634. [Google Scholar] [CrossRef]
- Nicot, C. Tumor Suppressor Inactivation in the Pathogenesis of Adult T-Cell Leukemia. J. Oncol. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bourgonje, A.R.; Abdulle, A.E.; Timens, W.; Hillebrands, J.L.; Navis, G.J.; Gordijn, S.J.; van der Voort, P.H. Angiotensin-converting enzyme-2 (ACE2), SARS-CoV-2 and pathophysiology of coronavirus disease 2019 (COVID-19). J. Pathol. 2020, 251, 228–248. [Google Scholar] [CrossRef] [PubMed]
- South, A.M.; Diz, D.I.; Chappell, M.C. COVID-19, ACE2, and the cardiovascular consequences. Am. J. Physiol. Circ. Physiol. 2020, 318, H1084–H1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciaglia, E.; Vecchione, C.; Puca, A.A. COVID-19 Infection and Circulating ACE2 Levels: Protective Role in Women and Children. Front. Pediatr. 2020, 8, 206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.; Yang, X.O. TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor Fedratinib. J. Microbiol. Immunol. Infect. 2020, 53, 368–370. [Google Scholar] [CrossRef] [PubMed]
- Tufan, A.; Güler, A.A.; Matucci-Cerinic, M. COVID-19, immune system response, hyperinflammation and repurposing antirheumatic drugs. Turk. J. Med. Sci. 2020, 50, 620–632. [Google Scholar] [CrossRef]
- Conti, P.; Ronconi, G.; Caraffa, A.; Gallenga, C.E.; Ross, R.; Frydas, I. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): Anti-inflammatory strategies. J. Biol. Regul. Homeost Agents 2020, 34, 1. [Google Scholar]
- Vardhana, S.; Wolchok, J.D. The many faces of the anti-COVID immune response. J. Exp. Med. 2020, 217. [Google Scholar] [CrossRef]
- Ooki, A.; Pena, M.D.C.R.; Marchionni, L.; Dinalankara, W.; Begum, A.; Hahn, N.M.; Vandenbussche, C.J.; Rasheed, Z.A.; Mao, S.; Netto, G.J.; et al. YAP1 and COX2 Coordinately Regulate Urothelial Cancer Stem-like Cells. Cancer Res. 2018, 78, 168–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadhukhan, P.; Saha, S.; Dutta, S.; Mahalanobish, S.; Sil, P.C. Nutraceuticals: An emerging therapeutic approach against the pathogenesis of Alzheimer’s disease. Pharmacol. Res. 2018, 129, 100–114. [Google Scholar] [CrossRef] [PubMed]
- Sadhukhan, P.; Saha, S.; Dutta, S.; Sil, P.C. Mangiferin Ameliorates Cisplatin Induced Acute Kidney Injury by Upregulating Nrf-2 via the Activation of PI3K and Exhibits Synergistic Anticancer Activity with Cisplatin. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef]
- Shibata, M.; Ooki, A.; Inokawa, Y.; Sadhukhan, P.; Ugurlu, M.T.; Izumchenko, E.; Munari, E.; Bogina, G.; Rudin, C.M.; Gabrielson, E.; et al. Concurrent Targeting of Potential Cancer Stem Cells Regulating Pathways Sensitizes Lung Adenocarcinoma to Standard Chemotherapy. Mol. Cancer Ther. 2020. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; MacFadyen, J.G.; Thuren, T.; Everett, B.M.; Libby, P.; Glynn, R.J.; Lorenzatti, A.; Krum, H.; Varigos, J.; Siostrzonek, P.; et al. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: Exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet 2017, 390, 1833–1842. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, Z.; Li, J.-W.; Zhao, H.; Gui-Qiang, W. Cytokine release syndrome in severe COVID-19: Interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int. J. Antimicrob. Agents 2020, 55, 105954. [Google Scholar] [CrossRef]
- Gosain, R.; Abdou, Y.; Singh, A.; Rana, N.; Puzanov, I.; Ernstoff, M.S. COVID-19 and Cancer: A Comprehensive Review. Curr. Oncol. Rep. 2020, 22, 1–15. [Google Scholar] [CrossRef]
- Osman, M.S.; Van Eeden, C.; Tervaert, J.W.C. Fatal COVID-19 infections: Is NK cell dysfunction a link with autoimmune HLH? Autoimmun. Rev. 2020, 19, 102561. [Google Scholar] [CrossRef]
- Liang, W.; Guan, W.; Chen, R.; Wang, W.; Li, J.; Xu, K. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol. 2020, 21, 335–337. [Google Scholar] [CrossRef]
- Luo, J.; Rizvi, H.; Preeshagul, I.R.; Egger, J.V.; Hoyos, D.; Bandlamudi, C.; McCarthy, C.G.; Falcon, C.J.; Schoenfeld, A.J.; Arbour, K.C.; et al. COVID-19 in patients with lung cancer. Ann. Oncol. 2020, 31, 1386–1396. [Google Scholar] [CrossRef]
- Dai, M.-Y.; Liu, D.-B.; Liu, M.; Zhou, F.-X.; Li, G.-L.; Chen, Z.; Zhang, Z.-A.; Md, H.-Y.; Wu, M.; Zheng, Q.-C.; et al. Abstract CT406: Patients with Cancer Appear More Vulnerable to SARS-COV-2: A Multi-Center Study during the COVID-19 Outbreak; AACR: 2020. Available online: https://cancerres.aacrjournals.org/content/80/16_Supplement/CT406 (accessed on 18 December 2020).
- Lambertini, M.; Toss, A.; Passaro, A.; Criscitiello, C.; Cremolini, C.; Cardone, C.; Loupakis, F.; Viscardi, G.; Meattini, I.; Dieci, M.V.; et al. Cancer care during the spread of coronavirus disease 2019 (COVID-19) in Italy: Young oncologists’ perspective. ESMO Open 2020, 5, e000759. [Google Scholar] [CrossRef] [Green Version]
- Calabrò, L.; Peters, S.; Soria, J.-C.; Di Giacomo, A.M.; Barlesi, F.; Covre, A.; Altomonte, M.; Vegni, V.; Gridelli, C.; Reck, M.; et al. Challenges in lung cancer therapy during the COVID-19 pandemic. Lancet Respir. Med. 2020, 8, 542–544. [Google Scholar] [CrossRef]
- Hirsch, F.R.; Scagliotti, G.V.; Mulshine, J.L.; Kwon, R.; Curran, W.J.; Wu, Y.-L.; Paz-Ares, L. Lung cancer: Current therapies and new targeted treatments. Lancet 2017, 389, 299–311. [Google Scholar] [CrossRef]
- Fung, T.S.; Liu, D.X. Human Coronavirus: Host-Pathogen Interaction. Annu. Rev. Microbiol. 2019, 73, 529–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, Q.; Song, D.; Li, H.; He, M.-L. Stress proteins: The biological functions in virus infection, present and challenges for target-based antiviral drug development. Signal Transduct. Target. Ther. 2020, 5, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Qin, L.; Xu, Z.; Yin, Y.; Wang, X.; Kong, B.; Bai, J.; Lu, Y.; Fang, Z.; Song, Q.; et al. Using Artificial Intelligence to Detect COVID-19 and Community-acquired Pneumonia Based on Pulmonary CT: Evaluation of the Diagnostic Accuracy. Radiology 2020, 296, E65–E71. [Google Scholar] [CrossRef]
- Xu, X.; Yu, C.; Qu, J.; Zhang, L.; Jiang, S.; Huang, D. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1275–1280. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Shen, J.; Li, L.; Yu, C. Radiographic and Clinical Features of Children with Coronavirus Disease (COVID-19) Pneumonia. Indian Pediatr. 2020, 57, 423–426. [Google Scholar] [CrossRef]
- Hirano, C.; Ohshimo, S.; Horimasu, Y.; Iwamoto, H.; Fujitaka, K.; Hamada, H.; Kohno, N.; Komoto, D.; Awai, K.; Shime, N.; et al. Baseline High-Resolution CT Findings Predict Acute Exacerbation of Idiopathic Pulmonary Fibrosis: German and Japanese Cohort Study. J. Clin. Med. 2019, 8, 2069. [Google Scholar] [CrossRef] [Green Version]
- Ostrow, K.L.; Hoque, M.O.; Loyo, M.; Brait, M.; Greenberg, A.; Siegfried, J.M.; Grandis, J.R.; Davis, A.G.; Bigbee, W.L.; Rom, W.; et al. Molecular Analysis of Plasma DNA for the Early Detection of Lung Cancer by Quantitative Methylation-Specific PCR. Clin. Cancer Res. 2010, 16, 3463–3472. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Han, X.; Zheng, C. Evolution of CT Manifestations in a Patient Recovered from 2019 Novel Coronavirus (2019-nCoV) Pneumonia in Wuhan, China. Radiology 2020, 295, 20. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhang, N.; Li, Y.; Xu, X. A systematic review of chest imaging findings in COVID-19. Quant. Imaging Med. Surg. 2020, 10, 1058–1079. [Google Scholar] [CrossRef] [PubMed]
- Migliore, M.; Fornito, M.; Palazzolo, M.; Criscione, A.; Gangemi, M.; Borrata, F.; Vigneri, P.; Nardini, M.; Dunning, J. Ground glass opacities management in the lung cancer screening era. Ann. Transl. Med. 2018, 6, 90. [Google Scholar] [CrossRef] [Green Version]
- Lee, L.; Iyer, S.; Jose, R.J.; Manuel, A. COVID-19 follow-up planning: What will we be missing? ERJ Open Res. 2020, 6, 00198–02020. [Google Scholar] [CrossRef]
- Lee, H.Y.; Choi, Y.-L.; Lee, K.S.; Han, J.; Zo, J.I.; Shim, Y.M.; Moon, J.W. Pure Ground-Glass Opacity Neoplastic Lung Nodules: Histopathology, Imaging, and Management. Am. J. Roentgenol. 2014, 202, W224–W233. [Google Scholar] [CrossRef]
- Asamura, H.; Hishida, T.; Suzuki, K.; Koike, T.; Nakamura, K.; Kusumoto, M.; Nagai, K.; Tada, H.; Mitsudomi, T.; Tsuboi, M.; et al. Radiographically determined noninvasive adenocarcinoma of the lung: Survival outcomes of Japan Clinical Oncology Group 0201. J. Thorac. Cardiovasc. Surg. 2013, 146, 24–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzo, S.; Petrella, F.; Buscarino, V.; De Maria, F.; Raimondi, S.; Barberis, M.; Fumagalli, C.; Spitaleri, G.; Rampinelli, C.; De Marinis, F.; et al. CT Radiogenomic Characterization of EGFR, K-RAS, and ALK Mutations in Non-Small Cell Lung Cancer. Eur. Radiol. 2016, 26, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.-W.; Rizzo, S.; Ma, L.-H.; Qiu, X.-Y.; Warth, A.; Seki, N.; Hasegawa, M.; Zou, J.-W.; Li, Q.; Femia, M.; et al. Pulmonary ground-glass opacity: Computed tomography features, histopathology and molecular pathology. Transl. Lung Cancer Res. 2017, 6, 68–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izumchenko, E.; Chang, X.; Brait, M.; Fertig, E.; Kagohara, L.T.; Bedi, A.; Marchionni, L.; Agrawal, N.; Ravi, R.; Jones, S.; et al. Targeted sequencing reveals clonal genetic changes in the progression of early lung neoplasms and paired circulating DNA. Nat. Commun. 2015, 6, 8258. [Google Scholar] [CrossRef]
- Park, C.M.; Goo, J.M.; Lee, H.J.; Lee, C.H.; Chun, E.J.; Im, J.-G. Nodular Ground-Glass Opacity at Thin-Section CT: Histologic Correlation and Evaluation of Change at Follow-up. Radiogram 2007, 27, 391–408. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Callejo, D.; Romero, A.; Provencio, M.; Torrente, M. Liquid biopsy based biomarkers in non-small cell lung cancer for diagnosis and treatment monitoring. Transl. Lung Cancer Res. 2016, 5, 455–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu-Welliver, M.; Carbone, D.P. Blood-based biomarkers in lung cancer: Prognosis and treatment decisions. Transl. Lung Cancer Res. 2017, 6, 708–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, M.; Teixeira, A.L.; Coelho, A.; Araujo, A.; Medeiros, R. The role of inflammation in lung cancer. Adv. Exp. Med. Biol. 2014, 816, 1–23. [Google Scholar] [PubMed]
- Crusz, S.M.; Balkwill, F.R. Inflammation and cancer: Advances and new agents. Nat. Rev. Clin. Oncol. 2015, 12, 584–596. [Google Scholar] [CrossRef]
- Schottenfeld, D.; Beebe-Dimmer, J. Chronic Inflammation: A Common and Important Factor in the Pathogenesis of Neoplasia. CA Cancer J. Clin. 2006, 56, 69–83. [Google Scholar] [CrossRef]
- Kundu, J.K.; Surh, Y.-J. Inflammation: Gearing the journey to cancer. Mutat. Res. Mutat. Res. 2008, 659, 15–30. [Google Scholar] [CrossRef]
- Costela-Ruiz, V.J.; Illescas-Montes, R.; Puerta-Puerta, J.M.; Ruiz, C.; Melguizo-Rodríguez, L. SARS-CoV-2 infection: The role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020, 54, 62–75. [Google Scholar] [CrossRef]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; MacAry, P.A.; Ng, L.F. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 2020, 1–12. [Google Scholar]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Pinlaor, S.; Sripa, B.; Ma, N.; Hiraku, Y.; Yongvanit, P.; Wongkham, S.; Pairojkul, C.; Bhudhisawasdi, V.; Oikawa, S.; Murata, M.; et al. Nitrative and oxidative DNA damage in intrahepatic cholangiocarcinoma patients in relation to tumor invasion. World J. Gastroenterol. 2005, 11, 4644–4649. [Google Scholar] [CrossRef]
- Kawanishi, S.; Ohnishi, S.; Mariko, M.; Hiraku, Y.; Oikawa, S.; Murata, M. Nitrative and oxidative DNA damage in infection-related carcinogenesis in relation to cancer stem cells. Genes Environ. 2016, 38, 26. [Google Scholar] [CrossRef]
- Liu, B. Free DNA, a reason for severe COVID-19 infection? Med. Hypotheses 2020, 142, 109812. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, A. Signal transduction of inflammatory cytokines and tumor development. Cancer Sci. 2006, 97, 439–447. [Google Scholar] [CrossRef]
- Montfort, A.; Colacios, C.; Levade, T.; Andrieu-Abadie, N.; Meyer, N.; Ségui, B. The TNF Paradox in Cancer Progression and Immunotherapy. Front. Immunol. 2019, 10, 1818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheen-Chen, S.-M.; Chen, W.-J.; Eng, H.-L.; Chou, F.-F. Serum concentration of tumor necrosis factor in patients with breast cancer. Breast Cancer Res. Treat. 1997, 43, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Szlosarek, P.; Charles, K.A.; Balkwill, F.R. Tumour necrosis factor-α as a tumour promoter. Eur. J. Cancer 2006, 42, 745–750. [Google Scholar] [CrossRef]
- Yan, B.; Wang, H.; Rabbani, Z.N.; Dewhirst, M.W.; Li, C. Tumor necrosis factor-α is a potent endogenous mutagen that promotes cellular transformation. J. Clin. Oncol. 2007, 25, 21104. [Google Scholar] [CrossRef]
- Taniguchi, K.; Karin, M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin. Immunol. 2014, 26, 54–74. [Google Scholar] [CrossRef]
- Kobayashi, S.; Werneburg, N.W.; Bronk, S.F.; Kaufmann, S.H.; Gores, G.J. Interleukin-6 Contributes to Mcl-1 Up-regulation and TRAIL Resistance via an Akt-Signaling Pathway in Cholangiocarcinoma Cells. Gastroenterology 2005, 128, 2054–2065. [Google Scholar] [CrossRef]
- Kai, H.; Kitadai, Y.; Kodama, M.; Cho, S.; Kuroda, T.; Ito, M.; Tanaka, S.; Ohmoto, Y.; Chayama, K. Involvement of proinflammatory cytokines IL-1beta and IL-6 in progression of human gastric carcinoma. Anticancer. Res. 2005, 25, 709–713. [Google Scholar]
- Wang, L.; Cao, L.; Wang, H.; Liu, B.; Zhang, Q.; Meng, Z.; Wu, X.; Zhou, Q.; Xu, K. Cancer-associated fibroblasts enhance metastatic potential of lung cancer cells through IL-6/STAT3 signaling pathway. Oncotarget 2017, 8, 76116–76128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, H.; Sheng, J.; Gao, D.; Li, F.; Durrans, A.; Ryu, S.; Lee, S.B.; Narula, N.; Rafii, S.; Elemento, O.; et al. Transcriptome Analysis of Individual Stromal Cell Populations Identifies Stroma-Tumor Crosstalk in Mouse Lung Cancer Model. Cell Rep. 2015, 10, 1187–1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.-H.; Lee, H.-S.; Yoon, D.; Berry, G.; Wheeler, T.M.; Sugarbaker, D.J.; Kheradmand, F.; Engleman, E.; Burt, B.M. Progression of EGFR-Mutant Lung Adenocarcinoma is Driven By Alveolar Macrophages. Clin. Cancer Res. 2016, 23, 778–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolf, J.S.; Chen, Z.; Dong, G.; Sunwoo, J.B.; Bancroft, C.C.; Capo, D.E. IL (interleukin)-1alpha promotes nuclear factor-kappaB and AP-1-induced IL-8 expression, cell survival, and proliferation in head and neck squamous cell carcinomas. Clin. Cancer Res. 2001, 7, 1812–1820. [Google Scholar]
- McLoed, A.G.; Sherrill, T.P.; Cheng, D.-S.; Han, W.; Saxon, J.A.; Gleaves, L.A. Neutrophil-Derived IL-1β Impairs the Efficacy of NF-κB Inhibitors against Lung Cancer. Cell Rep. 2016, 16, 120–132. [Google Scholar] [CrossRef] [Green Version]
- Numasaki, M.; Watanabe, M.; Suzuki, T.; Takahashi, H.; Nakamura, A.; McAllister, F.; Hishinuma, T.; Goto, J.; Lotze, M.T.; Kolls, J.K.; et al. IL-17 Enhances the Net Angiogenic Activity and In Vivo Growth of Human Non-Small Cell Lung Cancer in SCID Mice through Promoting CXCR-2-Dependent Angiogenesis. J. Immunol. 2005, 175, 6177–6189. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Liu, S.; Liu, J.; Zhang, Z.; Wan, X.; Huang, B.; Chen, Y.; Zhang, Y. COVID-19: Immunopathogenesis and Immunotherapeutics. Signal Transduct. Target. Ther. 2020, 5, 1–8. [Google Scholar] [CrossRef]
- Sun, L.; Louie, M.C.; Vannella, K.M.; Wilke, C.A.; Levine, A.M.; Moore, B.B.; Shanley, T.P. New concepts of IL-10-induced lung fibrosis: Fibrocyte recruitment and M2 activation in a CCL2/CCR2 axis. Am. J. Physiol. Cell. Mol. Physiol. 2011, 300, L341–L353. [Google Scholar] [CrossRef] [Green Version]
- Liao, M.; Liu, Y.; Yuan, J.; Wen, Y.; Xu, G.; Zhao, J. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 2020, 26, 842–844. [Google Scholar] [CrossRef]
- Wu, J. Tackle the free radicals damage in COVID-19. Nitric Oxide 2020, 102, 39–41. [Google Scholar] [CrossRef]
- Szczerba, B.M.; Castro-Giner, F.; Vetter, M.; Krol, I.; Gkountela, S.; Landin, J. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature 2019, 566, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.-L.; Wang, X.-M.; Feng, Y.-G.; Kang, P.-M.; Li, Q.-Y.; Sun, T.-Y.; Tan, Q.-Y.; Deng, B. Is the presence of lung injury in COVID-19 an independent risk factor for secondary lung cancer? Med. Hypotheses 2020, 143, 110074. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, B.J.; Yang, J.C.; Wang, M.Y.; Chen, C.; Luo, G.X. Research advances in the mechanism of pulmonary fibrosis induced by coronavirus disease 2019 and the corresponding therapeutic measures. Zhonghua Shao Shang Za Zhi 2020, 36, 691–697. [Google Scholar]
- Ojo, A.S.; Balogun, S.A.; Williams, O.T.; Ojo, O.S. Pulmonary Fibrosis in COVID-19 Survivors: Predictive Factors and Risk Reduction Strategies. Pulm. Med. 2020, 2020, 1–10. [Google Scholar] [CrossRef]
- Spagnolo, P.; Balestro, E.; Aliberti, S.; Cocconcelli, E.; Biondini, D.; Della Casa, G. Pulmonary fibrosis secondary to COVID-19: A call to arms? Lancet Respir. Med. 2020, 8, 750–752. [Google Scholar] [CrossRef]
- Channappanavar, R.; Perlman, S. Pathogenic human coronavirus infections: Causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017, 39, 529–539. [Google Scholar] [CrossRef]
- Jose, R.J.; Manuel, A. COVID-19 cytokine storm: The Interplay between inflammation and coagulation. Lancet Respir. Med. 2020, 8, e46–e47. [Google Scholar] [CrossRef]
- Godlee, F. Living with Covid-19; British Medical Journal Publishing Group: 2020. Available online: https://www.bmj.com/content/370/bmj.m3392 (accessed on 18 December 2020).
- Tian, S.; Hu, W.; Niu, L.; Liu, H.; Xu, H.; Xiao, S. Pulmonary Pathology of Early-Phase 2019 Novel Coronavirus (COVID-19) Pneumonia in Two Patients With Lung Cancer. J. Thorac. Oncol. 2020, 15, 700–704. [Google Scholar] [CrossRef]
- Chang, H.L.; Wei, P.J.; Wu, K.L.; Huang, H.L.; Yang, C.J. Checkpoint inhibitor pneumonitis mimicking COVID-19 infection during the COVID-19 pandemic. Lung Cancer 2020, 146, 376–377. [Google Scholar] [CrossRef]
- Delaunay, M.; Prévot, G.; Collot, S.; Guilleminault, L.; Didier, A.; Mazieres, J. Management of pulmonary toxicity associated with immune checkpoint inhibitors. Eur. Respir. Rev. 2019, 28, 190012. [Google Scholar] [CrossRef]
- Remon, J.; Passiglia, F.; Ahn, M.-J.; Barlesi, F.; Forde, P.M.; Garon, E.B.; Gettinger, S.; Goldberg, S.B.; Herbst, R.S.; Horn, L.; et al. Immune Checkpoint Inhibitors in Thoracic Malignancies: Review of the Existing Evidence by an IASLC Expert Panel and Recommendations. J. Thorac. Oncol. 2020, 15, 914–947. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Kalantar-Zadeh, K.; Anker, S.D. COVID-19: A major cause of cachexia and sarcopenia? J. Cachex- Sarcopenia Muscle 2020, 11, 863–865. [Google Scholar] [CrossRef] [PubMed]
- Dunne, R.F.; Loh, K.P.; Williams, G.R.; Jatoi, A.; Mustian, K.M.; Mohile, S.G. Cachexia and Sarcopenia in Older Adults with Cancer: A Comprehensive Review. Cancers 2019, 11, 1861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, A.; Kumar, A. ER Stress and Unfolded Protein Response in Cancer Cachexia. Cancers 2019, 11, 1929. [Google Scholar] [CrossRef] [Green Version]
- Miller, A.; McLeod, L.; Alhayyani, S.; Szczepny, A.; Watkins, D.N.; Chen, W.; Enriori, P.; Ferlin, W.; Ruwanpura, S.M.; Jenkins, B.J. Blockade of the IL-6 trans-signalling/STAT3 axis suppresses cachexia in Kras-induced lung adenocarcinoma. Oncogene 2016, 36, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, R.; Okazaki, T.; Ebihara, S.; Kobayashi, M.; Tsukita, Y.; Nihei, M.; Sugiura, H.; Niu, K.; Ebihara, T.; Ichinose, M. Aspiration pneumonia induces muscle atrophy in the respiratory, skeletal, and swallowing systems. J. Cachex-Sarcopenia Muscle 2018, 9, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Bonomi, P.; Fidler, M.J.; Shah, P.; Borgia, J.A. Theoretical and Practical Implications of Treating Cachexia in Advanced Lung Cancer Patients. Cancers 2019, 11, 1619. [Google Scholar] [CrossRef] [Green Version]
- Seljelid, R.; Jozefowski, S.; Sveinbjörnsson, B. Tumor stroma. Anticancer Res. 1999, 19, 4809–4822. [Google Scholar]
- Soy, M.; Keser, G.; Atagündüz, P.; Tabak, F.; Atagündüz, I.; Kayhan, S. Cytokine storm in COVID-19: Pathogenesis and overview of anti-inflammatory agents used in treatment. Clin. Rheumatol. 2020, 39, 2085–2094. [Google Scholar] [CrossRef]
- Singh, A.; Bass, J.; Lindner, D.H. Rare Complication of Pneumomediastinum and Pneumopericardium in a Patient with COVID-19 Pneumonia. Case Rep. Pulmonol. 2020, 2020, 1–4. [Google Scholar] [CrossRef]
- Hu, Z.-J.; Xu, J.; Yin, J.-M.; Li, L.; Hou, W.; Zhang, L.-L.; Zhou, Z.; Yu, Y.-Z.; Li, H.-J.; Feng, Y.-M.; et al. Lower Circulating Interferon-Gamma Is a Risk Factor for Lung Fibrosis in COVID-19 Patients. Front. Immunol. 2020, 11, 585647. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Shi, N.; Shan, F.; Zhang, Z.; Shen, J.; Lu, H.; Ling, Y.; Jiang, Y.; Shi, Y. Emerging 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology 2020, 295, 210–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkataraman, T.; Frieman, M.B. The role of epidermal growth factor receptor (EGFR) signaling in SARS coronavirus-induced pulmonary fibrosis. Antivir. Res. 2017, 143, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, H.; Pillat, M.M. CD147 as a Target for COVID-19 Treatment: Suggested Effects of Azithromycin and Stem Cell Engagement. Stem. Cell Rev. Rep. 2020, 16, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Grass, G.D.; Dai, L.; Qin, Z.; Parsons, C.; Toole, B.P. CD147: Regulator of Hyaluronan Signaling in Invasiveness and Chemoresistance. Advances in Cancer Research; Elsevier: Amsterdam, The Netherlands, 2014; pp. 351–373. [Google Scholar]
- Park, W.B.; Kwon, N.J.; Choi, S.J.; Kang, C.K.; Choe, P.G.; Kim, J.Y. Virus Isolation from the First Patient with SARS-CoV-2 in Korea. J. Korean Med. Sci. 2020, 35, e84. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Guan, Z.; Li, H.; Ye, M.; Chen, X. SARS-CoV-2 triggers inflammatory responses and cell death through caspase-8 activation. Signal Transduct. Target. Ther. 2020, 5, 235. [Google Scholar] [CrossRef]
- Jamilloux, Y.; Henry, T.; Belot, A.; Viel, S.; Fauter, M.; El Jammal, T.; Walzer, T.; François, B.; Sève, P. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun. Rev. 2020, 19, 102567. [Google Scholar] [CrossRef]
- Heaton, N.S. Revisiting the concept of a cytopathic viral infection. PLoS Pathog. 2017, 13, e1006409. [Google Scholar] [CrossRef]
- Fonville, J.M.; Marshall, N.; Tao, H.; Steel, J.; Lowen, A.C. Influenza Virus Reassortment Is Enhanced by Semi-infectious Particles but Can Be Suppressed by Defective Interfering Particles. PLoS Pathog. 2015, 11, e1005204. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Zhong, L.; Zhang, S.; Wang, J.; Zhao, X.; Wang, K.; Ding, W.; Xing, Z.; Shen, J. Analysis of Chest CT Results of Coronavirus Disease 2019 (COVID-19) Patients at First Follow-Up. Can. Respir. J. 2020, 2020, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-M.; Shang, Y.-M.; Song, W.-B.; Li, Q.-Q.; Xie, H.; Xu, Q.-F.; Jia, J.-L.; Li, L.-M.; Mao, H.-L.; Zhou, X.-M.; et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine 2020, 25, 100463. [Google Scholar] [CrossRef] [PubMed]

| Virus | Nuclear Material | Route of Infection | Risk of Cancer |
|---|---|---|---|
| Epstein-Barr virus (EBV) | DNA Virus | Respiratory secretions, Saliva | Lymphoma, nasopharynx, gastric cancer |
| Hepatitis B virus (HBV) | Blood and sexual route, perinatal | Hepatocellular carcinoma | |
| Human Papilloma virus (HPV) | Sexual route | Anal, cervical, head and neck, oral, penile, vaginal, vulvar | |
| Human herpes virus 8 (HHV-8) | Saliva, sexual route, blood | Kaposi sarcoma | |
| Merkel cell polyomavirus (MCV) | Respiratory secretions, saliva, skin? | Merkel cell carcinoma (MCC) | |
| Hepatitis C virus (HCV) | RNA Virus | Blood and body fluids | Hepatocellular carcinoma |
| Human T-lymphotrophic virus-1 (HTLV-1) | semen, vaginal fluids, blood and breast milk | T-cell leukemia/lymphoma (ATL) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadhukhan, P.; Ugurlu, M.T.; Hoque, M.O. Effect of COVID-19 on Lungs: Focusing on Prospective Malignant Phenotypes. Cancers 2020, 12, 3822. https://doi.org/10.3390/cancers12123822
Sadhukhan P, Ugurlu MT, Hoque MO. Effect of COVID-19 on Lungs: Focusing on Prospective Malignant Phenotypes. Cancers. 2020; 12(12):3822. https://doi.org/10.3390/cancers12123822
Chicago/Turabian StyleSadhukhan, Pritam, M. Talha Ugurlu, and Mohammad O. Hoque. 2020. "Effect of COVID-19 on Lungs: Focusing on Prospective Malignant Phenotypes" Cancers 12, no. 12: 3822. https://doi.org/10.3390/cancers12123822






