Identification of Dietary Pattern Networks Associated with Gastric Cancer Using Gaussian Graphical Models: A Case-Control Study
Abstract
:1. Introduction
2. Results
2.1. General Characteristics
2.2. Comparison of the Intake of Food Groups
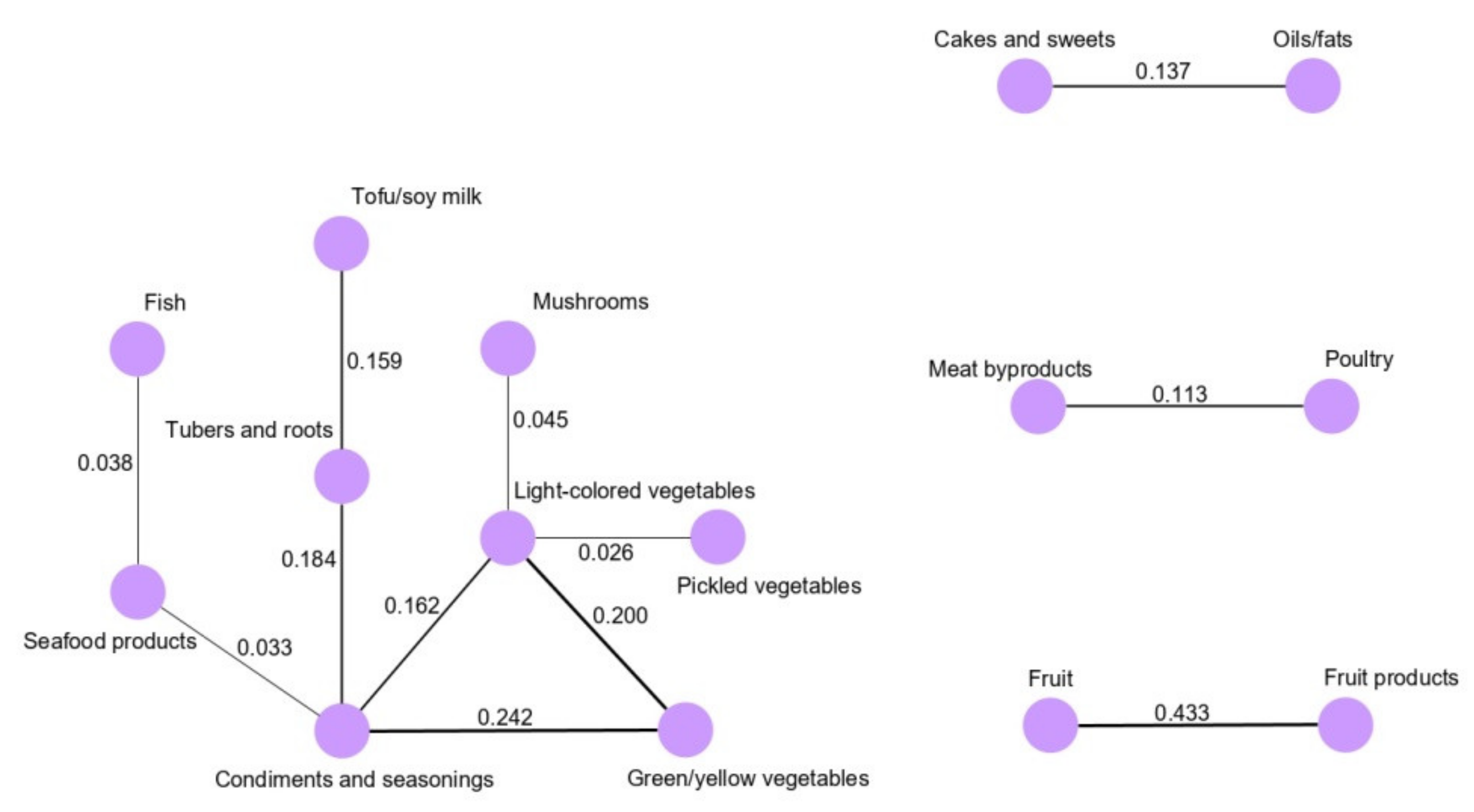
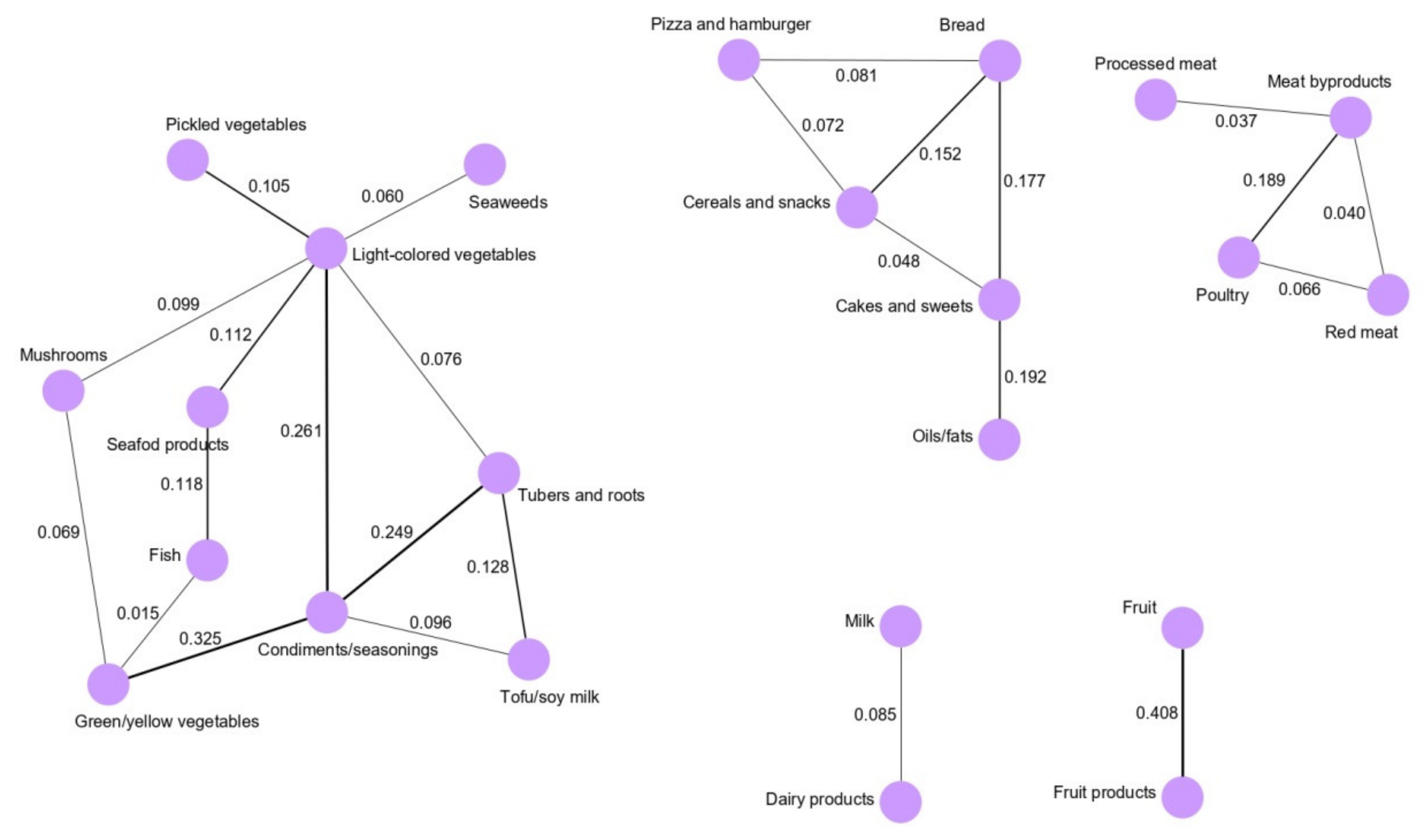
2.3. Dietary Pattern Networks Derived by GGMs
2.4. Association Between GGM-Derived Dietary Pattern Networks and GC Risk
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Data Collection
4.3. Statistical Analysis
4.3.1. Demographic and Dietary Intake Assessments
4.3.2. Assessment of Dietary Patterns by GGMs
4.3.3. Association between GGM-Identified Networks and GC Risk
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA-Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferlay, J.; Shin, H.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D. GLOBOCAN, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 [Internet]; International Agency for Research on Cancer: Lyon, France, 2013; Available online: http://globocan.iarc.fr (accessed on 13 March 2017).
- Jung, K.-W.; Won, Y.-J.; Kong, H.-J.; Lee, E.S. Cancer statistics in Korea: Incidence, mortality, survival, and prevalence in 2016. Cancer Res. Treat. 2019, 51, 417–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, K.-W.; Won, Y.-J.; Kong, H.-J.; Lee, E.S. Prediction of cancer incidence and mortality in Korea, 2019. Cancer Res. Treat. 2019, 51, 431. [Google Scholar] [CrossRef]
- World Cancer Research Fund International, Continuous Updated Project. Diet, Nutrition Physical Activity and Stomach Cancer. Revised 2018. Available online: http://www.Wcrf.Org/int/continuous-updateproject/cup-findings-reports/stomach-cancer (accessed on 21 September 2019).
- Naja, F.; Itani, L.; Hwalla, N.; Sibai, A.M.; Kharroubi, S.A. Identification of dietary patterns associated with elevated blood pressure among Lebanese men: A comparison of principal component analysis with reduced rank regression and partial least square methods. PLoS ONE 2019, 14, e0220942. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Garrett, W.S.; Chan, A.T. Nutrients, foods, and colorectal cancer prevention. Gastroenterology 2015, 148, 1244–1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGee, E.E.; Kiblawi, R.; Playdon, M.C.; Eliassen, A.H. Nutritional metabolomics in cancer epidemiology: Current trends, challenges, and future directions. Curr. Nutr. Rep. 2019, 8, 187–201. [Google Scholar] [CrossRef]
- Hoffmann, K.; Schulze, M.B.; Schienkiewitz, A.; Nöthlings, U.; Boeing, H. Application of a new statistical method to derive dietary patterns in nutritional epidemiology. Am. J. Epidemiol. 2004, 159, 935–944. [Google Scholar] [CrossRef]
- Nutrition Evidence Library, Technical Expert Collaborative on Study of Dietary Patterns. 2017. Available online: https://www.fns.usda.gov/nutrition-evidence-library-dietary-patterns-systematic-review-project (accessed on 1 September 2019).
- Iqbal, K.; Buijsse, B.; Wirth, J.; Schulze, M.B.; Floegel, A.; Boeing, H. Gaussian graphical models identify networks of dietary intake in a German adult population. J. Nutr. 2016, 146, 646–652. [Google Scholar] [CrossRef]
- Iqbal, K.; Schwingshackl, L.; Floegel, A.; Schwedhelm, C.; Stelmach-Mardas, M.; Wittenbecher, C. Gaussian graphical models identified food intake networks and risk of type 2 diabetes, CVD, and cancer in the EPIC-Potsdam study. Eur. J. Nutr. 2019, 58, 1673–1686. [Google Scholar] [CrossRef]
- Yusof, A.S.; Isa, Z.M.; Shah, S.A. Dietary patterns and risk of colorectal cancer: A systematic review of cohort studies (2000–2011). Asian Pac. J. Cancer Prev. 2012, 13, 4713–4717. [Google Scholar] [CrossRef] [Green Version]
- Bertuccio, P.; Rosato, V.; Andreano, A.; Ferraroni, M.; Decarli, A.; Edefonti, V. Dietary patterns and gastric cancer risk: A systematic review and meta-analysis. Ann. Oncol. 2013, 24, 1450–1458. [Google Scholar] [CrossRef]
- Bastos, J.; Lunet, N.; Peleteiro, B.; Lopes, C.; Barros, H. Dietary patterns and gastric cancer in a Portuguese urban population. Int. J. Cancer 2010, 127, 433–441. [Google Scholar] [CrossRef]
- Masaki, M.; Sugimori, H.; Nakamura, K.-I.; Tadera, M. Dietary patterns and stomach cancer among middle-aged male workers in Tokyo. Asian Pac. J. Cancer Prev. 2003, 4, 61–66. [Google Scholar]
- Hoang, B.V.; Lee, J.; Choi, I.J.; Kim, Y.-W.; Ryu, K.W.; Kim, J. Effect of dietary vitamin C on gastric cancer risk in the Korean population. World J. Gastroenterol. 2016, 22, 6257. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, J.; Choi, I.J.; Kim, Y.I.; Kwon, O.; Kim, H. Dietary carotenoids intake and the risk of gastric cancer: A case-control study in Korea. Nutrients 2018, 10, 1031. [Google Scholar] [CrossRef] [Green Version]
- Bahmanyar, S.; Ye, W. Dietary patterns and risk of squamous-cell carcinoma and adenocarcinoma of the esophagus and adenocarcinoma of the gastric cardia: A population-based case-control study in Sweden. Nutr. Cancer 2006, 54, 171–178. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.K.; Sasaki, S.; Sasazuki, S.; Tsugane, S. Prospective study of three major dietary patterns and risk of gastric cancer in Japan. Int. J. Cancer 2004, 110, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Wie, G.A.; Cho, Y.A.; Kang, H.H.; Ryu, K.A.; Yoo, M.K.; Kim, J. Identification of major dietary patterns in Korean adults and their association with cancer risk in the Cancer Screening Examination Cohort. Eur. J. Clin. Nutr. 2017, 71, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Lee, J.; Oh, J.H.; Shin, A.; Kim, J. Dietary patterns and colorectal cancer risk in a Korean population: A case-control study. Medicine 2016, 95, e3759. [Google Scholar] [CrossRef]
- Jang, B.Y.; Bu, S.Y. A vegetable and fish dietary pattern is positively associated with skeletal muscle mass in Korean men. Clin. Nutr. Res. 2019, 8, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Hoang, T.; Bu, S.Y.; Kim, J.-M.; Choi, J.-H.; Park, E. Associations of Dietary Intake with Cardiovascular Disease, Blood Pressure, and Lipid Profile in the Korean Population: A Systematic Review and Meta-Analysis. J. Lipid Atheroscler. 2020, 9, 205–229. [Google Scholar] [CrossRef]
- Shin, J.-Y.; Kim, J.-M.; Kim, Y. Associations between dietary patterns and hypertension among Korean adults: The Korean National Health and Nutrition Examination Survey (2008–2010). Nutr. Res. Pract. 2013, 7, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Palli, D.; Russo, A.; Decarli, A. Dietary patterns, nutrient intake and gastric cancer in a high-risk area of Italy. Cancer Causes Control 2001, 12, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Denova-Gutiérrez, E.; Hernández-Ramírez, R.U.; López-Carrillo, L. Dietary patterns and gastric cancer risk in Mexico. Nutr. Cancer 2014, 66, 369–376. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. Schistosomes LfaHpImoteocrth; IARC Press: Lyon, France, 1994; Volume 61. [Google Scholar]
- Shin, A.; Kim, J.; Park, S. Gastric cancer epidemiology in Korea. J. Gastric Cancer 2011, 11, 135–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Park, S.; Nam, B.-H. Gastric cancer and salt preference: A population-based cohort study in Korea. Am. J. Clin. Nutr. 2010, 91, 1289–1293. [Google Scholar] [CrossRef]
- Woo, H.; Lee, J.; Choi, I.; Kim, C.; Lee, J.; Kwon, O. Dietary flavonoids and gastric cancer risk in a Korean population. Nutrients 2014, 6, 4961–4973. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.; Woo, H.D.; Lee, J.; Choi, I.J.; Kim, Y.W.; Sung, J. Dietary folate, one-carbon metabolism-related genes, and gastric cancer risk in Korea. Mol. Nutr. Food Res. 2016, 60, 337–345. [Google Scholar] [CrossRef]
- Choi, J.-H.; Lee, J.; Choi, I.J.; Kim, Y.-W.; Ryu, K.W.; Kim, J. Genetic variation in the TAS2R38 bitter taste receptor and gastric cancer risk in Koreans. Sci. Rep. 2016, 6, 26904. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.H.; Lee, J.; Choi, I.J.; Kim, Y.W.; Ryu, K.W.; Kim, J. Variations in TAS1R taste receptor gene family modify food intake and gastric cancer risk in a Korean population. Mol. Nutr. Food Res. 2016, 60, 2433–2445. [Google Scholar] [CrossRef]
- Yang, S.; Lee, J.; Choi, I.J.; Kim, Y.W.; Ryu, K.W.; Sung, J. Effects of alcohol consumption, ALDH2 rs671 polymorphism, and Helicobacter pylori infection on the gastric cancer risk in a Korean population. Oncotarget 2017, 8, 6630. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, J.; Choi, I.J.; Kim, Y.-W.; Ryu, K.W.; Kim, Y.-I. Dietary inflammatory index and the risk of gastric cancer in a Korean population. Oncotarget 2017, 8, 85452. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Park, Y.; Lee, J.; Choi, I.J.; Kim, Y.W.; Ryu, K.W. Effects of soy product intake and interleukin genetic polymorphisms on early gastric cancer risk in Korea: A case-control study. Cancer Res. Treat. 2017, 49, 1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Lee, J.; Choi, I.J.; Kim, Y.-W.; Ryu, K.W.; Kim, Y.-I. Dietary n-3 and n-6 polyunsaturated fatty acids, the FADS gene, and the risk of gastric cancer in a Korean population. Sci. Rep. 2018, 8, 3823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunathilake, M.; Lee, J.; Jang, A.; Choi, I.; Kim, Y.-I.; Kim, J. Physical activity and gastric cancer risk in patients with and without Helicobacter pylori infection in a Korean population: A hospital-based case-control study. Cancers 2018, 10, 369. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Lee, J.; Woo, H.D.; Kim, D.W.; Choi, I.J.; Kim, Y.-I. Association between dietary cadmium intake and early gastric cancer risk in a Korean population: A case–control study. Eur. J. Nutr. 2019, 58, 3255–3266. [Google Scholar] [CrossRef]
- Dicken, B.J.; Saunders, L.D.; Jhangri, G.S.; De Gara, C.; Cass, C.; Andrews, S. Gastric cancer: Establishing predictors of biologic behavior with use of population-based data. Ann. Surg. Oncol. 2004, 11, 629–635. [Google Scholar] [CrossRef]
- Ahn, Y.; Kwon, E.; Shim, J.; Park, M.; Joo, Y.; Kimm, K. Validation and reproducibility of food frequency questionnaire for Korean genome epidemiologic study. Eur. J. Clin. Nutr. 2007, 61, 1435–1441. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Liu, H.; Roeder, K.; Lafferty, J.; Wasserman, L. The huge package for high-dimensional undirected graph estimation in R. J. Mach. Learn. Res. 2012, 13, 1059–1062. [Google Scholar] [PubMed]
- Friedman, J.; Hastie, T.; Tibshirani, R. Sparse inverse covariance estimation with the graphical lasso. Biostatistics 2008, 9, 432–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- yWorks. yEd Software Version 3.19 [Internet]. Available online: https://www.yworks.com/products/yed (accessed on 27 September 2019).

| All (n = 1245) | Male (n = 810) | Female (n = 435) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Controls (n = 830) | Cases (n = 415) | p-value b | Controls ((n = 540) | Cases (n = 270) | p-value b | Controls (n = 290) | Cases (n = 145) | p-value b |
| Age (y) | 53.7 ± 9.0 | 53.8 ± 9.3 | 0.89 | 54.8 ± 8.4 | 54.9 ± 8.7 | 0.91 | 51.6 ± 9.8 | 51.7 ± 9.9 | 0.94 |
| <50 | 285 (34.3) | 139 (33.5) | 0.78 | 153 (28.33) | 77 (28.5) | 0.96 | 132 (45.5) | 62 (42.8) | 0.59 |
| ≥50 | 545 (65.7) | 276 (66.5) | 387 (71.7) | 193 (71.5) | 158 (54.5) | 83 (57.2) | |||
| Sex [n (%)] | 0.99 | ||||||||
| Male | 540 (65.1) | 270 (65.1) | |||||||
| Female | 290 (34.9) | 145 (34.9) | |||||||
| Body mass index (kg/m2) [n (%)] | 23.9 ± 2.9 | 23.9 ± 3.0 | 0.63 | 24.4 ± 2.7 | 24.2 ± 3.0 | 0.39 | 23.1 ± 3.1 | 23.2 ± 3.0 | 0.79 |
| <23 | 314 (37.8) | 159 (38.3) | 0.98 | 161 (29.8) | 91 (33.7) | 0.51 | 153 (52.8) | 68 (46.9) | 0.53 |
| 23–25 | 249 (30.0) | 122 (29.4) | 170 (31.5) | 78 (28.9) | 79 (27.2) | 44 (30.3) | |||
| ≥25 | 266 (32.1) | 133 (32.1) | 209 (38.7) | 101 (37.4) | 57 (19.7) | 32 (22.1) | |||
| Missing | 1 (0.1) | 1 (0.2) | 0 (0.0) | 0 (0.0) | 1 (0.3) | 1 (0.7) | |||
| Smoking status [n (%)] | <0.001 | <0.001 | 0.021 | ||||||
| Current smoker | 162 (19.5) | 128 (30.8) | 157 (29.1) | 121 (44.8) | 5 (1.7) | 7 (4.8) | |||
| Ex-smoker | 284 (34.2) | 119 (28.7) | 277 (51.3) | 110 (40.7) | 7 (2.4) | 9 (6.2) | |||
| Non-smoker | 384 (46.3) | 167 (40.2) | 106 (19.6) | 39 (14.4) | 278 (95.9) | 128 (88.3) | |||
| Missing | 0 (0.0) | 1 (0.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.7) | |||
| Alcohol consumption [n (%)] | 0.24 | 0.28 | 0.82 | ||||||
| Current drinker | 534 (64.3) | 254 (61.2) | 404 (74.8) | 193 (71.5) | 130 (44.8) | 61 (42.1) | |||
| Ex-drinker | 60 (7.2) | 41 (9.9) | 47 (8.7) | 33 (12.2) | 13 (4.5) | 8 (5.5) | |||
| Non-drinker | 236 (28.4) | 119 (28.7) | 89 (16.5) | 44 (16.3) | 147 (50.7) | 75 (51.7) | |||
| Missing | 0 (0.0) | 1 (0.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.7) | |||
| First-degree family history of gastric cancer | <0.001 | 0.003 | 0.11 | ||||||
| Yes | 103 (12.4) | 82 (19.8) | 74 (13.7) | 60 (22.2) | 29 (10.0) | 22 (15.2) | |||
| No | 725 (87.4) | 332 (80.0) | 464 (85.9) | 209 (77.4) | 261 (90.0) | 123 (84.8) | |||
| Missing | 2 (0.2) | 1 (0.2) | 2 (0.4) | 1 (0.4) | 0 (0.0) | 0 (0.0) | |||
| Regular exercise [n (%)] | <0.001 | <0.001 | <0.001 | ||||||
| Yes | 466 (56.1) | 147 (35.4) | 303 (56.1) | 109 (40.4) | 163 (56.2) | 38 (26.2) | |||
| No | 361 (43.4) | 268 (64.6) | 234 (43.3) | 161 (59.6) | 127 (43.8) | 107 (73.8) | |||
| Missing | 3 (0.4) | 0 (0.0) | 3 (0.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| Educational level [n (%)] | <0.001 | <0.001 | <0.001 | ||||||
| Middle school | 119 (14.3) | 142 (34.2) | 71 (13.2) | 91 (33.7) | 48 (16.6) | 51 (35.2) | |||
| High school | 253 (30.5) | 174 (41.9) | 140 (25.9) | 112 (41.5) | 113 (38.9) | 62 (42.8) | |||
| College or more | 426 (51.3) | 97 (23.4) | 301 (55.7) | 66 (24.4) | 125 (43.1) | 31 (21.4) | |||
| Missing | 32 (3.9) | 2 (0.5) | 28 (5.2) | 1 (0.4) | 4 (1.4) | 1 (0.7) | |||
| Occupation [n (%)] | <0.001 | 0.009 | 0.002 | ||||||
| Group 1: Professional, administrative management | 156 (18.8) | 70 (16.9) | 117 (21.7) | 59 (21.9) | 39 (13.5) | 11 (7.6) | |||
| Group 2: Office, sales and service positions | 266 (32.1) | 122 (29.4) | 203 (37.6) | 81 (30.0) | 63 (21.7) | 41 (28.3) | |||
| Group 3: Agriculture, laborer | 128 (15.4) | 104 (25.1) | 111 (20.6) | 83 (30.7) | 17 (5.9) | 21 (14.5) | |||
| Group 4: Unemployed and other | 277 (33.4) | 117 (28.2) | 106 (19.6) | 46 (17.0) | 171 (58.9) | 71 (49.0) | |||
| Missing | 3 (0.4) | 2 (0.5) | 3 (0.6) | 1 (0.4) | 0 (0.0) | 1 (0.7) | |||
| Marital status [n (%)] | 0.61 | 0.48 | 0.98 | ||||||
| Married | 716 (86.3) | 361 (87.0) | 478 (88.5) | 243 (90.0) | 238 (82.1) | 118 (81.4) | |||
| Other (single, divorced, separated, widowed, cohabitating) | 113 (13.6) | 52 (12.5) | 61 (11.3) | 26 (9.6) | 52 (17.9) | 26 (17.9) | |||
| Missing | 1 (0.1) | 2 (0.5) | 1 (0.2) | 1 (0.4) | 0 (0.0) | 1 (0.7) | |||
| Monthly income [n (%)] a | <0.001 | <0.001 | 0.016 | ||||||
| <200 | 149 (18.0) | 133 (32.1) | 85 (15.7) | 85 (31.5) | 64 (22.1) | 48 (33.1) | |||
| 200–400 | 341 (41.1) | 148 (35.7) | 232 (43.0) | 106 (39.3) | 109 (37.6) | 42 (28.9) | |||
| ≥400 | 273 (32.9) | 96 (23.1) | 168 (31.1) | 55 (20.4) | 105 (36.2) | 41 (28.3) | |||
| Missing | 67 (8.1) | 38 (9.2) | 55 (10.2) | 24 (8.9) | 12 (4.1) | 14 (9.7) | |||
| H. pylori infection | <0.001 | <0.001 | <0.001 | ||||||
| Positive | 486 (58.6) | 382 (92.1) | 333 (61.7) | 252 (93.3) | 153 (52.8) | 130 (89.7) | |||
| Negative | 320 (38.6) | 33 (8.0) | 187 (34.6) | 18 (6.7) | 133 (45.9) | 15 (10.3) | |||
| Missing | 24 (2.9) | 0 (0.0) | 20 (3.7) | 0 (0.0) | 4 (1.4) | 0 (0.0) | |||
| Total energy intake (Kcal/day) | 1713.6 ± 545.5 | 1924.1 ± 612.9 | <0.001 | 1760.6 ± 541.5 | 2038.5 ± 634.8 | <0.001 | 1626.0 ± 543.1 | 1711.1 ± 507.0 | 0.12 |
| Total Population | Male Population | Female Population | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Food Group (g/Day) | Controls (n = 830) | Cases (n = 415) | p-Value b | Controls (n = 540) | Cases (n = 270) | p-Value b | Controls (n = 290) | Cases (n = 145) | p-Value b |
| Refined grains | 548.8 ± 179.6 | 602.6 ± 168.4 | <0.001 | 578.1 ± 166.7 | 610.0 ± 168.2 | 0.011 | 491.1 ± 189.9 | 588.7 ± 168.4 | <0.001 |
| Whole grains | 7.56 ± 6.61 | 7.13 ± 6.45 | 0.28 | 7.42 ± 6.79 | 6.69 ± 6.49 | 0.15 | 7.82 ± 6.27 | 7.95 ± 6.32 | 0.84 |
| Tubers and roots | 48.09 ± 48.51 | 41.23 ± 40.14 | 0.008 | 42.53 ± 38.92 | 34.39 ± 37.36 | 0.005 | 58.44 ± 61.32 | 53.95 ± 42.11 | 0.37 |
| Noodles | 48.11 ± 50.45 | 45.08 ± 43.52 | 0.27 | 52.25 ± 52.77 | 46.74 ± 42.95 | 0.11 | 40.41 ± 44.92 | 41.99 ± 44.56 | 0.73 |
| Rice cakes | 15.42 ± 41.95 | 11.30 ± 40.86 | 0.10 | 11.31 ± 33.19 | 8.89 ± 44.41 | 0.43 | 23.07 ± 53.87 | 15.77 ± 32.95 | 0.08 |
| Bread | 41.71 ± 130.70 | 21.22 ± 68.17 | <0.001 | 32.25 ± 108.6 | 15.79 ± 39.79 | 0.002 | 59.32 ± 162.8 | 31.31 ± 101.2 | 0.03 |
| Cereals and snacks | 11.76 ± 69.50 | 7.40 ± 37.86 | 0.15 | 6.18 ± 26.07 | 6.20 ± 41.05 | 0.99 | 22.15 ± 111.4 | 9.63 ± 31.06 | 0.08 |
| Pizza, hamburgers | 12.44 ± 54.79 | 15.80 ± 122.9 | 0.60 | 8.55 ± 37.34 | 7.57 ± 55.67 | 0.79 | 19.68 ± 77.01 | 31.12 ± 193.0 | 0.49 |
| Cakes and sweets | 12.26 ± 14.53 | 11.74 ± 15.44 | 0.56 | 12.19 ± 15.50 | 12.05 ± 16.67 | 0.90 | 12.36 ± 12.52 | 11.16 ± 12.86 | 0.35 |
| Legumes | 5.41 ± 10.19 | 3.84 ± 6.73 | 0.001 | 4.95 ± 9.36 | 3.79 ± 6.74 | 0.04 | 6.27 ± 11.55 | 3.92 ± 6.73 | 0.008 |
| Tofu/soymilk | 59.20 ± 74.65 | 50.81 ± 51.88 | 0.021 | 57.68 ± 75.25 | 48.79 ± 53.31 | 0.05 | 62.01 ± 73.56 | 54.56 ± 49.06 | 0.21 |
| Nuts and seeds | 4.34 ± 11.17 | 2.20 ± 5.58 | <0.001 | 2.97 ± 6.59 | 2.08 ± 5.85 | 0.05 | 6.91 ± 16.32 | 2.41 ± 5.04 | <0.001 |
| Red meat | 54.21 ± 36.03 | 52.79 ± 33.23 | 0.50 | 56.18 ± 34.88 | 55.25 ± 31.90 | 0.71 | 50.56 ± 37.86 | 48.23 ± 35.24 | 0.54 |
| Meat byproducts | 10.65 ± 66.16 | 5.89 ± 35.97 | 0.10 | 9.92 ± 66.34 | 3.60 ± 10.39 | 0.031 | 12.01 ± 65.94 | 10.14 ± 59.08 | 0.77 |
| Processed meat | 4.91 ± 24.94 | 1.62 ± 5.93 | <0.001 | 2.78 ± 10.17 | 1.66 ± 6.51 | 0.06 | 8.88 ± 39.58 | 1.55 ± 4.64 | 0.002 |
| Poultry | 9.85 ± 23.19 | 7.35 ± 17.22 | 0.033 | 8.35 ± 17.95 | 5.84 ± 12.58 | 0.021 | 12.63 ± 30.50 | 10.17 ± 23.35 | 0.35 |
| Fish | 21.44 ± 20.85 | 20.24 ± 17.37 | 0.28 | 21.60 ± 21.02 | 20.78 ± 17.19 | 0.55 | 21.14 ± 20.58 | 19.22 ± 17.69 | 0.32 |
| Seafood and seafood products | 18.45 ± 15.33 | 19.24 ± 21.22 | 0.50 | 17.50 ± 11.92 | 19.87 ± 24.63 | 0.14 | 20.22 ± 20.11 | 18.08 ± 12.60 | 0.18 |
| Seaweeds | 2.04 ± 1.79 | 2.02 ± 1.89 | 0.78 | 1.89 ± 1.75 | 1.78 ± 1.62 | 0.36 | 2.32 ± 1.82 | 2.45 ± 2.26 | 0.55 |
| Eggs | 19.27 ± 17.85 | 15.00 ± 17.13 | <0.001 | 18.53 ± 17.29 | 14.33 ± 16.95 | 0.001 | 20.64 ± 18.79 | 16.26 ± 17.46 | 0.019 |
| Milk | 260.60 ± 1009.0 | 141.20 ± 647.3 | 0.012 | 175.1 ± 853.8 | 82.32 ± 368.3 | 0.032 | 419.8 ± 1233.4 | 250.7 ± 965.7 | 0.12 |
| Dairy products | 71.99 ± 235.6 | 51.59 ± 276.5 | 0.20 | 65.54 ± 270.1 | 48.61 ± 330.9 | 0.468 | 84.03 ± 151.6 | 57.14 ± 123.2 | 0.05 |
| Fruit | 156.00 ± 173.1 | 110.70 ± 134.6 | <0.001 | 122.5 ± 132.9 | 94.98 ± 125.3 | 0.005 | 218.4 ± 216.7 | 140.1 ± 146.3 | <0.001 |
| Fruit products | 36.87 ± 50.82 | 25.03 ± 40.27 | <0.001 | 30.28 ± 43.92 | 20.03 ± 29.22 | <0.001 | 49.14 ± 59.82 | 34.33 ± 54.16 | 0.01 |
| Green/yellow vegetables | 95.41 ± 78.40 | 82.32 ± 64.71 | 0.002 | 86.62 ± 76.04 | 76.11 ± 57.62 | 0.028 | 111.8 ± 80.23 | 93.89 ± 75.03 | 0.03 |
| Light-colored vegetables | 86.59 ± 59.87 | 78.69 ± 51.24 | 0.016 | 83.23 ± 53.06 | 74.04 ± 47.52 | 0.013 | 92.85 ± 70.51 | 87.36 ± 56.68 | 0.38 |
| Pickled vegetables | 4.11 ± 8.52 | 4.22 ± 8.64 | 0.84 | 3.91 ± 7.34 | 4.35 ± 8.28 | 0.46 | 4.49 ± 10.38 | 3.96 ± 9.28 | 0.61 |
| Kimchi | 145.6 ± 105.7 | 161.5 ± 131.8 | 0.033 | 148.6 ± 106.7 | 161.1 ± 131.4 | 0.18 | 139.9 ± 103.9 | 162.3 ± 133.0 | 0.08 |
| Mushrooms | 9.25 ± 11.39 | 7.56 ± 9.41 | 0.005 | 7.58 ± 8.48 | 6.24 ± 6.47 | 0.013 | 12.35 ± 14.95 | 10.01 ± 12.93 | 0.09 |
| Oils/fats | 4.80 ± 4.76 | 4.67 ± 5.55 | 0.68 | 5.09 ± 4.73 | 5.23 ± 6.18 | 0.75 | 4.26 ± 4.76 | 3.62 ± 3.89 | 0.14 |
| Condiments/seasonings | 17.79 ± 13.74 | 15.54 ± 10.64 | 0.002 | 17.12 ± 13.69 | 14.98 ± 10.15 | 0.013 | 19.03 ± 13.75 | 16.56 ± 11.46 | 0.049 |
| Carbonated beverages | 135.2 ± 1170.7 | 124.9 ± 1259.5 | 0.89 | 134.1 ± 1157.3 | 168.6 ± 1554.5 | 0.75 | 137.3 ± 1197.3 | 43.53 ± 189.7 | 0.19 |
| Coffee/tea | 68.40 ± 108.4 | 51.29 ± 103.3 | 0.007 | 60.16 ± 87.46 | 43.22 ± 95.77 | 0.012 | 83.75 ± 138.2 | 66.34 ± 115.0 | 0.17 |
| Dietary Patterns | No. of Controls | No. of Cases | Model I OR (95% CI) | Model II OR (95% CI) | Model III OR (95% CI) |
|---|---|---|---|---|---|
| Vegetables and seafood | |||||
| T1 (low) | 276 (33.3) | 165 (39.8) | 1.00 | 1.00 | 1.00 |
| T2 (medium) | 277 (33.4) | 154 (37.1) | 0.93 (0.71–1.23) | 0.98 (0.72–1.34) | 1.03 (0.74–1.44) |
| T3 (high) | 277 (33.4) | 96 (23.1) | 0.58 (0.43–0.78) | 0.66 (0.47–0.93) | 0.72 (0.50–1.03) |
| p for trend | <0.001 | 0.018 | 0.07 | ||
| Snacks and fats | |||||
| T1 (low) | 276 (33.3) | 171 (41.2) | 1.00 | 1.00 | 1.00 |
| T2 (medium) | 278 (33.5) | 144 (34.7) | 0.84 (0.63–1.10) | 0.89 (0.66–1.22) | 1.03 (0.74–1.44) |
| T3 (high) | 276 (33.3) | 100 (24.1) | 0.59 (0.43–0.79) | 0.88 (0.61–1.27) | 0.93 (0.64–1.37) |
| p for trend | <0.001 | 0.53 | 0.70 | ||
| Dairy | |||||
| T1 (low) | 276 (33.3) | 197 (47.5) | 1.00 | 1.00 | 1.00 |
| T2 (medium) | 277 (33.4) | 125 (30.1) | 0.63 (0.48–0.84) | 0.77 (0.56–1.05) | 0.68 (0.48–0.95) |
| T3 (high) | 277 (33.4) | 93 (22.4) | 0.47 (0.35–0.63) | 0.87 (0.60–1.26) | 0.88 (0.59–1.31) |
| p for trend | <0.001 | 0.73 | 0.96 | ||
| Meat | |||||
| T1 (low) | 277 (33.4) | 172 (41.5) | 1.00 | 1.00 | 1.00 |
| T2 (medium) | 276 (33.3) | 139 (33.5) | 0.81 (0.61–1.07) | 0.89 (0.64–1.24) | 0.81 (0.57–1.14) |
| T3 (high) | 277 (33.4) | 104(25.1) | 0.61 (0.45–0.81) | 0.88 (0.59–1.31) | 0.83 (0.55–1.28) |
| p for trend | 0.001 | 0.59 | 0.54 | ||
| Fruit | |||||
| T1 (low) | 277 (33.4) | 202 (48.7) | 1.00 | 1.00 | 1.00 |
| T2 (medium) | 276 (33.3) | 134 (32.3) | 0.67 (0.51–0.88) | 0.84 (0.62-1.15) | 0.85 (0.61–1.18) |
| T3 (high) | 277 (33.4) | 79 (19.0) | 0.39 (0.29-0.53) | 0.54 (0.38–0.77) | 0.56 (0.38–0.81) |
| p for trend | <0.001 | <0.001 | 0.002 |
| Dietary Patterns | No. of Controls | No. of Cases | Model I OR (95% CI) | Model II OR (95% CI) | Model III OR (95% CI) |
|---|---|---|---|---|---|
| Males | |||||
| Vegetables and seafood | |||||
| T1 (low) | 180 (33.3) | 104 (38.5) | 1.00 | 1.00 | 1.00 |
| T2 (medium) | 179 (33.2) | 115 (42.6) | 1.11 (0.79–1.56) | 1.22 (0.82–1.80) | 1.25 (0.82–1.91) |
| T3 (high) | 181 (33.5) | 51 (18.9) | 0.48 (0.33–0.72) | 0.51 (0.32–0.81) | 0.55 (0.34–0.89) |
| p for trend | <0.001 | 0.003 | 0.012 | ||
| Snacks and fats | |||||
| T1 (low) | 179 (33.2) | 99 (36.7) | 1.00 | 1.00 | 1.00 |
| T2 (medium) | 180 (33.3) | 100 (37.0) | 1.00 (0.71–1.42) | 1.07 (0.72–1.60) | 1.03 (0.67–1.58) |
| T3 (high) | 181 (33.5) | 71 (26.3) | 0.71 (0.49–1.03) | 0.78 (0.50–1.20) | 0.80 (0.50–1.28) |
| p for trend | 0.05 | 0.22 | 0.31 | ||
| Meat | |||||
| T1 (low) | 180 (33.3) | 119 (44.1) | 1.00 | 1.00 | 1.00 |
| T2 (medium) | 180 (33.3) | 82 (30.4) | 0.69 (0.48–0.98) | 0.84 (0.56–1.27) | 0.93 (0.60–1.44) |
| T3 (high) | 180 (33.3) | 69 (25.6) | 0.58 (0.40–0.83) | 1.17 (0.72–1.90) | 1.23 (0.74–2.06) |
| p for trend | 0.01 | 0.34 | 0.33 | ||
| Fruit | |||||
| T1 (low) | 180 (33.3) | 124 (45.9) | 1.00 | 1.00 | 1.00 |
| T2 (medium) | 180 (33.3) | 80 (29.6) | 0.65 (0.46–0.91) | 0.81 (0.54–1.21) | 0.77 (0.50–1.19) |
| T3 (high) | 180 (33.3) | 66 (24.4) | 0.53 (0.37–0.77) | 0.77 (0.50–1.17) | 0.76 (0.48–1.19) |
| p for trend | 0.002 | 0.25 | 0.29 | ||
| Females | |||||
| Vegetables and seafood | |||||
| T1 (low) | 97 (33.5) | 61 (42.1) | 1.00 | 1.00 | 1.00 |
| T2 (medium) | 96 (33.1) | 47 (32.4) | 0.78 (0.48–1.25) | 0.85 (0.50–1.45) | 1.04 (0.58–1.84) |
| T3 (high) | 97 (33.5) | 37 (25.5) | 0.61 (0.37–0.99) | 0.76 (0.43–1.34) | 0.82 (0.45–1.51) |
| p for trend | 0.049 | 0.34 | 0.52 | ||
| Snacks and fat | |||||
| T1 (low) | 96 (33.1) | 55 (37.9) | 1.00 | 1.00 | 1.00 |
| T2 (medium) | 97 (33.5) | 60 (41.4) | 1.08 (0.68–1.71) | 1.09 (0.65–1.85) | 1.29 (0.73–2.27) |
| T3 (high) | 97 (33.5) | 30 (20.7) | 0.54 (0.32–0.91) | 0.62 (0.31–1.22) | 0.65 (0.32–1.34) |
| p for trend | 0.009 | 0.11 | 0.15 | ||
| Meat | |||||
| T1 (low) | 97 (33.5) | 57 (39.3) | 1.00 | 1.00 | 1.00 |
| T2 (medium) | 96 (33.1) | 38 (26.2) | 0.67 (0.41–1.11) | 0.72 (0.40–1.30) | 0.67 (0.36–1.27) |
| T3 (high) | 97 (33.5) | 50 (34.5) | 0.88 (0.55–1.41) | 0.85 (0.46–1.56) | 0.65 (0.34–1.23) |
| p for trend | 0.81 | 0.79 | 0.26 | ||
| Dairy | |||||
| T1 (low) | 97 (33.5) | 61 (42.1) | 1.00 | 1.00 | 1.00 |
| T2 (medium) | 97 (33.5) | 50 (34.5) | 0.82 (0.51–1.31) | 0.94 (0.56–1.59) | 0.89 (0.51–1.56) |
| T3 (high) | 96 (33.1) | 34 (23.5) | 0.56 (0.34–0.93) | 0.82 (0.43–1.57) | 0.80 (0.40–1.59) |
| p for trend | 0.032 | 0.57 | 0.57 | ||
| Fruit | |||||
| T1 (low) | 97 (33.5) | 82 (56.6) | 1.00 | 1.00 | 1.00 |
| T2 (medium) | 96 (33.1) | 35 (24.1) | 0.43 (0.27–0.70) | 0.54 (0.32–0.93) | 0.59 (0.33–1.05) |
| T3 (high) | 97 (33.5) | 28 (19.3) | 0.34 (0.20–0.57) | 0.56 (0.32–1.00) | 0.62 (0.34–1.14) |
| p for trend | <0.001 | 0.06 | 0.15 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gunathilake, M.; Lee, J.; Choi, I.J.; Kim, Y.-I.; Kim, J. Identification of Dietary Pattern Networks Associated with Gastric Cancer Using Gaussian Graphical Models: A Case-Control Study. Cancers 2020, 12, 1044. https://doi.org/10.3390/cancers12041044
Gunathilake M, Lee J, Choi IJ, Kim Y-I, Kim J. Identification of Dietary Pattern Networks Associated with Gastric Cancer Using Gaussian Graphical Models: A Case-Control Study. Cancers. 2020; 12(4):1044. https://doi.org/10.3390/cancers12041044
Chicago/Turabian StyleGunathilake, Madhawa, Jeonghee Lee, Il Ju Choi, Young-Il Kim, and Jeongseon Kim. 2020. "Identification of Dietary Pattern Networks Associated with Gastric Cancer Using Gaussian Graphical Models: A Case-Control Study" Cancers 12, no. 4: 1044. https://doi.org/10.3390/cancers12041044





