Nanoparticles to Improve the Efficacy of Peptide-Based Cancer Vaccines
Abstract
:1. Introduction
2. Nanomaterials and Nanoparticles for Cancer Vaccines
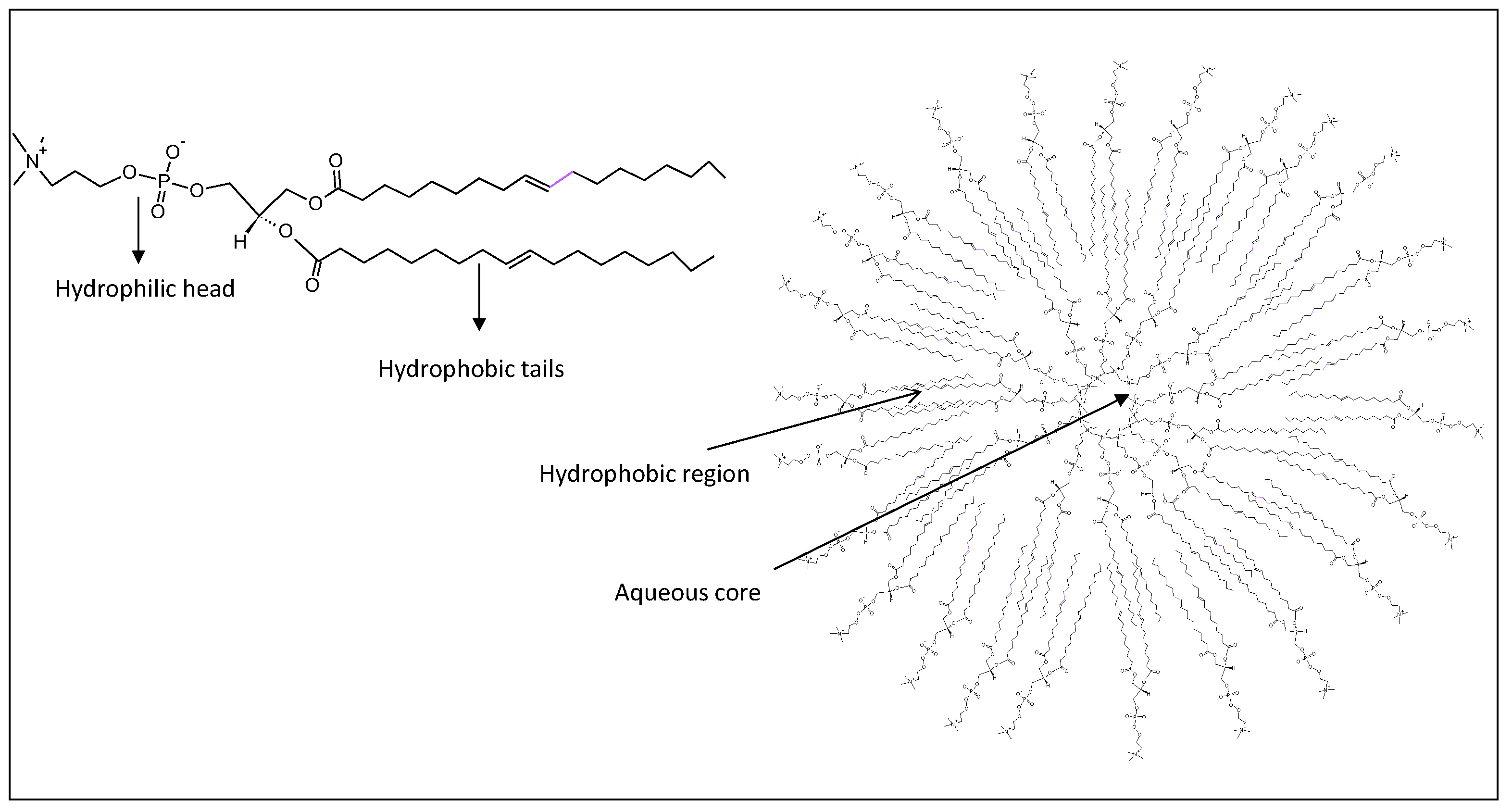
2.1. Liposome
2.2. Polymeric Nanoparticles
2.3. Hydrogels, Nanogels, Micelles, Dendrimers
2.3.1. Hydrogel Nanoparticles
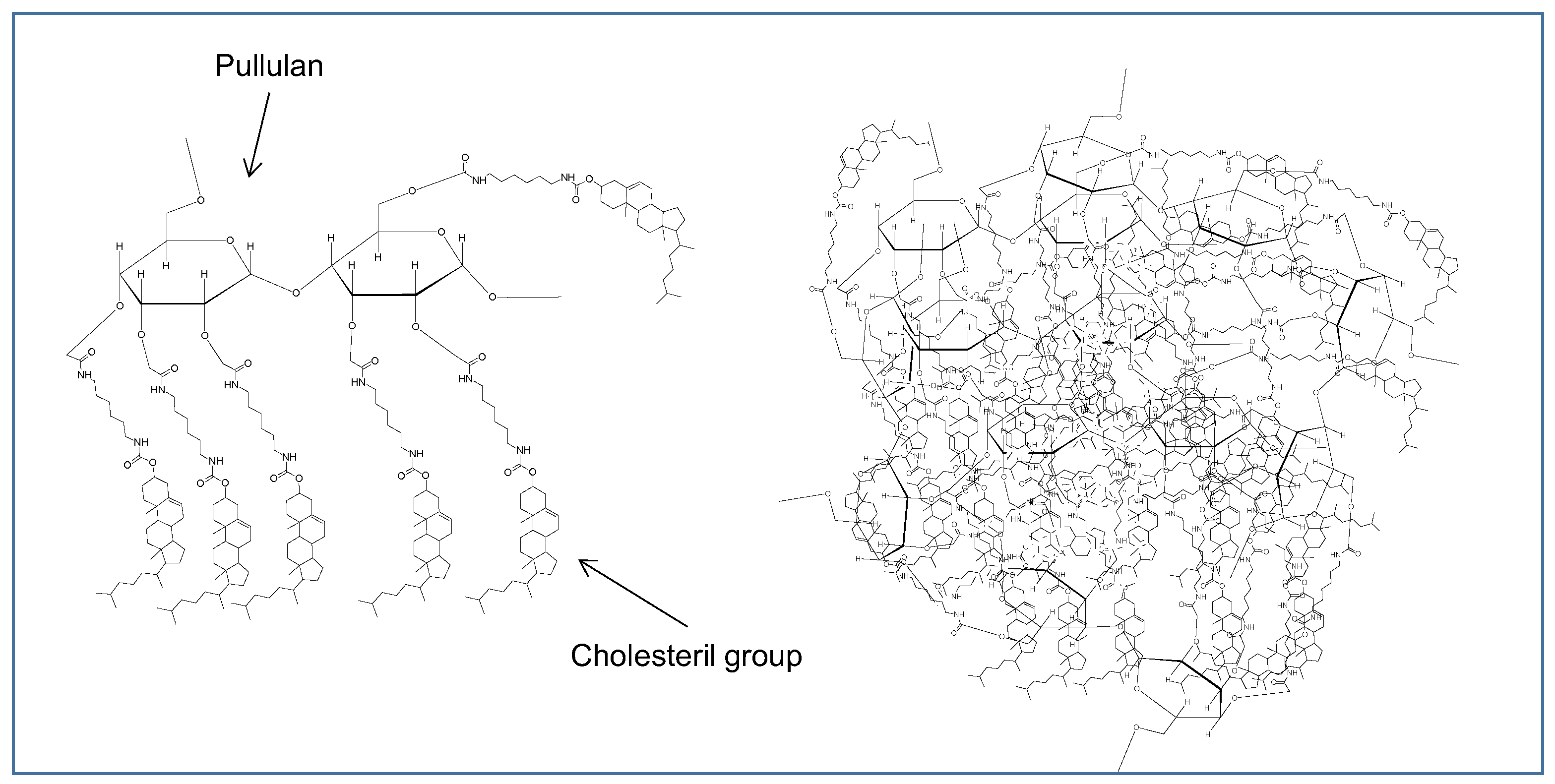
2.3.2. Nanogel Nanoparticles
2.3.3. Polimeric Micelles
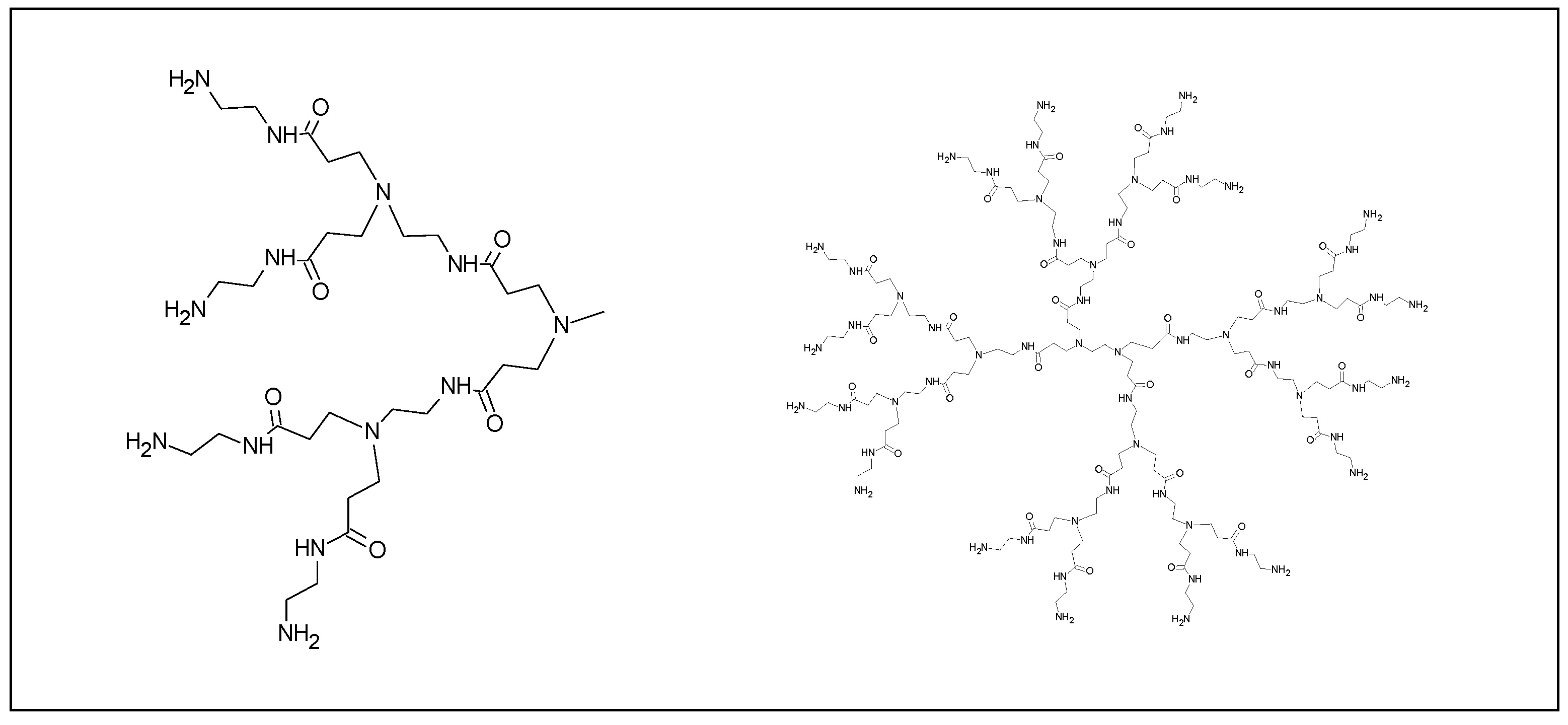
2.3.4. Dendrimer Nanoparticles
2.4. Inorganic Nanoparticles
2.4.1. Gold Nanoparticles
2.4.2. Iron Oxide Nanoparticles
2.4.3. Aluminum Salts (Alum)
2.4.4. Other Inorganic Nanoparticles
3. Virus-Like Particles, Proteinlike Nanoparticles and Peptides
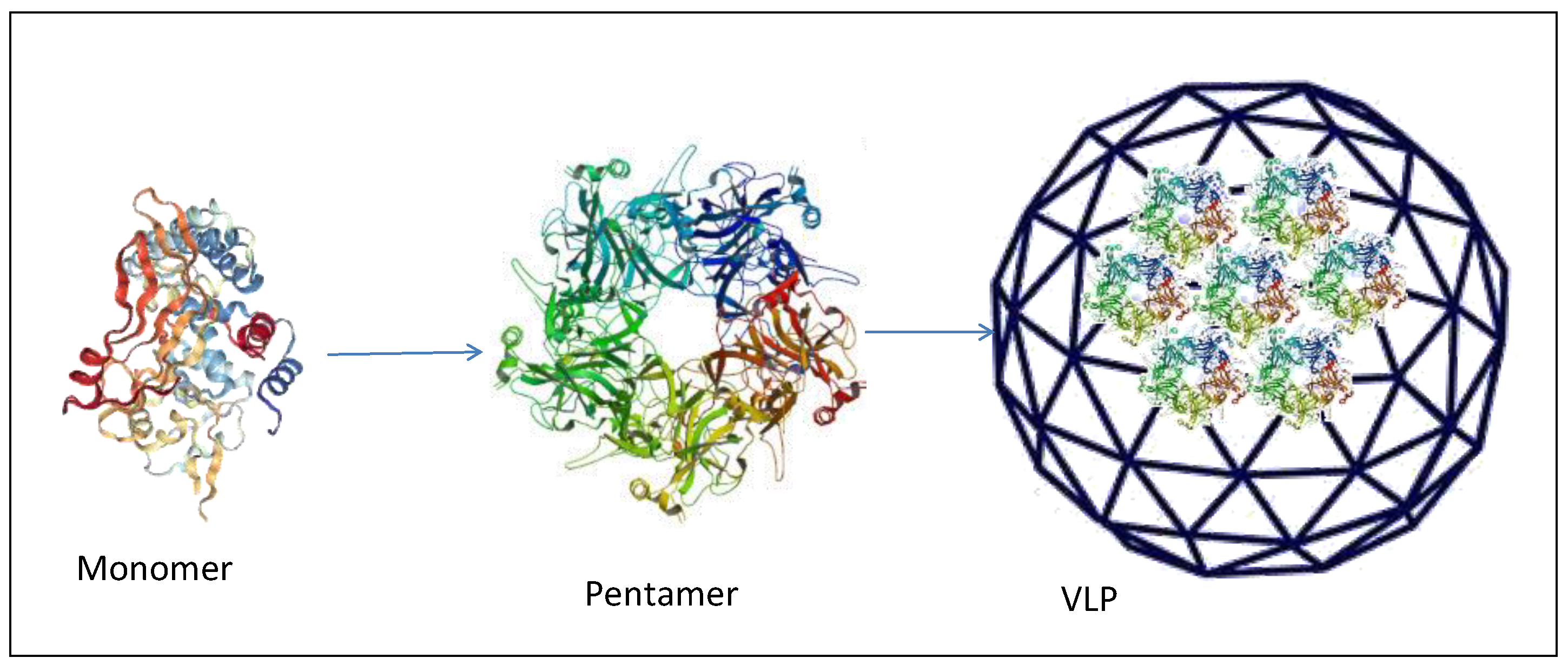
3.1. Virus-Like Particles
3.2. Protein and Peptide Particles
4. Nanoparticles in Clinical Trials
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hoebe, K.; Janssen, E.; Beutler, B. The interface between innate and adaptive immunity. Nat. Immunol. 2004, 5, 971–974. [Google Scholar] [CrossRef] [PubMed]
- Surendran, S.P.; Moon, M.J.; Park, R.; Jeong, Y.Y. Bioactive Nanoparticles for Cancer Immunotherapy. Int. J. Mol. Sci. 2018, 19, 3877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reche, P.; Flower, D.R.; Fridkis-Hareli, M.; Hoshino, Y. Peptide-Based Immunotherapeutics and Vaccines 2017. J. Immunol. Res. 2018, 2018, 4568239. [Google Scholar] [CrossRef] [Green Version]
- Finn, O.J. The dawn of vaccines for cancer prevention. Nat. Rev. Immunol. 2018, 18, 183–194. [Google Scholar] [CrossRef]
- Calvo, T.M.; Allard, M.; Dutoit, V.; Dietrich, P.Y.; Walker, P.R. Peptides as cancer vaccines. Curr. Opin. Pharmacol. 2019, 47, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Gregory, A.E.; Titball, R.; Williamson, D. Vaccine delivery using nanoparticles. Front. Cell. Infect. Microbiol. 2013, 3, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachmann, M.F.; Jennings, G.T. Vaccine delivery: A matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 2010, 10, 787–796. [Google Scholar] [CrossRef]
- Guo, Y.; Lei, K.; Tang, L. Neoantigen Vaccine Delivery for Personalized Anticancer Immunotherapy. Front. Immunol. 2018, 9, 1499. [Google Scholar] [CrossRef] [Green Version]
- Biswas, S.; Torchilin, V.P. Nanopreparations for organelle-specific delivery in cancer. Adv. Drug Deliv. Rev. 2014, 66, 26–41. [Google Scholar] [CrossRef] [Green Version]
- Koshy, S.T.; Mooney, D.J. Biomaterials for enhancing anti-cancer immunity. Curr. Opin. Biotechnol. 2016, 40, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Fujita, Y.; Taguchi, H. Current status of multiple antigen-presenting peptide vaccine systems: Application of organic and inorganic nanoparticles. Chem. Cent. J. 2011, 5, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwendener, R.A. Liposomes as vaccine delivery systems: A review of the recent advances. Ther. Adv. Vaccines 2014, 2, 159–182. [Google Scholar] [CrossRef] [PubMed]
- Shariat, S.; Badiee, A.; Jalali, S.A.; Mansourian, M.; Yazdani, M.; Mortazavi, S.A.; Jaafari, M.R. P5 HER2/neu-derived peptide conjugated to liposomes containing MPL adjuvant as an effective prophylactic vaccine formulation for breast cancer. Cancer Lett. 2014, 355, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Pauli, G.; Tang, W.L.; Li, S.D. Development and Characterization of the Solvent-Assisted Active Loading Technology (SALT) for Liposomal Loading of Poorly Water-Soluble Compounds. Pharmaceutics 2019, 11, 465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riaz, M.K.; Riaz, M.A.; Zhang, X.; Lin, C.; Wong, K.H.; Chen, X.; Zhang, G.; Lu, A.; Yang, Z. Surface Functionalization and Targeting Strategies of Liposomes in Solid Tumor Therapy: A Review. Int. J. Mol. Sci. 2018, 19, 195. [Google Scholar] [CrossRef] [Green Version]
- He, H.; Lu, Y.; Qi, J.; Zhu, Q.; Chen, Z.; Wu, W. Adapting liposomes for oral drug delivery. Acta Pharm. Sin. B 2019, 9, 36–48. [Google Scholar] [CrossRef]
- Gao, J.; Ochyl, L.J.; Yang, E.; Moon, J.J. Cationic liposomes promote antigen cross-presentation in dendritic cells by alkalizing the lysosomal pH and limiting the degradation of antigens. Int. J. Nanomed. 2017, 12, 1251–1264. [Google Scholar] [CrossRef] [Green Version]
- Heuts, J.; Varypataki, E.M.; van der Maaden, K.; Romeijn, S.; Drijfhout, J.W.; van Scheltinga, A.T.; Ossendorp, F.; Jiskoot, W. Cationic Liposomes: A Flexible Vaccine Delivery System for Physicochemically Diverse Antigenic Peptides. Pharm. Res. 2018, 35, 207. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Kim, D.; Wu, G.; Jung, H.; Park, J.A.; Kwon, H.J.; Lee, Y. A peptide-CpG-DNA-liposome complex vaccine targeting TM4SF5 suppresses growth of pancreatic cancer in a mouse allograft model. OncoTargets Ther. 2018, 11, 8655–8672. [Google Scholar] [CrossRef] [Green Version]
- Miura, N.; Akita, H.; Tateshita, N.; Nakamura, T.; Harashima, H. Modifying Antigen-Encapsulating Liposomes with KALA Facilitates MHC Class I Antigen Presentation and Enhances Anti-tumor Effects. Mol. Ther. 2017, 25, 1003–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, T.; Ono, K.; Suzuki, Y.; Moriguchi, R.; Kogure, K.; Harashima, H. Octaarginine-modified liposomes enhance cross-presentation by promoting the C-terminal trimming of antigen peptide. Mol. Pharm. 2014, 11, 2787–2795. [Google Scholar] [CrossRef] [PubMed]
- Razazan, A.; Behravan, J.; Arab, A.; Barati, N.; Arabi, L.; Gholizadeh, Z.; Hatamipour, M.; Reza, N.A.; Momtazi-Borojeni, A.A.; Mosaffa, F.; et al. Conjugated nanoliposome with the HER2/neu-derived peptide GP2 as an effective vaccine against breast cancer in mice xenograft model. PLoS ONE 2017, 12, e0185099. [Google Scholar] [CrossRef] [PubMed]
- Farzad, N.; Barati, N.; Momtazi-Borojeni, A.A.; Yazdani, M.; Arab, A.; Razazan, A.; Shariat, S.; Mansourian, M.; Abbasi, A.; Saberi, Z.; et al. P435 HER2/neu-derived peptide conjugated to liposomes containing DOPE as an effective prophylactic vaccine formulation for breast cancer. Artif. Cells Nanomed. Biotechnol. 2019, 47, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.; Duan, S.; Ye, F.; Hou, X.; Li, X.; Zhao, J.; Yu, X.; Hu, Z.; Tang, Z.; Mo, F.; et al. The enhanced antitumor-specific immune response with mannose- and CpG-ODN-coated liposomes delivering TRP2 peptide. Theranostics 2018, 8, 1723–1739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezvantalab, S.; Drude, N.I.; Moraveji, M.K.; Guvener, N.; Koons, E.K.; Shi, Y.; Lammers, T.; Kiessling, F. PLGA-Based Nanoparticles in Cancer Treatment. Front. Pharmacol. 2018, 9, 1260. [Google Scholar] [CrossRef] [Green Version]
- Petrizzo, A.; Conte, C.; Tagliamonte, M.; Napolitano, M.; Bifulco, K.; Carriero, V.; De, S.A.; Tornesello, M.L.; Buonaguro, F.M.; Quaglia, F.; et al. Functional characterization of biodegradable nanoparticles as antigen delivery system. J. Exp. Clin. Cancer Res. 2015, 34, 114. [Google Scholar] [CrossRef] [Green Version]
- Qiu, F.; Becker, K.W.; Knight, F.C.; Baljon, J.J.; Sevimli, S.; Shae, D.; Gilchuk, P.; Joyce, S.; Wilson, J.T. Poly(propylacrylic acid)-peptide nanoplexes as a platform for enhancing the immunogenicity of neoantigen cancer vaccines. Biomaterials 2018, 182, 82–91. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef]
- Galliverti, G.; Tichet, M.; Domingos-Pereira, S.; Hauert, S.; Nardelli-Haefliger, D.; Swartz, M.A.; Hanahan, D.; Wullschleger, S. Nanoparticle Conjugation of Human Papillomavirus 16 E7-long Peptides Enhances Therapeutic Vaccine Efficacy against Solid Tumors in Mice. Cancer Immunol. Res. 2018, 6, 1301–1313. [Google Scholar] [CrossRef]
- Bae, J.; Parayath, N.; Ma, W.; Amiji, M.; Munshi, N.; Anderson, K. BCMA peptide-engineered nanoparticles enhance induction and function of antigen-specific CD8(+) cytotoxic T lymphocytes against multiple myeloma: Clinical applications. Leukemia 2020, 34, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.D.; Gao, Q.; Wilson, K.L.; Heyerick, A.; Plebanski, M. A Nanoparticle Based Sp17 Peptide Vaccine Exposes New Immuno-Dominant and Species Cross-reactive B Cell Epitopes. Vaccines 2015, 3, 875–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiang, S.D.; Wilson, K.L.; Goubier, A.; Heyerick, A.; Plebanski, M. Design of Peptide-Based Nanovaccines Targeting Leading Antigens from Gynecological Cancers to Induce HLA-A2. 1 Restricted CD8(+) T Cell Responses. Front. Immunol. 2018, 9, 2968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dannert, C.; Stokke, B.T.; Dias, R.S. Nanoparticle-Hydrogel Composites: From Molecular Interactions to Macroscopic Behavior. Polymers 2019, 11, 275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akhtar, M.F.; Hanif, M.; Ranjha, N.M. Methods of synthesis of hydrogels…A review. Saudi Pharm. J. 2016, 24, 554–559. [Google Scholar] [CrossRef] [Green Version]
- Kousalova, J.; Etrych, T. Polymeric nanogels as drug delivery systems. Physiol. Res. 2018, 67, S305–S317. [Google Scholar] [CrossRef]
- Muraoka, D.; Harada, N.; Hayashi, T.; Tahara, Y.; Momose, F.; Sawada, S.; Mukai, S.A.; Akiyoshi, K.; Shiku, H. Nanogel-based immunologically stealth vaccine targets macrophages in the medulla of lymph node and induces potent antitumor immunity. ACS Nano 2014, 8, 9209–9218. [Google Scholar] [CrossRef]
- Kordalivand, N.; Tondini, E.; Lau, C.Y.J.; Vermonden, T.; Mastrobattista, E.; Hennink, W.E.; Ossendorp, F.; Nostrum, C.F.V. Cationic synthetic long peptides-loaded nanogels: An efficient therapeutic vaccine formulation for induction of T-cell responses. J. Control. Release 2019, 315, 114–125. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Hanafy, N.A.N.; El-Kemary, M.; Leporatti, S. Micelles Structure Development as a Strategy to Improve Smart Cancer Therapy. Cancers 2018, 10, 238. [Google Scholar] [CrossRef] [Green Version]
- Clay, N.E.; Whittenberg, J.J.; Leong, J.; Kumar, V.; Chen, J.; Choi, I.; Liamas, E.; Schieferstein, J.M.; Jeong, J.H.; Kim, D.H.; et al. Chemical and mechanical modulation of polymeric micelle assembly. Nanoscale 2017, 9, 5194–5204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ventola, C.L. Progress in Nanomedicine: Approved and Investigational Nanodrugs. Pharm. Ther. 2017, 42, 742–755. [Google Scholar]
- Zeng, Q.; Li, H.; Jiang, H.; Yu, J.; Wang, Y.; Ke, H.; Gong, T.; Zhang, Z.; Sun, X. Tailoring polymeric hybrid micelles with lymph node targeting ability to improve the potency of cancer vaccines. Biomaterials 2017, 122, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, A.S.; Chauhan, P.N.; Noolvi, M.N.; Chaturvedi, K.; Ganguly, K.; Shukla, S.S.; Nadagouda, M.N.; Aminabhavi, T.M. Polymeric micelles: Basic research to clinical practice. Int. J. Pharm. 2017, 532, 249–268. [Google Scholar] [CrossRef]
- Heegaard, P.M.; Boas, U.; Sorensen, N.S. Dendrimers for vaccine and immunostimulatory uses. A review. Bioconjug. Chem. 2010, 21, 405–418. [Google Scholar] [CrossRef]
- Duinkerken, S.; Horrevorts, S.K.; Kalay, H.; Ambrosini, M.; Rutte, L.; de Gruijl, T.D.; Garcia-Vallejo, J.J.; van Kooyk, Y. Glyco-Dendrimers as Intradermal Anti-Tumor Vaccine Targeting Multiple Skin DC Subsets. Theranostics 2019, 9, 5797–5809. [Google Scholar] [CrossRef]
- Male, K.B.; Lachance, B.; Hrapovic, S.; Sunahara, G.; Luong, J.H. Assessment of cytotoxicity of quantum dots and gold nanoparticles using cell-based impedance spectroscopy. Anal. Chem. 2008, 80, 5487–5493. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.; Ahn, S.; Lee, J.; Kim, J.Y.; Choi, M.; Gujrati, V.; Kim, H.; Kim, J.; Shin, E.C.; Jon, S. Effects of gold nanoparticle-based vaccine size on lymph node delivery and cytotoxic T-lymphocyte responses. J. Control. Release 2017, 256, 56–67. [Google Scholar] [CrossRef]
- Lin, A.Y.; Lunsford, J.; Bear, A.S.; Young, J.K.; Eckels, P.; Luo, L.; Foster, A.E.; Drezek, R.A. High-density sub-100-nm peptide-gold nanoparticle complexes improve vaccine presentation by dendritic cells in vitro. Nanoscale Res. Lett. 2013, 8, 72. [Google Scholar] [CrossRef] [Green Version]
- Tavernaro, I.; Hartmann, S.; Sommer, L.; Hausmann, H.; Rohner, C.; Ruehl, M.; Hoffmann-Roeder, A.; Schlecht, S. Synthesis of tumor-associated MUC1-glycopeptides and their multivalent presentation by functionalized gold colloids. Org. Biomol. Chem. 2015, 13, 81–97. [Google Scholar] [CrossRef] [Green Version]
- Mocan, T.; Matea, C.; Tabaran, F.; Iancu, C.; Orasan, R.; Mocan, L. In Vitro Administration of Gold Nanoparticles Functionalized with MUC-1 Protein Fragment Generates Anticancer Vaccine Response via Macrophage Activation and Polarization Mechanism. J. Cancer 2015, 6, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Parry, A.L.; Clemson, N.A.; Ellis, J.; Bernhard, S.S.; Davis, B.G.; Cameron, N.R. ‘Multicopy multivalent’ glycopolymer-stabilized gold nanoparticles as potential synthetic cancer vaccines. J. Am. Chem. Soc. 2013, 135, 9362–9365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fytianos, K.; Rodriguez-Lorenzo, L.; Clift, M.J.; Blank, F.; Vanhecke, D.; von Garnier, C.; Petri-Fink, A.; Rothen-Rutishauser, B. Uptake efficiency of surface modified gold nanoparticles does not correlate with functional changes and cytokine secretion in human dendritic cells in vitro. Nanomedicine 2015, 11, 633–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida, J.P.M.; Lin, A.Y.; Figueroa, E.R.; Foster, A.E.; Drezek, R.A. In vivo gold nanoparticle delivery of peptide vaccine induces anti-tumor immune response in prophylactic and therapeutic tumor models. Small 2015, 11, 1453–1459. [Google Scholar] [CrossRef] [Green Version]
- Liang, R.; Xie, J.; Li, J.; Wang, K.; Liu, L.; Gao, Y.; Hussain, M.; Shen, G.; Zhu, J.; Tao, J. Liposomes-coated gold nanocages with antigens and adjuvants targeted delivery to dendritic cells for enhancing antitumor immune response. Biomaterials 2017, 149, 41–50. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, X.; Cheng, Y.; Guo, X.; Yuan, W. Iron Oxide Nanoparticles-Based Vaccine Delivery for Cancer Treatment. Mol. Pharm. 2018, 15, 1791–1799. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, F.; Men, K.; Huang, R.; Zhou, B.; Zhang, R.; Zou, R.; Yang, L. Modified Fe3O4 Magnetic Nanoparticle Delivery of CpG Inhibits Tumor Growth and Spontaneous Pulmonary Metastases to Enhance Immunotherapy. Nanoscale Res. Lett. 2018, 13, 240. [Google Scholar] [CrossRef] [Green Version]
- Gavin, A.L.; Hoebe, K.; Duong, B.; Ota, T.; Martin, C.; Beutler, B.; Nemazee, D. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science 2006, 314, 1936–1938. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Li, M.; Yu, C.; Zhang, R.; Zhang, X.; Huang, R.; Lu, L.; Yuan, F.; Fan, Y.; Zhou, B.; et al. The novel complex combination of alum, CpG ODN and HH2 as adjuvant in cancer vaccine effectively suppresses tumor growth in vivo. Oncotarget 2017, 8, 45951–45964. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Aldayel, A.M.; Cui, Z. Aluminum hydroxide nanoparticles show a stronger vaccine adjuvant activity than traditional aluminum hydroxide microparticles. J. Control. Release 2014, 173, 148–157. [Google Scholar] [CrossRef] [Green Version]
- Hesse, C.; Kollenda, S.; Rotan, O.; Pastille, E.; Adamczyk, A.; Wenzek, C.; Hansen, W.; Epple, M.; Buer, J.; Westendorf, A.M.; et al. A Tumor-Peptide-Based Nanoparticle Vaccine Elicits Efficient Tumor Growth Control in Antitumor Immunotherapy. Mol. Cancer Ther. 2019, 18, 1069–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, H.; Qian, Y.; Dai, Y.; Qiao, S.; Huang, C.; Lu, L.; Luo, Q.; Chen, J.; Zhang, Z. Magnetic Enrichment of Dendritic Cell Vaccine in Lymph Node with Fluorescent-Magnetic Nanoparticles Enhanced Cancer Immunotherapy. Theranostics 2016, 6, 2000–2014. [Google Scholar] [CrossRef] [PubMed]
- Bharti, C.; Nagaich, U.; Pal, A.K.; Gulati, N. Mesoporous silica nanoparticles in target drug delivery system: A review. Int. J. Pharm. Investig. 2015, 5, 124–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dellacherie, M.O.; Li, A.W.; Lu, B.Y.; Mooney, D.J. Covalent Conjugation of Peptide Antigen to Mesoporous Silica Rods to Enhance Cellular Responses. Bioconjug. Chem. 2018, 29, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Cha, B.G.; Jeong, J.H.; Kim, J. Extra-Large Pore Mesoporous Silica Nanoparticles Enabling Co-Delivery of High Amounts of Protein Antigen and Toll-like Receptor 9 Agonist for Enhanced Cancer Vaccine Efficacy. ACS Cent. Sci. 2018, 4, 484–492. [Google Scholar] [CrossRef] [Green Version]
- Buonaguro, L.; Tagliamonte, M.; Tornesello, M.L.; Buonaguro, F.M. Developments in virus-like particle-based vaccines for infectious diseases and cancer. Expert Rev. Vaccines 2011, 10, 1569–1583. [Google Scholar] [CrossRef]
- Harper, D.M.; Franco, E.L.; Wheeler, C.M.; Moscicki, A.B.; Romanowski, B.; Roteli-Martins, C.M.; Jenkins, D.; Schuind, A.; Costa Clemens, S.A.; Dubin, G. Sustained efficacy up to 4. 5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: Follow-up from a randomised control trial. Lancet 2006, 367, 1247–1255. [Google Scholar] [CrossRef] [Green Version]
- Dawar, M.; Deeks, S.; Dobson, S. Human papillomavirus vaccines launch a new era in cervical cancer prevention. CMAJ 2007, 177, 456–461. [Google Scholar] [CrossRef] [Green Version]
- Storni, T.; Ruedl, C.; Schwarz, K.; Schwendener, R.A.; Renner, W.A.; Bachmann, M.F. Nonmethylated CG motifs packaged into virus-like particles induce protective cytotoxic T cell responses in the absence of systemic side effects. J. Immunol. 2004, 172, 1777–1785. [Google Scholar] [CrossRef] [Green Version]
- Shukla, S.; Jandzinski, M.; Wang, C.; Gong, X.; Bonk, K.W.; Keri, R.A.; Steinmetz, N.F. A Viral Nanoparticle Cancer Vaccine Delays Tumor Progression and Prolongs Survival in a HER2+ Tumor Mouse Model. Adv. Ther. 2019, 2, 1800139. [Google Scholar] [CrossRef]
- Borrelli, A.; Tornesello, A.L.; Tornesello, M.L.; Buonaguro, F.M. Cell Penetrating Peptides as Molecular Carriers for Anti-Cancer Agents. Molecules 2018, 23, 295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohsen, M.O.; Heath, M.D.; Cabral-Miranda, G.; Lipp, C.; Zeltins, A.; Sande, M.; Stein, J.V.; Riether, C.; Roesti, E.; Zha, L.; et al. Vaccination with nanoparticles combined with micro-adjuvants protects against cancer. J. Immunother. Cancer 2019, 7, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Huang, Y.; Lindstrom, A.R.; Lin, T.Y.; Lam, K.S.; Li, Y. Peptide-based materials for cancer immunotherapy. Theranostics 2019, 9, 7807–7825. [Google Scholar] [CrossRef] [PubMed]
- Skwarczynski, M.; Toth, I. Peptide-based synthetic vaccines. Chem. Sci. 2016, 7, 842–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bird, G.H.; Irimia, A.; Ofek, G.; Kwong, P.D.; Wilson, I.A.; Walensky, L.D. Stapled HIV-1 peptides recapitulate antigenic structures and engage broadly neutralizing antibodies. Nat. Struct. Mol. Biol. 2014, 21, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, R.E.; Jansen, K. Turning the corner on therapeutic cancer vaccines. NPJ Vaccines 2019, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Miles, D.; Roche, H.; Martin, M.; Perren, T.J.; Cameron, D.A.; Glaspy, J.; Dodwell, D.; Parker, J.; Mayordomo, J.; Tres, A.; et al. Phase III multicenter clinical trial of the sialyl-TN (STn)-keyhole limpet hemocyanin (KLH) vaccine for metastatic breast cancer. Oncologist 2011, 16, 1092–1100. [Google Scholar] [CrossRef] [Green Version]
- Fenstermaker, R.A.; Ciesielski, M.J.; Qiu, J.; Yang, N.; Frank, C.L.; Lee, K.P.; Mechtler, L.R.; Belal, A.; Ahluwalia, M.S.; Hutson, A.D. Clinical study of a survivin long peptide vaccine (SurVaxM) in patients with recurrent malignant glioma. Cancer Immunol. Immunother. 2016, 65, 1339–1352. [Google Scholar] [CrossRef] [Green Version]
- Belnoue, E.; Di Berardino-Besson, W.; Gaertner, H.; Carboni, S.; Dunand-Sauthier, I.; Cerini, F.; Suso-Inderberg, E.M.; Walchli, S.; Konig, S.; Salazar, A.M.; et al. Enhancing Antitumor Immune Responses by Optimized Combinations of Cell-penetrating Peptide-based Vaccines and Adjuvants. Mol. Ther. 2016, 24, 1675–1685. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Luo, Y.; Shibu, M.A.; Toth, I.; Skwarczynskia, M. Cell-penetrating Peptides: Efficient Vectors for Vaccine Delivery. Curr. Drug Deliv. 2019, 16, 430–443. [Google Scholar] [CrossRef]
- Skwarczynski, M.; Toth, I. Cell-penetrating peptides in vaccine delivery: Facts, challenges and perspectives. Ther. Deliv. 2019, 10, 465–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alizadeh, S.; Irani, S.; Bolhassani, A.; Sadat, S.M. Simultaneous use of natural adjuvants and cell penetrating peptides improves HCV NS3 antigen-specific immune responses. Immunol. Lett. 2019, 212, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, S.; Bolhassani, A. Comparison of six cell penetrating peptides with different properties for in vitro and in vivo delivery of HPV16 E7 antigen in therapeutic vaccines. Int. Immunopharmacol. 2018, 62, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Grippin, A.J.; Sayour, E.J.; Mitchell, D.A. Translational nanoparticle engineering for cancer vaccines. Oncoimmunology 2017, 6, e1290036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Campos, F.; Candini, D.; Carrasco, E.; Berenguer Frances, M.A. Nanoparticles applied to cancer immunoregulation. Rep. Pract. Oncol. Radiother. 2019, 24, 47–55. [Google Scholar] [CrossRef]
- Gao, S.; Yang, D.; Fang, Y.; Lin, X.; Jin, X.; Wang, Q.; Wang, X.; Ke, L.; Shi, K. Engineering Nanoparticles for Targeted Remodeling of the Tumor Microenvironment to Improve Cancer Immunotherapy. Theranostics 2019, 9, 126–151. [Google Scholar] [CrossRef]
- Butts, C.; Maksymiuk, A.; Goss, G.; Soulieres, D.; Marshall, E.; Cormier, Y.; Ellis, P.M.; Price, A.; Sawhney, R.; Beier, F.; et al. Updated survival analysis in patients with stage IIIB or IV non-small-cell lung cancer receiving BLP25 liposome vaccine (L-BLP25): Phase IIB randomized, multicenter, open-label trial. J. Cancer Res. Clin. Oncol. 2011, 137, 1337–1342. [Google Scholar] [CrossRef]
- Butts, C.; Murray, R.N.; Smith, C.J.; Ellis, P.M.; Jasas, K.; Maksymiuk, A.; Goss, G.; Ely, G.; Beier, F.; Soulieres, D. A multicenter open-label study to assess the safety of a new formulation of BLP25 liposome vaccine in patients with unresectable stage III non-small-cell lung cancer. Clin. Lung Cancer 2010, 11, 391–395. [Google Scholar] [CrossRef]
- Butts, C.; Murray, N.; Maksymiuk, A.; Goss, G.; Marshall, E.; Soulieres, D.; Cormier, Y.; Ellis, P.; Price, A.; Sawhney, R.; et al. Randomized phase IIB trial of BLP25 liposome vaccine in stage IIIB and IV non-small-cell lung cancer. J. Clin. Oncol. 2005, 23, 6674–6681. [Google Scholar] [CrossRef] [Green Version]
- Kruit, W.H.; Suciu, S.; Dreno, B.; Mortier, L.; Robert, C.; Chiarion-Sileni, V.; Maio, M.; Testori, A.; Dorval, T.; Grob, J.J.; et al. Selection of immunostimulant AS15 for active immunization with MAGE-A3 protein: Results of a randomized phase II study of the European Organisation for Research and Treatment of Cancer Melanoma Group in Metastatic Melanoma. J. Clin. Oncol. 2013, 31, 2413–2420. [Google Scholar] [CrossRef]
- McQuade, J.L.; Homsi, J.; Torres-Cabala, C.A.; Bassett, R.; Popuri, R.M.; James, M.L.; Vence, L.M.; Hwu, W.J. A phase II trial of recombinant MAGE-A3 protein with immunostimulant AS15 in combination with high-dose Interleukin-2 (HDIL2) induction therapy in metastatic melanoma. BMC Cancer 2018, 18, 1274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vansteenkiste, J.F.; Cho, B.C.; Vanakesa, T.; De, P.T.; Zielinski, M.; Kim, M.S.; Jassem, J.; Yoshimura, M.; Dahabreh, J.; Nakayama, H.; et al. Efficacy of the MAGE-A3 cancer immunotherapeutic as adjuvant therapy in patients with resected MAGE-A3-positive non-small-cell lung cancer (MAGRIT): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016, 17, 822–835. [Google Scholar] [CrossRef]
- Berinstein, N.L.; Karkada, M.; Oza, A.M.; Odunsi, K.; Villella, J.A.; Nemunaitis, J.J.; Morse, M.A.; Pejovic, T.; Bentley, J.; Buyse, M.; et al. Survivin-targeted immunotherapy drives robust polyfunctional T cell generation and differentiation in advanced ovarian cancer patients. Oncoimmunology 2015, 4, e1026529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berinstein, N.L.; Karkada, M.; Morse, M.A.; Nemunaitis, J.J.; Chatta, G.; Kaufman, H.; Odunsi, K.; Nigam, R.; Sammatur, L.; MacDonald, L.D.; et al. First-in-man application of a novel therapeutic cancer vaccine formulation with the capacity to induce multi-functional T cell responses in ovarian, breast and prostate cancer patients. J. Transl. Med. 2012, 10, 156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karkada, M.; Berinstein, N.L.; Mansour, M. Therapeutic vaccines and cancer: Focus on DPX-0907. Biologics 2014, 8, 27–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, O.; Davis, I.D.; McArthur, G.A.; Chen, L.; Haydon, A.; Parente, P.; Dimopoulos, N.; Jackson, H.; Xiao, K.; Maraskovsky, E.; et al. Low-dose cyclophosphamide enhances antigen-specific CD4(+) T cell responses to NY-ESO-1/ISCOMATRIX vaccine in patients with advanced melanoma. Cancer Immunol. Immunother. 2015, 64, 507–518. [Google Scholar] [CrossRef]
- Kitano, S.; Kageyama, S.; Nagata, Y.; Miyahara, Y.; Hiasa, A.; Naota, H.; Okumura, S.; Imai, H.; Shiraishi, T.; Masuya, M.; et al. HER2-specific T-cell immune responses in patients vaccinated with truncated HER2 protein complexed with nanogels of cholesteryl pullulan. Clin. Cancer Res. 2006, 12, 7397–7405. [Google Scholar] [CrossRef] [Green Version]
- Goldinger, S.M.; Dummer, R.; Baumgaertner, P.; Mihic-Probst, D.; Schwarz, K.; Hammann-Haenni, A.; Willers, J.; Geldhof, C.; Prior, J.O.; Kundig, T.M.; et al. Nano-particle vaccination combined with TLR-7 and -9 ligands triggers memory and effector CD8(+) T-cell responses in melanoma patients. Eur. J. Immunol. 2012, 42, 3049–3061. [Google Scholar] [CrossRef] [Green Version]
- Speiser, D.E.; Schwarz, K.; Baumgaertner, P.; Manolova, V.; Devevre, E.; Sterry, W.; Walden, P.; Zippelius, A.; Conzett, K.B.; Senti, G.; et al. Memory and effector CD8 T-cell responses after nanoparticle vaccination of melanoma patients. J. Immunother. 2010, 33, 848–858. [Google Scholar] [CrossRef]



| Nanomaterials | Peptide Sequence | Adjuvant | Tumor | REF |
|---|---|---|---|---|
| Liposome | SLP OVA24: (SIINFEKL) | poly(inosinic-polycytidylic acid) (poly(I:C) | N/A | [16] |
| Liposome | p5 (ELAAWCRWGFLLALLPPGIAG), p373 (KIFGSLAFL), p435(IRGRILHDGAYSLTLQGLGIH), p1209 (SPPHPSPAFSPAFDNLYYWDQ) | CpG ODN 1826 (5-TCCATGACGTTCCTGACGTT-3) MPL | Breast cancer | [17,18] |
| Liposome | GP2 (654–662: IISAVVGIL) | MPL | Breast cancer | [18] |
| Liposome | GP2 (Ac-CGGGIISAVVGIL) | MPL | Breast cancer | [18] |
| Liposome | TM4SF5 peptide (TAGAYLLNRTLWDRCEAPPRVVPWNVT), hTM4SF5EC2-C: (TACAYLLNRTLWDRCEAPPRVVPWNcT) | CpG-DNA or flagella | HCC and colon cancer Pancreatic cancer | [19,20] |
| Liposome | TRP-2180–188 (SVYDFFVWL) | CpG-ODN | Melanoma | [21] |
| Polymeric nanoparticles | HPV E7 SLP (GQAEPDRAHYNIVTFCCKCDSTLRLCVQSTHVDIR) | CpG | HPV cancer | [22,23] |
| Polymeric nanoparticles | BCMA72−80 (YLMFLLRKI) | Myeloma | [24] | |
| Polystyrene | hSp17111-142 (KEKEEVAAVKIQAAFRGHIAREEAKKMKTNSL), HPV16-E782−94 and HPV16-E641−65 (LLMGTLGIVCPICKQQLLRREVYDFAFRDLCIVYRDGN), WT1126−134 (RMFPNAPYL), SV90−124 (KKQFEELTLGEFLKLDRERAKNKIAKETNNKKKEF), SV2−36 (GAPTLPPAWQPFLKDHRISTFKNWPFLEGCACTPE) | CpG | Gynecological cancer | [25] |
| Hydrogel | (KVPRNQDWL) | CpG | Melanoma | [26] |
| Hydrogel | CTL (DEWSGLEQLESIINFEKLAAAAAK), Help (DEWEISQAVHAAHAEINEAGRE) | Poly(I:C) | Different cancers | [27] |
| Micelles | Trp2180-188 (SVYDFFVWL) | CpG ODN | Melanoma | [28] |
| Inorganic Nanoparticles | TRP2180-188 (SVYDFFVWL) | MPL | Melanoma | [29] |
| Inorganic nanoparticles | OVA (ISQAVHAAHAEINEAGR) | Colon adenocarcinoma | [30] | |
| Inorganic nanoparticles | HA110–120 (SVSSFERFERFEIFPKESS) HA512–520 (YQILAIYSTVASSLVLL) | CpG | Colorectal cancer | [31,32] |
| Inorganic nanoparticles | APgp100 peptide (gp10025-33) (KVPRNQDWL) | Lymphoma | [33] | |
| VLPs | gp33 (KAVYNFATM) | CpG | Fibrosarcoma | [34] |
| Viral nanoparticles (CPMV) | human163-182 (YQDTILWKDIFHKNNQLALT) rat167-186 (YQDMVLWKDVFRKNNQLAPV) | Breast carcinomas | [35] | |
| VLPs | P33 (KAVYNFATMGGCK) | MCT | Melanoma | [36] |
| Nanoparticles | Payloads | Clinical Stages | Indications | Ref |
|---|---|---|---|---|
| Liposome (L-BLP25) | MUC-1, tecemotide monophosphoryl lipid A | Terminated after phase III | NSCLC | [87,88,89] |
| Liposome (AS15) | MAGE-A3, CpG 7909 monophosphoryl lipid A | Terminated after phase III | Melanoma, NSCLC | [90,91,92] |
| Liposome (ISCOMATRIX) | NY-ESO-1 | Terminated after Phase II | Melanoma | [96] |
| Liposome (DPX) | HLA-A2, Survivin polynucleotide | Phase I/II | Ovarian cancer | [93,94,95] |
| Cholesteryl pullulan (CHP) | HER-2 protein | Phase I/II | Esophageal cancer | [97] |
| Virus-like particles (VLPs) | Melan-A/MART-1, CpG | Phase I/II | Melanoma | [98,99] |
| Nanoparticles | Advantages | Disadvantages | Manufacturing Limitations |
|---|---|---|---|
| Liposomes |
|
| High cost |
| Polymeric Nanoparticles |
|
| Low scale-up |
| Hydrogels, Nanogels |
|
| Reproducibility Difficult to sterilize |
| Micelles |
|
| Reproducibility costs |
| Dendrimers |
|
| High cost |
| Inorganic Nanoparticles(e.g., Gold NPs) |
|
| Low scale-up High cost |
| Mesoporous Silicas |
|
| High cost |
| VLPs |
|
| Low stability |
| CPPS |
|
| No limitation |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tornesello, A.L.; Tagliamonte, M.; Tornesello, M.L.; Buonaguro, F.M.; Buonaguro, L. Nanoparticles to Improve the Efficacy of Peptide-Based Cancer Vaccines. Cancers 2020, 12, 1049. https://doi.org/10.3390/cancers12041049
Tornesello AL, Tagliamonte M, Tornesello ML, Buonaguro FM, Buonaguro L. Nanoparticles to Improve the Efficacy of Peptide-Based Cancer Vaccines. Cancers. 2020; 12(4):1049. https://doi.org/10.3390/cancers12041049
Chicago/Turabian StyleTornesello, Anna Lucia, Maria Tagliamonte, Maria Lina Tornesello, Franco M. Buonaguro, and Luigi Buonaguro. 2020. "Nanoparticles to Improve the Efficacy of Peptide-Based Cancer Vaccines" Cancers 12, no. 4: 1049. https://doi.org/10.3390/cancers12041049








