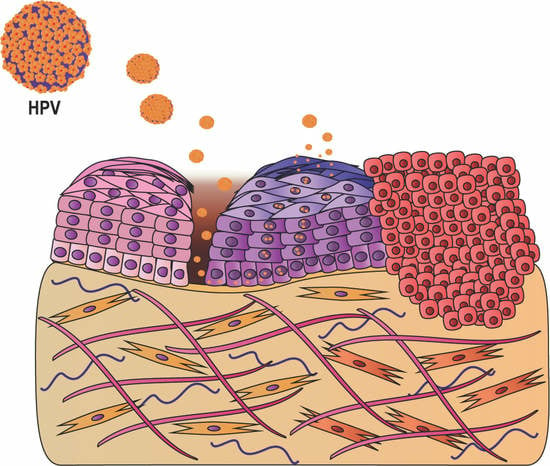
In Vitro Organotypic Systems to Model Tumor Microenvironment in Human Papillomavirus (HPV)-Related Cancers
Abstract
:1. Introduction
2. HPV-Related Cervical Diseases
2.1. In Vitro Model to Reproduce HPV Life Cycle in HPV-Related Cervical Cancers
2.2. In Vitro Organotypic Systems to Model Tumor Microenvironment in HPV-Related Cervical Cancer
3. HPV-Related Human Malignancies Arising from Mucosal Squamous Epithelia: Head and Neck and Anogenital-Tract Cancers
3.1. Head and Neck Cancers
3.2. Anogenital HPV-Associated Cancers in Males and Females
3.2.1. Vulva and Vagina
3.2.2. Anus
3.2.3. Penis and Penile Urethra
4. Other HPV-Related Cancers (Non-Melanoma Skin Cancer)
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Doorbar, J. Molecular biology of human papillomavirus infection and cervical cancer. Clin. Sci. (Lond.) 2006, 110, 525–541. [Google Scholar] [CrossRef] [Green Version]
- Cubie, H.A. Diseases associated with human papillomavirus infection. Virology 2013, 445, 21–34. [Google Scholar] [CrossRef] [Green Version]
- Syrjanen, S. Human papillomavirus (HPV) in head and neck cancer. J. Clin. Virol. 2005, 32, S59–S66. [Google Scholar] [CrossRef] [PubMed]
- De Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef] [Green Version]
- Bansal, A.; Singh, M.P.; Rai, B. Human papillomavirus-associated cancers: A growing global problem. Int. J. Appl. Basic. Med. Res. 2016, 6, 84–89. [Google Scholar] [PubMed] [Green Version]
- Gillison, M.L.; Chaturvedi, A.K.; Lowy, D.R. HPV prophylactic vaccines and the potential prevention of noncervical cancers in both men and women. Cancer 2008, 113, 3036–3046. [Google Scholar] [CrossRef]
- Chan, P.K.; Chang, A.R.; Cheung, J.L.; Chan, D.P.; Xu, L.Y.; Tang, N.L.; Cheng, A.F. Determinants of cervical human papillomavirus infection: Differences between high– and low–oncogenic risk types. J. Infect. Dis. 2002, 185, 28–35. [Google Scholar] [CrossRef]
- Hartwig, S.; Baldauf, J.-J.; Dominiak-Felden, G.; Simondon, F.; Alemany, L.; de Sanjosé, S.; Castellsagué, X. Estimation of the epidemiological burden of HPV-related anogenital cancers, precancerous lesions, and genital warts in women and men in Europe: Potential additional benefit of a nine-valent second generation HPV vaccine compared to first generation HPV vaccines. Papillomavirus Res. 2015, 1, 90–100. [Google Scholar]
- Bosch, F.X.; Broker, T.R.; Forman, D.; Moscicki, A.B.; Gillison, M.L.; Doorbar, J.; Stern, P.L.; Stanley, M.; Arbyn, M.; Poljak, M.; et al. Comprehensive control of human papillomavirus infections and related diseases. Vaccine 2013, 31, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Hoots, B.E.; Palefsky, J.M.; Pimenta, J.M.; Smith, J.S. Human papillomavirus type distribution in anal cancer and anal intraepithelial lesions. Int. J. Cancer 2009, 124, 2375–2383. [Google Scholar] [CrossRef]
- Munoz, N.; Bosch, F.X.; de Sanjose, S.; Herrero, R.; Castellsague, X.; Shah, K.V.; Snijders, P.J.; Meijer, C.J.; International Agency for Research on Cancer Multicenter Cervical Cancer Study Group. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N. Engl. J. Med. 2003, 348, 518–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghittoni, R.; Accardi, R.; Chiocca, S.; Tommasino, M. Role of human papillomaviruses in carcinogenesis. Ecancermedicalscience 2015, 9, 526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartwig, S.; St Guily, J.L.; Dominiak-Felden, G.; Alemany, L.; de Sanjose, S. Estimation of the overall burden of cancers, precancerous lesions, and genital warts attributable to 9-valent HPV vaccine types in women and men in Europe. Infect. Agent. Cancer 2017, 12, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steben, M.; Duarte-Franco, E. Human papillomavirus infection: Epidemiology and pathophysiology. Gynecol. Oncol. 2007, 107, S2–S5. [Google Scholar] [CrossRef] [PubMed]
- Shanmugasundaram, S.; You, J. Targeting Persistent Human Papillomavirus Infection. Viruses 2017, 9, 229. [Google Scholar] [CrossRef] [Green Version]
- Doorbar, J.; Griffin, H. Refining our understanding of cervical neoplasia and its cellular origins. Papillomavirus Res. 2019, 7, 176–179. [Google Scholar] [CrossRef]
- Burd, E.M. Human papillomavirus and cervical cancer. Clin. Microbiol. Rev. 2003, 16, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Rajendra, S.; Sharma, P. Transforming human papillomavirus infection and the esophageal transformation zone: Prime time for total excision/ablative therapy? Dis. Esophagus. 2019, 32. [Google Scholar] [CrossRef]
- Egawa, N.; Egawa, K.; Griffin, H.; Doorbar, J. Human Papillomaviruses; Epithelial Tropisms, and the Development of Neoplasia. Viruses 2015, 7, 3863–3890. [Google Scholar] [CrossRef] [Green Version]
- Stanley, M.A. Epithelial cell responses to infection with human papillomavirus. Clin. Microbiol. Rev. 2012, 25, 215–222. [Google Scholar] [CrossRef] [Green Version]
- Graham, S.V. Human papillomavirus: Gene expression, regulation and prospects for novel diagnostic methods and antiviral therapies. Future Microbiol. 2010, 5, 1493–1506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlecht, N.F.; Platt, R.W.; Duarte-Franco, E.; Costa, M.C.; Sobrinho, J.P.; Prado, J.C.; Ferenczy, A.; Rohan, T.E.; Villa, L.L.; Franco, E.L. Human papillomavirus infection and time to progression and regression of cervical intraepithelial neoplasia. J. Natl. Cancer Inst. 2003, 95, 1336–1343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMurray, H.R.; Nguyen, D.; Westbrook, T.F.; McAnce, D.J. Biology of human papillomaviruses. Int. J. Exp. Pathol. 2001, 82, 15–33. [Google Scholar] [CrossRef] [PubMed]
- McBride, A.A. Replication and partitioning of papillomavirus genomes. Adv. Virus. Res. 2008, 72, 155–205. [Google Scholar] [PubMed] [Green Version]
- Tornesello, M.L.; Buonaguro, L.; Giorgi-Rossi, P.; Buonaguro, F.M. Viral and cellular biomarkers in the diagnosis of cervical intraepithelial neoplasia and cancer. Biomed. Res. Int. 2013, 2013, 519619. [Google Scholar] [CrossRef]
- Howley, P.M.; Livingston, D.M. Small DNA tumor viruses: Large contributors to biomedical sciences. Virology 2009, 384, 256–259. [Google Scholar] [CrossRef] [Green Version]
- Spurgeon, M.E.; Lambert, P.F. Human Papillomavirus and the Stroma: Bidirectional Crosstalk during the Virus Life Cycle and Carcinogenesis. Viruses 2017, 9, 219. [Google Scholar] [CrossRef] [Green Version]
- Surviladze, Z.; Sterkand, R.T.; Ozbun, M.A. Interaction of human papillomavirus type 16 particles with heparan sulfate and syndecan-1 molecules in the keratinocyte extracellular matrix plays an active role in infection. J. Gen. Virol. 2015, 96, 2232–2241. [Google Scholar] [CrossRef]
- Cerqueira, C.; Liu, Y.; Kuhling, L.; Chai, W.; Hafezi, W.; van Kuppevelt, T.H.; Kuhn, J.E.; Feizi, T.; Schelhaas, M. Heparin increases the infectivity of Human Papillomavirus type 16 independent of cell surface proteoglycans and induces L1 epitope exposure. Cell Microbiol. 2013, 15, 1818–1836. [Google Scholar] [CrossRef] [Green Version]
- Maeda, N.; Maenaka, K. The Roles of Matricellular Proteins in Oncogenic Virus-Induced Cancers and Their Potential Utilities as Therapeutic Targets. Int. J. Mol. Sci. 2017, 18, 2198. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Langenheim, J.F.; Chen, W.Y. Stromal–epithelial interactions modulate cross-talk between prolactin receptor and HER2/Neu in breast cancer. Breast Cancer Res. Treat. 2012, 134, 157–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodby, B.; Scott, M.; Bodily, J. The Interaction Between Human Papillomaviruses and the Stromal Microenvironment. Prog. Mol. Biol. Transl. Sci. 2016, 144, 169–238. [Google Scholar] [PubMed] [Green Version]
- Fiori, M.E.; Di Franco, S.; Villanova, L.; Bianca, P.; Stassi, G.; De Maria, R. Cancer–associated fibroblasts as abettors of tumor progression at the crossroads of EMT and therapy resistance. Mol. Cancer 2019, 18, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef] [PubMed]
- Hemmings, C. Is carcinoma a mesenchymal disease? The role of the stromal microenvironment in carcinogenesis. Pathology 2013, 45, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, O.; Bray, F.; Coleman, M.P.; Vanderpuye, V.; Eniu, A.; Kotha, S.R.; Sarker, M.; Huong, T.T.; Allemani, C.; Dvaladze, A.; et al. The global burden of women’s cancers: A grand challenge in global health. Lancet 2017, 389, 847–860. [Google Scholar] [CrossRef]
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjose, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef] [Green Version]
- Lacey, J.V., Jr.; Swanson, C.A.; Brinton, L.A.; Altekruse, S.F.; Barnes, W.A.; Gravitt, P.E.; Greenberg, M.D.; Hadjimichael, O.C.; McGowan, L.; Mortel, R.; et al. Obesity as a potential risk factor for adenocarcinomas and squamous cell carcinomas of the uterine cervix. Cancer 2003, 98, 814–821. [Google Scholar] [CrossRef]
- Koskela, P.; Anttila, T.; Bjorge, T.; Brunsvig, A.; Dillner, J.; Hakama, M.; Hakulinen, T.; Jellum, E.; Lehtinen, M.; Lenner, P.; et al. Chlamydia trachomatis infection as a risk factor for invasive cervical cancer. Int. J. Cancer 2000, 85, 35–39. [Google Scholar] [CrossRef]
- Waggoner, S.E. Cervical cancer. Lancet 2003, 361, 2217–2225. [Google Scholar] [CrossRef]
- Muntean, M.; Simionescu, C.; Taslica, R.; Gruia, C.; Comanescu, A.; Patrana, N.; Fota, G. Cytological and histopathological aspects concerning preinvasive squamous cervical lesions. Curr. Health Sci. J. 2010, 36, 26–32. [Google Scholar] [PubMed]
- Moody, C. Mechanisms by which HPV Induces a Replication Competent Environment in Differentiating Keratinocytes. Viruses 2017, 9, 261. [Google Scholar] [CrossRef] [PubMed]
- McBride, A.A. Mechanisms and strategies of papillomavirus replication. Biol. Chem. 2017, 398, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, R.; Hirt, B.; Bechtold, V.; Beard, P.; Raj, K. Different modes of human papillomavirus DNA replication during maintenance. J. Virol. 2006, 80, 4431–4439. [Google Scholar] [CrossRef] [Green Version]
- Bonnez, W.; Rose, R.C.; Da Rin, C.; Borkhuis, C.; de Mesy Jensen, K.L.; Reichman, R.C. Propagation of human papillomavirus type 11 in human xenografts using the severe combined immunodeficiency (SCID) mouse and comparison to the nude mouse model. Virology 1993, 197, 455–458. [Google Scholar] [CrossRef]
- Kreider, J.W.; Howett, M.K.; Leure-Dupree, A.E.; Zaino, R.J.; Weber, J.A. Laboratory production in vivo of infectious human papillomavirus type 11. J. Virol. 1987, 61, 590–593. [Google Scholar] [CrossRef] [Green Version]
- Doorbar, J. Model systems of human papillomavirus–associated disease. J. Pathol. 2016, 238, 166–179. [Google Scholar] [CrossRef] [Green Version]
- Doorbar, J.; Egawa, N.; Griffin, H.; Kranjec, C.; Murakami, I. Human papillomavirus molecular biology and disease association. Rev. Med. Virol. 2015, 25, 2–23. [Google Scholar] [CrossRef] [Green Version]
- Delvenne, P.; Hubert, P.; Jacobs, N.; Giannini, S.L.; Havard, L.; Renard, I.; Saboulard, D.; Boniver, J. The organotypic culture of HPV–transformed keratinocytes: An effective in vitro model for the development of new immunotherapeutic approaches for mucosal (pre)neoplastic lesions. Vaccine 2001, 19, 2557–2564. [Google Scholar] [CrossRef] [Green Version]
- McLaughlin-Drubin, M.E.; Meyers, C. Propagation of infectious, high-risk HPV in organotypic “raft” culture. Methods Mol. Med. 2005, 119, 171–186. [Google Scholar]
- Arnette, C.; Koetsier, J.L.; Hoover, P.; Getsios, S.; Green, K.J. In Vitro Model of the Epidermis: Connecting Protein Function to 3D Structure. Methods Enzymol. 2016, 569, 287–308. [Google Scholar] [PubMed] [Green Version]
- Dollard, S.C.; Wilson, J.L.; Demeter, L.M.; Bonnez, W.; Reichman, R.C.; Broker, T.R.; Chow, L.T. Production of human papillomavirus and modulation of the infectious program in epithelial raft cultures. OFF. Genes Dev. 1992, 6, 1131–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bubb, V.; McCance, D.J.; Schlegel, R. DNA sequence of the HPV–16 E5 ORF and the structural conservation of its encoded protein. Virology 1988, 163, 243–246. [Google Scholar] [CrossRef]
- Asselineau, D.; Prunieras, M. Reconstruction of ‘simplified’ skin: Control of fabrication. Br. J. Dermatol. 1984, 111, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Kopan, R.; Traska, G.; Fuchs, E. Retinoids as important regulators of terminal differentiation: Examining keratin expression in individual epidermal cells at various stages of keratinization. J. Cell Biol. 1987, 105, 427–440. [Google Scholar] [CrossRef] [Green Version]
- Kirnbauer, R.; Booy, F.; Cheng, N.; Lowy, D.R.; Schiller, J.T. Papillomavirus L1 major capsid protein self–assembles into virus-like particles that are highly immunogenic. Proc. Natl. Acad. Sci. USA 1992, 89, 12180–12184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buck, C.B.; Pastrana, D.V.; Lowy, D.R.; Schiller, J.T. Efficient intracellular assembly of papillomaviral vectors. J. Virol. 2004, 78, 751–757. [Google Scholar] [CrossRef] [Green Version]
- Culp, T.D.; Christensen, N.D. Kinetics of in vitro adsorption and entry of papillomavirus virions. Virology 2004, 319, 152–161. [Google Scholar] [CrossRef] [Green Version]
- Conway, M.J.; Alam, S.; Ryndock, E.J.; Cruz, L.; Christensen, N.D.; Roden, R.B.; Meyers, C. Tissue-spanning redox gradient-dependent assembly of native human papillomavirus type 16 virions. J. Virol. 2009, 83, 10515–10526. [Google Scholar] [CrossRef] [Green Version]
- Ozbun, M.A.; Patterson, N.A. Using organotypic (raft) epithelial tissue cultures for the biosynthesis and isolation of infectious human papillomaviruses. Curr. Protoc. Microbiol. 2014, 34, 14B.3.1–14B.3.18. [Google Scholar]
- El Awady, M.K.; Kaplan, J.B.; O’Brien, S.J.; Burk, R.D. Molecular analysis of integrated human papillomavirus 16 sequences in the cervical cancer cell line SiHa. Virology 1987, 159, 389–398. [Google Scholar] [CrossRef]
- Pattillo, R.A.; Hussa, R.O.; Story, M.T.; Ruckert, A.C.; Shalaby, M.R.; Mattingly, R.F. Tumor antigen and human chorionic gonadotropin in CaSki cells: A new epidermoid cervical cancer cell line. Science 1977, 196, 1456–1458. [Google Scholar] [CrossRef] [PubMed]
- Badal, S.; Badal, V.; Calleja-Macias, I.E.; Kalantari, M.; Chuang, L.S.; Li, B.F.; Bernard, H.U. The human papillomavirus–18 genome is efficiently targeted by cellular DNA methylation. Virology 2004, 324, 483–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veeraraghavalu, K.; Pett, M.; Kumar, R.V.; Nair, P.; Rangarajan, A.; Stanley, M.A.; Krishna, S. Papillomavirus-mediated neoplastic progression is associated with reciprocal changes in JAGGED1 and manic fringe expression linked to notch activation. J. Virol. 2004, 78, 8687–8700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheffner, M.; Munger, K.; Byrne, J.C.; Howley, P.M. The state of the p53 and retinoblastoma genes in human cervical carcinoma cell lines. Proc. Natl. Acad. Sci. USA 1991, 88, 5523–5527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyers, C.; Frattini, M.G.; Hudson, J.B.; Laimins, L.A. Biosynthesis of human papillomavirus from a continuous cell line upon epithelial differentiation. Science 1992, 257, 971–973. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin-Drubin, M.E.; Wilson, S.; Mullikin, B.; Suzich, J.; Meyers, C. Human papillomavirus type 45 propagation, infection, and neutralization. Virology 2003, 312, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Isaacson Wechsler, E.; Wang, Q.; Roberts, I.; Pagliarulo, E.; Jackson, D.; Untersperger, C.; Coleman, N.; Griffin, H.; Doorbar, J. Reconstruction of human papillomavirus type 16-mediated early-stage neoplasia implicates E6/E7 deregulation and the loss of contact inhibition in neoplastic progression. J. Virol. 2012, 86, 6358–6364. [Google Scholar] [CrossRef] [Green Version]
- Duenas-Gonzalez, A.; Lizano, M.; Candelaria, M.; Cetina, L.; Arce, C.; Cervera, E. Epigenetics of cervical cancer. An overview and therapeutic perspectives. Mol. Cancer 2005, 4, 38. [Google Scholar] [CrossRef] [Green Version]
- Stanley, M.A.; Browne, H.M.; Appleby, M.; Minson, A.C. Properties of a non–tumorigenic human cervical keratinocyte cell line. Int. J. Cancer 1989, 43, 672–676. [Google Scholar] [CrossRef]
- Gray, E.; Pett, M.R.; Ward, D.; Winder, D.M.; Stanley, M.A.; Roberts, I.; Scarpini, C.G.; Coleman, N. In vitro progression of human papillomavirus 16 episome-associated cervical neoplasia displays fundamental similarities to integrant–associated carcinogenesis. Cancer Res. 2010, 70, 4081–4091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoppler, H.; Hartmann, D.P.; Sherman, L.; Schlegel, R. The human papillomavirus type 16 E6 and E7 oncoproteins dissociate cellular telomerase activity from the maintenance of telomere length. J. Biol. Chem. 1997, 272, 13332–13337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiyono, T.; Foster, S.A.; Koop, J.I.; McDougall, J.K.; Galloway, D.A.; Klingelhutz, A.J. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature 1998, 396, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Blanton, R.A.; Perez-Reyes, N.; Merrick, D.T.; McDougall, J.K. Epithelial cells immortalized by human papillomaviruses have premalignant characteristics in organotypic culture. Am. J. Pathol. 1991, 138, 673–685. [Google Scholar]
- Farwell, D.G.; Shera, K.A.; Koop, J.I.; Bonnet, G.A.; Matthews, C.P.; Reuther, G.W.; Coltrera, M.D.; McDougall, J.K.; Klingelhutz, A.J. Genetic and epigenetic changes in human epithelial cells immortalized by telomerase. Am. J. Pathol. 2000, 156, 1537–1547. [Google Scholar] [CrossRef] [Green Version]
- Schutze, D.M.; Snijders, P.J.; Bosch, L.; Kramer, D.; Meijer, C.J.; Steenbergen, R.D. Differential in vitro immortalization capacity of eleven (probable) [corrected] high-risk human papillomavirus types. J. Virol. 2014, 88, 1714–1724. [Google Scholar] [CrossRef] [Green Version]
- Steenbergen, R.D.; Walboomers, J.M.; Meijer, C.J.; van der Raaij-Helmer, E.M.; Parker, J.N.; Chow, L.T.; Broker, T.R.; Snijders, P.J. Transition of human papillomavirus type 16 and 18 transfected human foreskin keratinocytes towards immortality: Activation of telomerase and allele losses at 3p, 10p, 11q and/or 18q. Oncogene 1996, 13, 1249–1257. [Google Scholar]
- Bosch, F.X.; Leube, R.E.; Achtstatter, T.; Moll, R.; Franke, W.W. Expression of simple epithelial type cytokeratins in stratified epithelia as detected by immunolocalization and hybridization in situ. J. Cell Biol. 1988, 106, 1635–1648. [Google Scholar] [CrossRef]
- Gasparoni, A.; Squier, C.A.; Fonzi, L. Cytokeratin changes in cell culture systems of epithelial cells isolated from oral mucosa: A short review. Ital. J. Anat. Embryol. 2005, 110, 75–82. [Google Scholar]
- Roberts, S.; Evans, D.; Mehanna, H.; Parish, J.L. Modelling human papillomavirus biology in oropharyngeal keratinocytes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20180289. [Google Scholar] [CrossRef] [Green Version]
- Wira, C.R.; Rodriguez-Garcia, M.; Patel, M.V. The role of sex hormones in immune protection of the female reproductive tract. Nat. Rev. Immunol. 2015, 15, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Z.; Wang, T.T.; Zhang, Y.Z. A modified method for the culture of naturally HPV–infected high–grade cervical intraepithelial neoplasia keratinocytes from human neoplastic cervical biopsies. Oncol. Lett. 2016, 11, 1457–1462. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, V.; Imparato, G.; Urciuolo, F.; Tornesello, M.L.; Annunziata, C.; Buonaguro, F.M.; Netti, P.A. An Engineered Cell-Instructive Stroma for the Fabrication of a Novel Full Thickness Human Cervix Equivalent In Vitro. Adv. Healthc. Mater. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Villa, P.L.; Jackson, R.; Eade, S.; Escott, N.; Zehbe, I. Isolation of Biopsy–Derived, Human Cervical Keratinocytes Propagated as Monolayer and Organoid Cultures. Sci. Rep. 2018, 8, 17869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bissell, M.J.; Radisky, D. Putting tumours in context. Nat. Rev. Cancer 2001, 1, 46–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, E.; Yan, T.; Xu, Z.; Shang, Z. Tumor Microenvironment and Cell Fusion. Biomed. Res. Int. 2019, 2019, 5013592. [Google Scholar] [CrossRef] [PubMed]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Poltavets, V.; Kochetkova, M.; Pitson, S.M.; Samuel, M.S. The Role of the Extracellular Matrix and Its Molecular and Cellular Regulators in Cancer Cell Plasticity. Front. Oncol. 2018, 8, 431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McBride, A.A.; Munger, K. Expert Views on HPV Infection. Viruses 2018, 10, 94. [Google Scholar] [CrossRef] [Green Version]
- Llames, S.; Garcia-Perez, E.; Meana, A.; Larcher, F.; del Rio, M. Feeder Layer Cell Actions and Applications. Tissue Eng. Part B. Rev. 2015, 21, 345–353. [Google Scholar] [CrossRef] [Green Version]
- Schumacher, M.; Schuster, C.; Rogon, Z.M.; Bauer, T.; Caushaj, N.; Baars, S.; Szabowski, S.; Bauer, C.; Schorpp-Kistner, M.; Hess, J.; et al. Efficient keratinocyte differentiation strictly depends on JNK–induced soluble factors in fibroblasts. J. Invest. Dermatol. 2014, 134, 1332–1341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maas-Szabowski, N.; Shimotoyodome, A.; Fusenig, N.E. Keratinocyte growth regulation in fibroblast cocultures via a double paracrine mechanism. J. Cell Sci. 1999, 112, 1843–1853. [Google Scholar] [PubMed]
- Maas-Szabowski, N.; Stark, H.J.; Fusenig, N.E. Keratinocyte growth regulation in defined organotypic cultures through IL-1-induced keratinocyte growth factor expression in resting fibroblasts. J. Invest. Dermatol. 2000, 114, 1075–1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dall, K.L.; Scarpini, C.G.; Roberts, I.; Winder, D.M.; Stanley, M.A.; Muralidhar, B.; Herdman, M.T.; Pett, M.R.; Coleman, N. Characterization of naturally occurring HPV16 integration sites isolated from cervical keratinocytes under noncompetitive conditions. Cancer Res. 2008, 68, 8249–8259. [Google Scholar] [CrossRef] [Green Version]
- Deng, H.; Hillpot, E.; Mondal, S.; Khurana, K.K.; Woodworth, C.D. HPV16–Immortalized Cells from Human Transformation Zone and Endocervix are More Dysplastic than Ectocervical Cells in Organotypic Culture. Sci. Rep. 2018, 8, 15402. [Google Scholar] [CrossRef]
- Rijal, G.; Li, W. Native-mimicking in vitro microenvironment: An elusive and seductive future for tumor modeling and tissue engineering. J. Biol. Eng. 2018, 12, 20. [Google Scholar] [CrossRef] [Green Version]
- Maufort, J.P.; Shai, A.; Pitot, H.C.; Lambert, P.F. A role for HPV16 E5 in cervical carcinogenesis. Cancer Res. 2010, 70, 2924–2931. [Google Scholar] [CrossRef] [Green Version]
- Hogervorst, M.; Rietveld, M.; de Gruijl, F.; El Ghalbzouri, A. A shift from papillary to reticular fibroblasts enables tumour–stroma interaction and invasion. Br. J. Cancer 2018, 118, 1089–1097. [Google Scholar] [CrossRef] [Green Version]
- Ridky, T.W.; Chow, J.M.; Wong, D.J.; Khavari, P.A. Invasive three–dimensional organotypic neoplasia from multiple normal human epithelia. Nat. Med. 2010, 16, 1450–1455. [Google Scholar] [CrossRef] [Green Version]
- Pickard, A.; Cichon, A.C.; Barry, A.; Kieran, D.; Patel, D.; Hamilton, P.; Salto-Tellez, M.; James, J.; McCance, D.J. Inactivation of Rb in stromal fibroblasts promotes epithelial cell invasion. EMBO J. 2012, 31, 3092–3103. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.; Li, F.; Zhao, X.; Ma, Y.; Li, Y.; Lin, M.; Jin, G.; Lu, T.J.; Genin, G.M.; Xu, F. Functional and Biomimetic Materials for Engineering of the Three–Dimensional Cell Microenvironment. Chem. Rev. 2017, 117, 12764–12850. [Google Scholar] [CrossRef] [PubMed]
- Oxford, J.T.; Reeck, J.C.; Hardy, M.J. Extracellular Matrix in Development and Disease. Int. J. Mol. Sci. 2019, 20, 205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amar, S.; Smith, L.; Fields, G.B. Matrix metalloproteinase collagenolysis in health and disease. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1940–1951. [Google Scholar] [CrossRef]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef] [Green Version]
- Fullar, A.; Dudas, J.; Olah, L.; Hollosi, P.; Papp, Z.; Sobel, G.; Karaszi, K.; Paku, S.; Baghy, K.; Kovalszky, I. Remodeling of extracellular matrix by normal and tumor–associated fibroblasts promotes cervical cancer progression. BMC Cancer 2015, 15, 256. [Google Scholar] [CrossRef] [Green Version]
- Bergmann, S.; Schoenen, H.; Hammerschmidt, S. The interaction between bacterial enolase and plasminogen promotes adherence of Streptococcus pneumoniae to epithelial and endothelial cells. Int. J. Med. Microbiol. 2013, 303, 452–462. [Google Scholar] [CrossRef]
- Zhai, Y.; Hotary, K.B.; Nan, B.; Bosch, F.X.; Munoz, N.; Weiss, S.J.; Cho, K.R. Expression of membrane type 1 matrix metalloproteinase is associated with cervical carcinoma progression and invasion. Cancer Res. 2005, 65, 6543–6550. [Google Scholar] [CrossRef] [Green Version]
- Sahebali, S.; Van den Eynden, G.; Murta, E.F.; Michelin, M.A.; Cusumano, P.; Petignat, P.; Bogers, J.J. Stromal issues in cervical cancer: A review of the role and function of basement membrane, stroma, immune response and angiogenesis in cervical cancer development. Eur. J. Cancer Prev. 2010, 19, 204–215. [Google Scholar] [CrossRef]
- Ayala, G.E.; Dai, H.; Powell, M.; Li, R.; Ding, Y.; Wheeler, T.M.; Shine, D.; Kadmon, D.; Thompson, T.; Miles, B.J.; et al. Cancer-related axonogenesis and neurogenesis in prostate cancer. Clin. Cancer Res. 2008, 14, 7593–7603. [Google Scholar] [CrossRef] [Green Version]
- Magnon, C.; Hall, S.J.; Lin, J.; Xue, X.; Gerber, L.; Freedland, S.J.; Frenette, P.S. Autonomic nerve development contributes to prostate cancer progression. Science 2013, 341, 1236361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucido, C.T.; Wynja, E.; Madeo, M.; Williamson, C.S.; Schwartz, L.E.; Imblum, B.A.; Drapkin, R.; Vermeer, P.D. Innervation of cervical carcinoma is mediated by cancer–derived exosomes. Gynecol. Oncol. 2019, 154, 228–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.S.; Kim, H.S.; Park, J.T.; Lee, M.C.; Park, C.S. Expression of vascular endothelial growth factor in the progression of cervical neoplasia and its relation to angiogenesis and p53 status. Anal. Quant. Cytol. Histol. 2003, 25, 303–311. [Google Scholar] [PubMed]
- Smith-McCune, K.K.; Weidner, N. Demonstration and characterization of the angiogenic properties of cervical dysplasia. Cancer Res. 1994, 54, 800–804. [Google Scholar]
- Chen, W.; Li, F.; Mead, L.; White, H.; Walker, J.; Ingram, D.A.; Roman, A. Human papillomavirus causes an angiogenic switch in keratinocytes which is sufficient to alter endothelial cell behavior. Virology 2007, 367, 168–174. [Google Scholar] [CrossRef] [Green Version]
- Mazio, C.; Casale, C.; Imparato, G.; Urciuolo, F.; Attanasio, C.; De Gregorio, M.; Rescigno, F.; Netti, P.A. Pre–vascularized dermis model for fast and functional anastomosis with host vasculature. Biomaterials 2019, 192, 159–170. [Google Scholar] [CrossRef]
- D’Anna, R.; Le Buanec, H.; Alessandri, G.; Caruso, A.; Burny, A.; Gallo, R.; Zagury, J.F.; Zagury, D.; D’Alessio, P. Selective activation of cervical microvascular endothelial cells by human papillomavirus 16-e7 oncoprotein. J. Natl. Cancer Inst. 2001, 93, 1843–1851. [Google Scholar] [CrossRef] [Green Version]
- Mazibrada, J.; Ritta, M.; Mondini, M.; De Andrea, M.; Azzimonti, B.; Borgogna, C.; Ciotti, M.; Orlando, A.; Surico, N.; Chiusa, L.; et al. Interaction between inflammation and angiogenesis during different stages of cervical carcinogenesis. Gynecol. Oncol. 2008, 108, 112–120. [Google Scholar] [CrossRef]
- Gun, S.Y.; Lee, S.W.L.; Sieow, J.L.; Wong, S.C. Targeting immune cells for cancer therapy. Redox Biol. 2019, 25, 101174. [Google Scholar] [CrossRef]
- Kyrgiou, M.; Mitra, A.; Moscicki, A.B. Does the vaginal microbiota play a role in the development of cervical cancer? Transl. Res. 2017, 179, 168–182. [Google Scholar] [CrossRef] [Green Version]
- Belkaid, Y.; Harrison, O.J. Homeostatic Immunity and the Microbiota. Immunity 2017, 46, 562–576. [Google Scholar] [CrossRef] [PubMed]
- Ayehunie, S.; Islam, A.; Cannon, C.; Landry, T.; Pudney, J.; Klausner, M.; Anderson, D.J. Characterization of a Hormone-Responsive Organotypic Human Vaginal Tissue Model: Morphologic and Immunologic Effects. Reprod. Sci. 2015, 22, 980–990. [Google Scholar] [CrossRef] [PubMed]
- Gorodeski, G.I.; Romero, M.F.; Hopfer, U.; Rorke, E.; Utian, W.H.; Eckert, R.L. Human uterine cervical epithelial cells grown on permeable support––A new model for the study of differentiation. Differentiation 1994, 56, 107–118. [Google Scholar] [PubMed]
- Heroiu Cataloiu, A.D.; Danciu, C.E.; Popescu, C.R. Multiple cancers of the head and neck. Maedica (Buchar.) 2013, 8, 80–85. [Google Scholar]
- Peltanova, B.; Raudenska, M.; Masarik, M. Effect of tumor microenvironment on pathogenesis of the head and neck squamous cell carcinoma: A systematic review. Mol. Cancer 2019, 18, 63. [Google Scholar] [CrossRef]
- Tumban, E. A Current Update on Human Papillomavirus-Associated Head and Neck Cancers. Viruses 2019, 11, 992. [Google Scholar] [CrossRef] [Green Version]
- Pan, C.; Issaeva, N.; Yarbrough, W.G. HPV-driven oropharyngeal cancer: Current knowledge of molecular biology and mechanisms of carcinogenesis. Cancers Head Neck 2018, 3, 12. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Lee, C.R.; Rigas, N.K.; Kim, R.H.; Kang, M.K.; Park, N.H.; Shin, K.H. Human papillomavirus 16 (HPV16) enhances tumor growth and cancer stemness of HPV-negative oral/oropharyngeal squamous cell carcinoma cells via miR-181 regulation. Papillomavirus Res. 2015, 1, 116–125. [Google Scholar] [CrossRef] [Green Version]
- Reddout, N.; Christensen, T.; Bunnell, A.; Jensen, D.; Johnson, D.; O’Malley, S.; Kingsley, K. High risk HPV types 18 and 16 are potent modulators of oral squamous cell carcinoma phenotypes in vitro. Infect. Agent Cancer 2007, 2, 21. [Google Scholar] [CrossRef] [Green Version]
- Scanlon, C.S.; Van Tubergen, E.A.; Chen, L.C.; Elahi, S.F.; Kuo, S.; Feinberg, S.; Mycek, M.A.; D’Silva, N.J. Characterization of squamous cell carcinoma in an organotypic culture via subsurface non–linear optical molecular imaging. Exp. Biol. Med. (Maywood) 2013, 238, 1233–1241. [Google Scholar] [CrossRef] [Green Version]
- Dalley, A.J.; AbdulMajeed, A.A.; Upton, Z.; Farah, C.S. Organotypic culture of normal, dysplastic and squamous cell carcinoma–derived oral cell lines reveals loss of spatial regulation of CD44 and p75 NTR in malignancy. J. Oral. Pathol. Med. 2013, 42, 37–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herfs, M.; Soong, T.R.; Delvenne, P.; Crum, C.P. Deciphering the Multifactorial Susceptibility of Mucosal Junction Cells to HPV Infection and Related Carcinogenesis. Viruses 2017, 9, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Israr, M.; Biryukov, J.; Ryndock, E.J.; Alam, S.; Meyers, C. Comparison of human papillomavirus type 16 replication in tonsil and foreskin epithelia. Virology 2016, 499, 82–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyford-Pike, S.; Peng, S.; Young, G.D.; Taube, J.M.; Westra, W.H.; Akpeng, B.; Bruno, T.C.; Richmon, J.D.; Wang, H.; Bishop, J.A.; et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 2013, 73, 1733–1741. [Google Scholar] [CrossRef] [Green Version]
- Ward, M.J.; Thirdborough, S.M.; Mellows, T.; Riley, C.; Harris, S.; Suchak, K.; Webb, A.; Hampton, C.; Patel, N.N.; Randall, C.J.; et al. Tumour-infiltrating lymphocytes predict for outcome in HPV-positive oropharyngeal cancer. Br. J. Cancer 2014, 110, 489–500. [Google Scholar] [CrossRef] [Green Version]
- Hoang, L.N.; Park, K.J.; Soslow, R.A.; Murali, R. Squamous precursor lesions of the vulva: Current classification and diagnostic challenges. Pathology 2016, 48, 291–302. [Google Scholar] [CrossRef] [Green Version]
- Alkatout, I.; Schubert, M.; Garbrecht, N.; Weigel, M.T.; Jonat, W.; Mundhenke, C.; Gunther, V. Vulvar cancer: Epidemiology, clinical presentation, and management options. Int. J. Womens Health 2015, 7, 305–313. [Google Scholar] [CrossRef] [Green Version]
- Del Pino, M.; Rodriguez-Carunchio, L.; Ordi, J. Pathways of vulvar intraepithelial neoplasia and squamous cell carcinoma. Histopathology 2013, 62, 161–175. [Google Scholar] [CrossRef]
- Bleeker, M.C.; Visser, P.J.; Overbeek, L.I.; van Beurden, M.; Berkhof, J. Lichen Sclerosus: Incidence and Risk of Vulvar Squamous Cell Carcinoma. Cancer Epidemiol. Biomarkers Prev. 2016, 25, 1224–1230. [Google Scholar] [CrossRef] [Green Version]
- Dongre, H.; Rana, N.; Fromreide, S.; Rajthala, S.; Boe Engelsen, I.; Paradis, J.; Gutkind, J.S.; Vintermyr, O.K.; Johannessen, A.C.; Bjorge, L.; et al. Establishment of a novel cancer cell line derived from vulvar carcinoma associated with lichen sclerosus exhibiting a fibroblast–dependent tumorigenic potential. Exp. Cell Res. 2020, 386, 111684. [Google Scholar] [CrossRef]
- Van Staveren, W.C.; Solis, D.Y.; Hebrant, A.; Detours, V.; Dumont, J.E.; Maenhaut, C. Human cancer cell lines: Experimental models for cancer cells in situ? For cancer stem cells? Biochim. Biophys. Acta 2009, 1795, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Jung, J. Human tumor xenograft models for preclinical assessment of anticancer drug development. Toxicol. Res. 2014, 30, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merbah, M.; Introini, A.; Fitzgerald, W.; Grivel, J.C.; Lisco, A.; Vanpouille, C.; Margolis, L. Cervico-vaginal tissue ex vivo as a model to study early events in HIV-1 infection. Am. J. Reprod. Immunol. 2011, 65, 268–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.; Geng, L.; Zhao, L.; Zuo, P.; Wang, J. Human papillomavirus E6-regulated microRNA-20b promotes invasion in cervical cancer by targeting tissue inhibitor of metalloproteinase 2. Mol. Med. Rep. 2017, 16, 5464–5470. [Google Scholar] [CrossRef]
- Yang, E.J.; Quick, M.C.; Hanamornroongruang, S.; Lai, K.; Doyle, L.A.; McKeon, F.D.; Xian, W.; Crum, C.P.; Herfs, M. Microanatomy of the cervical and anorectal squamocolumnar junctions: A proposed model for anatomical differences in HPV-related cancer risk. Mod. Pathol. 2015, 28, 994–1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genther, S.M.; Sterling, S.; Duensing, S.; Munger, K.; Sattler, C.; Lambert, P.F. Quantitative role of the human papillomavirus type 16 E5 gene during the productive stage of the viral life cycle. J. Virol. 2003, 77, 2832–2842. [Google Scholar] [CrossRef] [Green Version]
- Wechsler, E.I.; Tugizov, S.; Herrera, R.; Da Costa, M.; Palefsky, J.M. E5 can be expressed in anal cancer and leads to epidermal growth factor receptor–induced invasion in a human papillomavirus 16-transformed anal epithelial cell line. J. Gen. Virol. 2018, 99, 631–644. [Google Scholar] [CrossRef]
- Ganor, Y.; Zhou, Z.; Bodo, J.; Tudor, D.; Leibowitch, J.; Mathez, D.; Schmitt, A.; Vacher–Lavenu, M.C.; Revol, M.; Bomsel, M. The adult penile urethra is a novel entry site for HIV–1 that preferentially targets resident urethral macrophages. Mucosal. Immunol. 2013, 6, 776–786. [Google Scholar] [CrossRef]
- Ma, L.M.; Wang, Z.; Wang, H.; Li, R.S.; Zhou, J.; Liu, B.C.; Baskin, L.S. Estrogen effects on fetal penile and urethral development in organotypic mouse genital tubercle culture. J. Urol. 2009, 182, 2511–2517. [Google Scholar] [CrossRef]
- Pfister, H. Chapter 8: Human papillomavirus and skin cancer. J. Natl. Cancer Inst. Monogr. 2003, 31, 52–56. [Google Scholar] [CrossRef] [Green Version]
- Harwood, C.A.; McGregor, J.M.; Proby, C.M.; Breuer, J. Human papillomavirus and the development of non-melanoma skin cancer. J. Clin. Pathol. 1999, 52, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Akgul, B.; Cooke, J.C.; Storey, A. HPV-associated skin disease. J. Pathol. 2006, 208, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Akgul, B.; Garcia-Escudero, R.; Ghali, L.; Pfister, H.J.; Fuchs, P.G.; Navsaria, H.; Storey, A. The E7 protein of cutaneous human papillomavirus type 8 causes invasion of human keratinocytes into the dermis in organotypic cultures of skin. Cancer Res. 2005, 65, 2216–2223. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Cell Lines | HPV Types | Physical State | References |
|---|---|---|---|
| Epithelial Cells | |||
| SiHa | HPV16 | Int. | [61,64] |
| CaSki | HPV16/18 | Int. | [62,64] |
| HeLa | HPV18 | Int. | [63,64] |
| W12 | HPV16 | Int./Epi. | [70,71] |
| C-33a | - | - | [65] |
| NIKS | HPV16 | Epi. | [68] |
| Primary HFK | HPVs | Int. or Epi. | [72,73,74,75,76,77] |
| Primary HCK | HPVs | Int. or Epi. | [82,83,84] |
| Stromal cells | |||
| 3t3J2 fibroblasts | - | - | [90] |
| Primary HFF | - | - | [91,92,93] |
| Primary HCF | - | - | [83,84,95,106] |
| Cell Culture Systems | Advantages | Limitations | References |
|---|---|---|---|
2D cell culture | Simplified model/ Possibility of making co-culture/ Cost effective/ Easy to use | Inability to reproduce HPV life cycle/ Short time culture/ Low biological relevance/ | [56,57,58,61,62,63,65,66,70,106] |
Air-liquid interface culture | Recapitulate pluristratified epithelium/ Cell-to-cell-interactions/ HPV genetic studies/ Convenient/ Easy to use | Lack of connective tissue/ Genetic manipulation epithelial cell-dependent/ | [122,123] |
3D collagen cell culture | Resemble the epithelial architecture and differentiation/ Cell-to-ECM signaling/ More complex culture system/ | Hydrogel composition differs to real ECM/ HPV genetic studies epithelial cells-dependent/ Added expensive/ | [49,50,51,52,54,55,59,60,64,67,68,71,74,75,76,77,82] |
Complex 3D organotypic models | More accurate culture systems/ Cell-virus-ECM interactions/ ECM complexity/ Patient-specific models/ Long-time culture (3–4 weeks)/ Useful drug testing platform/ Useful for Invasion studies/ | Differences between specimens/ Endpoint assays (genomic, proteomic, metabolomics) dependent of cell culture used/ More expensive/ | [83,84,95,99] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Gregorio, V.; Urciuolo, F.; Netti, P.A.; Imparato, G. In Vitro Organotypic Systems to Model Tumor Microenvironment in Human Papillomavirus (HPV)-Related Cancers. Cancers 2020, 12, 1150. https://doi.org/10.3390/cancers12051150
De Gregorio V, Urciuolo F, Netti PA, Imparato G. In Vitro Organotypic Systems to Model Tumor Microenvironment in Human Papillomavirus (HPV)-Related Cancers. Cancers. 2020; 12(5):1150. https://doi.org/10.3390/cancers12051150
Chicago/Turabian StyleDe Gregorio, Vincenza, Francesco Urciuolo, Paolo Antonio Netti, and Giorgia Imparato. 2020. "In Vitro Organotypic Systems to Model Tumor Microenvironment in Human Papillomavirus (HPV)-Related Cancers" Cancers 12, no. 5: 1150. https://doi.org/10.3390/cancers12051150