Novel Molecular Targets for Tumor-Specific Imaging of Epithelial Ovarian Cancer Metastases
Abstract
:1. Introduction
2. Results
2.1. Patient Selection and Clinicopathological Characteristics
2.2. Evaluation of EGFR, VEGF-A and L1CAM Expression
2.3. Expression of EpCAM, αvβ6 and FRα in Tumor-Negative Tissues
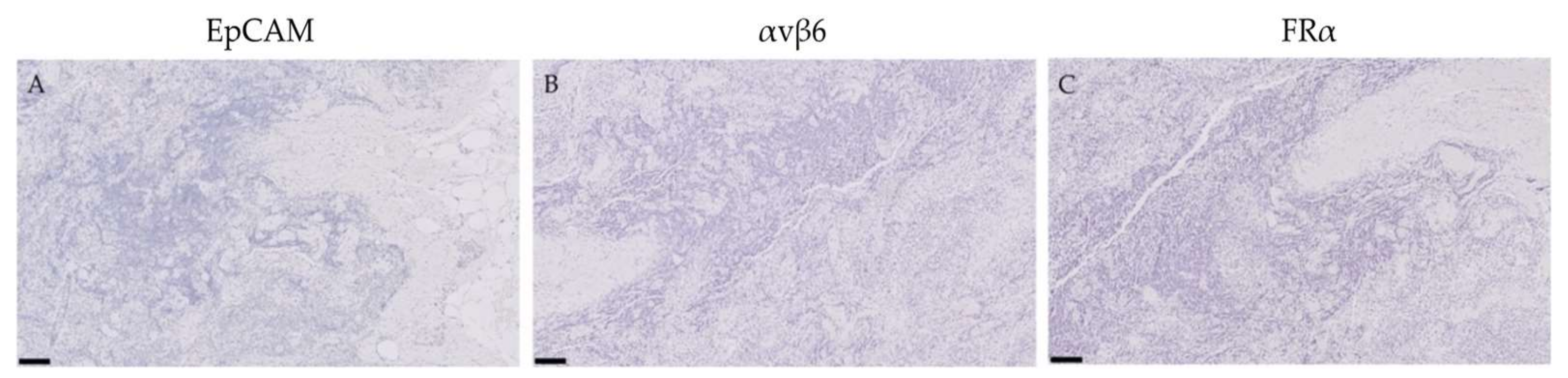
2.4. Expression of EpCAM, αvβ6 and FRα in Lymph Nodes False-Positively Illuminated with OTL-38
2.5. Expression of EpCAM, αvβ6 and FRα in Primary Ovarian Tumors and Metastases
2.6. Lymph Node Detection Accuracy of EpCAM, αvβ6 and FRα
2.7. Biomarker Expression after NACT
3. Discussion
4. Materials and Methods
4.1. Patient and Tissue Selection
4.2. Immunohistochemistry
4.3. Evaluation of Immunoreactivity
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Antibody | Clone Number | Species | Monoclonal/Polyclonal | Stock Concentration | Dilution | Company |
|---|---|---|---|---|---|---|
| Primary antibodies | ||||||
| Anti-EGFR | E30 | Mouse | Monoclonal | 286 µg/mL | 1:100 | Dako, Glostrup, Denmark |
| Anti-VEGF-A | RB9031 | Rabbit | Monoclonal | 200 µg/mL | 1:800 | Thermo Fisher Scientific, Waltham, MA, USA |
| Anti-L1CAM | 14.10 | Mouse | Monoclonal | 0.5 mg/mL | 1:500 | BioLegend, San Diego, CA, USA |
| Anti-EpCAM | 323/A3 | Mouse | Monoclonal | 0.4 mg/mL | 1:1600 | Department of Pathology, LUMC, The Netherlands |
| Anti-αvβ6 | 6.2A1 | Mouse | Monoclonal | 50 µg/mL | 1:100 | Biogen Idec MA Inc., Cambridge, MA, USA |
| Anti-FR-α | BRI 4006K AA (kit) | Mouse | Monoclonal | N/A | Ready-to-use | Biocare Medical, Pacheco, CA, USA |
Appendix B

References
- Du Bois, A.; Reuss, A.; Pujade-Lauraine, E.; Harter, P.; Ray-Coquard, I.; Pfisterer, J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: A combined exploratory analysis of 3 prospective randomized phase 3 multicenter trials. Cancer 2009, 115, 1234–1244. [Google Scholar] [CrossRef] [PubMed]
- Mutch, D.G.; Prat, J. 2014 FIGO staging for ovarian, fallopian tube and peritoneal cancer. Gynecol. Oncol. 2014, 133, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Reid, B.M.; Permuth, J.B.; Sellers, T.A. Epidemiology of ovarian cancer: A review. Cancer Biol. Med. 2017, 14, 9–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trimbos, B.; Timmers, P.; Pecorelli, S.; Coens, C.; Ven, K.; van der Burg, M.; Casado, A. Surgical staging and treatment of early ovarian cancer: Long-term analysis from a randomized trial. J. Natl. Cancer Inst. 2010, 102, 982–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, A.A.; Bohlke, K.; Armstrong, D.K.; Bookman, M.A.; Cliby, W.A.; Coleman, R.L.; Dizon, D.S.; Kash, J.J.; Meyer, L.A.; Moore, K.N.; et al. Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology Clinical Practice Guideline. Gynecol. Oncol. 2016, 143, 3–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hacker, N.F.; Rao, A. Surgery for advanced epithelial ovarian cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 41, 71–87. [Google Scholar] [CrossRef]
- Van Dam, G.M.; Themelis, G.; Crane, L.M.A.; Harlaar, N.J.; Pleijhuis, R.G.; Kelder, W.; Sarantopoulos, A.; de Jong, J.S.; Arts, H.J.G.; van der Zee, A.G.J.; et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-α targeting: First in-human results. Nat. Med. 2011, 17, 1315. [Google Scholar] [CrossRef]
- Gill, S.E.; McGree, M.E.; Weaver, A.L.; Cliby, W.A.; Langstraat, C.L. Optimizing the treatment of ovarian cancer: Neoadjuvant chemotherapy and interval debulking versus primary debulking surgery for epithelial ovarian cancers likely to have suboptimal resection. Gynecol. Oncol. 2017, 144, 266–273. [Google Scholar] [CrossRef]
- Spizzo, G.; Fong, D.; Wurm, M.; Ensinger, C.; Obrist, P.; Hofer, C.; Mazzoleni, G.; Gastl, G.; Went, P. EpCAM expression in primary tumour tissues and metastases: An immunohistochemical analysis. J. Clin. Pathol. 2011, 64, 415–420. [Google Scholar] [CrossRef] [Green Version]
- Vahrmeijer, A.L.; Hutteman, M.; van der Vorst, J.R.; van de Velde, C.J.; Frangioni, J.V. Image-guided cancer surgery using near-infrared fluorescence. Nat. Rev. Clin. Oncol 2013, 10, 507–518. [Google Scholar] [CrossRef] [Green Version]
- Hoogstins, C.E.; Tummers, Q.R.; Gaarenstroom, K.N.; de Kroon, C.D.; Trimbos, J.B.; Bosse, T.; Smit, V.T.; Vuyk, J.; van de Velde, C.J.; Cohen, A.F.; et al. A Novel Tumor-Specific Agent for Intraoperative Near-Infrared Fluorescence Imaging: A Translational Study in Healthy Volunteers and Patients with Ovarian Cancer. Clin. Cancer Res. 2016, 22, 2929–2938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mondal, S.B.; Gao, S.; Zhu, N.; Liang, R.; Gruev, V.; Achilefu, S. Real-time fluorescence image-guided oncologic surgery. Adv. Cancer Res. 2014, 124, 171–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crane, L.M.; Arts, H.J.; van Oosten, M.; Low, P.S.; van der Zee, A.G.; van Dam, G.M.; Bart, J. The effect of chemotherapy on expression of folate receptor-alpha in ovarian cancer. Cell Oncol. 2012, 35, 9–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Despierre, E.; Lambrechts, S.; Leunen, K.; Berteloot, P.; Neven, P.; Amant, F.; O’Shannessy, D.J.; Somers, E.B.; Vergote, I. Folate receptor alpha (FRA) expression remains unchanged in epithelial ovarian and endometrial cancer after chemotherapy. Gynecol. Oncol. 2013, 130, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Giulia, F.; Luffer, S.; Kumar, R.; Wu, B.; Tavallai, M.; Bekele, R.T.; Birrer, M.J. How can molecular abnormalities influence our clinical approach. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, viii16–viii24. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Hernot, S.; van Manen, L.; Debie, P.; Mieog, J.S.D.; Vahrmeijer, A.L. Latest developments in molecular tracers for fluorescence image-guided cancer surgery. Lancet Oncol. 2019, 20, e354–e367. [Google Scholar] [CrossRef]
- Sheng, Q.; Liu, J. The therapeutic potential of targeting the EGFR family in epithelial ovarian cancer. Br. J. Cancer 2011, 104, 1241–1245. [Google Scholar] [CrossRef] [Green Version]
- Ravikumar, G.; Crasta, J.A. Vascular endothelial growth factor expression in ovarian serous carcinomas and its effect on tumor proliferation. South Asian J. Cancer 2013, 2, 87–90. [Google Scholar] [CrossRef]
- Duncan, T.J.; Al-Attar, A.; Rolland, P.; Scott, I.V.; Deen, S.; Liu, D.T.Y.; Spendlove, I.; Durrant, L.G. Vascular Endothelial Growth Factor Expression in Ovarian Cancer: A Model for Targeted Use of Novel Therapies? Clin. Cancer Res. 2008, 14, 3030–3035. [Google Scholar] [CrossRef] [Green Version]
- Bandyopadhyay, A.; Raghavan, S. Defining the role of integrin alphavbeta6 in cancer. Curr. Drug Targets 2009, 10, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Woopen, H.; Pietzner, K.; Richter, R.; Fotopoulou, C.; Joens, T.; Braicu, E.I.; Mellstedt, H.; Mahner, S.; Lindhofer, H.; Darb-Esfahani, S.; et al. Overexpression of the epithelial cell adhesion molecule is associated with a more favorable prognosis and response to platinum-based chemotherapy in ovarian cancer. J. Gynecol. Oncol. 2014, 25, 221–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bondong, S.; Kiefel, H.; Hielscher, T.; Zeimet, A.G.; Zeillinger, R.; Pils, D.; Schuster, E.; Castillo-Tong, D.C.; Cadron, I.; Vergote, I.; et al. Prognostic significance of L1CAM in ovarian cancer and its role in constitutive NF-kappaB activation. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2012, 23, 1795–1802. [Google Scholar] [CrossRef]
- Shih, I.-M.; Kurman, R.J. Ovarian Tumorigenesis: A Proposed Model Based on Morphological and Molecular Genetic Analysis. Am. J. Pathol. 2004, 164, 1511–1518. [Google Scholar] [CrossRef]
- Peres, L.C.; Cushing-Haugen, K.L.; Kobel, M.; Harris, H.R.; Berchuck, A.; Rossing, M.A.; Schildkraut, J.M.; Doherty, J.A. Invasive Epithelial Ovarian Cancer Survival by Histotype and Disease Stage. J. Natl. Cancer Inst. 2019, 111, 60–68. [Google Scholar] [CrossRef]
- Hunter, K.W.; Amin, R.; Deasy, S.; Ha, N.-H.; Wakefield, L. Genetic insights into the morass of metastatic heterogeneity. Nat. Rev. Cancer 2018, 18, 211. [Google Scholar] [CrossRef] [Green Version]
- Tayama, S.; Motohara, T.; Narantuya, D.; Li, C.; Fujimoto, K.; Sakaguchi, I.; Tashiro, H.; Saya, H.; Nagano, O.; Katabuchi, H. The impact of EpCAM expression on response to chemotherapy and clinical outcomes in patients with epithelial ovarian cancer. Oncotarget 2017, 8, 44312–44325. [Google Scholar] [CrossRef] [Green Version]
- Rutten, M.J.; Leeflang, M.M.; Kenter, G.G.; Mol, B.W.; Buist, M. Laparoscopy for diagnosing resectability of disease in patients with advanced ovarian cancer. Cochrane Database Syst. Rev. 2014, CD009786. [Google Scholar] [CrossRef] [Green Version]
- Kyriazi, S.; Kaye, S.B.; de Souza, N.M. Imaging ovarian cancer and peritoneal metastases—Current and emerging techniques. Nat. Rev. Clin. Oncol 2010, 7, 381–393. [Google Scholar] [CrossRef]
- Petrillo, M.; Vizzielli, G.; Fanfani, F.; Gallotta, V.; Cosentino, F.; Chiantera, V.; Legge, F.; Carbone, V.; Scambia, G.; Fagotti, A. Definition of a dynamic laparoscopic model for the prediction of incomplete cytoreduction in advanced epithelial ovarian cancer: Proof of a concept. Gynecol. Oncol. 2015, 139, 5–9. [Google Scholar] [CrossRef]
- Van de Vrie, R.; van Meurs, H.S.; Rutten, M.J.; Naaktgeboren, C.A.; Opmeer, B.C.; Gaarenstroom, K.N.; van Gorp, T.; Ter Brugge, H.G.; Hofhuis, W.; Schreuder, H.W.R.; et al. Cost-effectiveness of laparoscopy as diagnostic tool before primary cytoreductive surgery in ovarian cancer. Gynecol. Oncol. 2017, 146, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Mehner, C.; Oberg, A.L.; Goergen, K.M.; Kalli, K.R.; Maurer, M.J.; Nassar, A.; Goode, E.L.; Keeney, G.L.; Jatoi, A.; Radisky, D.C.; et al. EGFR as a prognostic biomarker and therapeutic target in ovarian cancer: Evaluation of patient cohort and literature review. Genes Cancer 2017, 8, 589–599. [Google Scholar] [CrossRef] [PubMed]






| Clinicopathological Characteristics | Primary Debulking (42) | Interval Debulking (42) |
|---|---|---|
| Age in years, median (range) | 65 (29–83) | 67 (30–88) |
| FIGO 1 stage [2] | ||
| IIB | 3 | 0 |
| IIIA | 1 | 0 |
| IIIB | 6 | 1 |
| IIIC | 31 | 25 |
| IV | 1 | 16 |
| Histological subtype | ||
| HGSC 2 | 32 | 41 |
| Other 3 | 10 | 1 |
| LGSC 4 | 1 | 0 |
| Endometrioid adenocarcinoma | 4 | 0 |
| Clear cell carcinoma | 1 | 1 |
| Mucinous adenocarcinoma | 4 | 0 |
| BRCA carrier status | ||
| Positive, BRCA1 | 5 | 4 |
| Positive, BRCA2 | 1 | 1 |
| Negative | 16 | 20 |
| Unknown | 20 | 17 |
| Primary Debulking | |||||
|---|---|---|---|---|---|
| Biomarker | Sensitivity | Specificity | PPV 1 | NPV 2 | AUC 3 (95% CI) |
| EpCAM | 88% | 100% | 100% | 94% | 0.97 (0.90 to 1.00) |
| αvβ6 | 33% | 100% | 100% | 71% | 0.86 (0.70 to 1.00) |
| FRα | 88% | 100% | 100% | 94% | 0.97 (0.90 to 1.00) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Muynck, L.D.A.N.; Gaarenstroom, K.N.; Sier, C.F.M.; van Duijvenvoorde, M.; Bosse, T.; Mieog, J.S.D.; de Kroon, C.D.; Vahrmeijer, A.L.; Peters, I.T.A. Novel Molecular Targets for Tumor-Specific Imaging of Epithelial Ovarian Cancer Metastases. Cancers 2020, 12, 1562. https://doi.org/10.3390/cancers12061562
de Muynck LDAN, Gaarenstroom KN, Sier CFM, van Duijvenvoorde M, Bosse T, Mieog JSD, de Kroon CD, Vahrmeijer AL, Peters ITA. Novel Molecular Targets for Tumor-Specific Imaging of Epithelial Ovarian Cancer Metastases. Cancers. 2020; 12(6):1562. https://doi.org/10.3390/cancers12061562
Chicago/Turabian Stylede Muynck, Lysanne D. A. N., Katja N. Gaarenstroom, Cornelis F. M. Sier, Maurice van Duijvenvoorde, Tjalling Bosse, J. Sven D. Mieog, Cornelis D. de Kroon, Alexander L. Vahrmeijer, and Inge T. A. Peters. 2020. "Novel Molecular Targets for Tumor-Specific Imaging of Epithelial Ovarian Cancer Metastases" Cancers 12, no. 6: 1562. https://doi.org/10.3390/cancers12061562





