Cancer and SARS-CoV-2 Infection: Diagnostic and Therapeutic Challenges
Abstract
:1. Introduction
Incidence and Severity of SARS-CoV2 Infection in Cancer Patients
2. Pathophysiology of SARS-CoV 2 Infection in Cancer Patients
3. Diagnostic Challenges in the Recognition of SARS-CoV-2 Infection and Neoplastic Disease in Cancer-Infected Patients
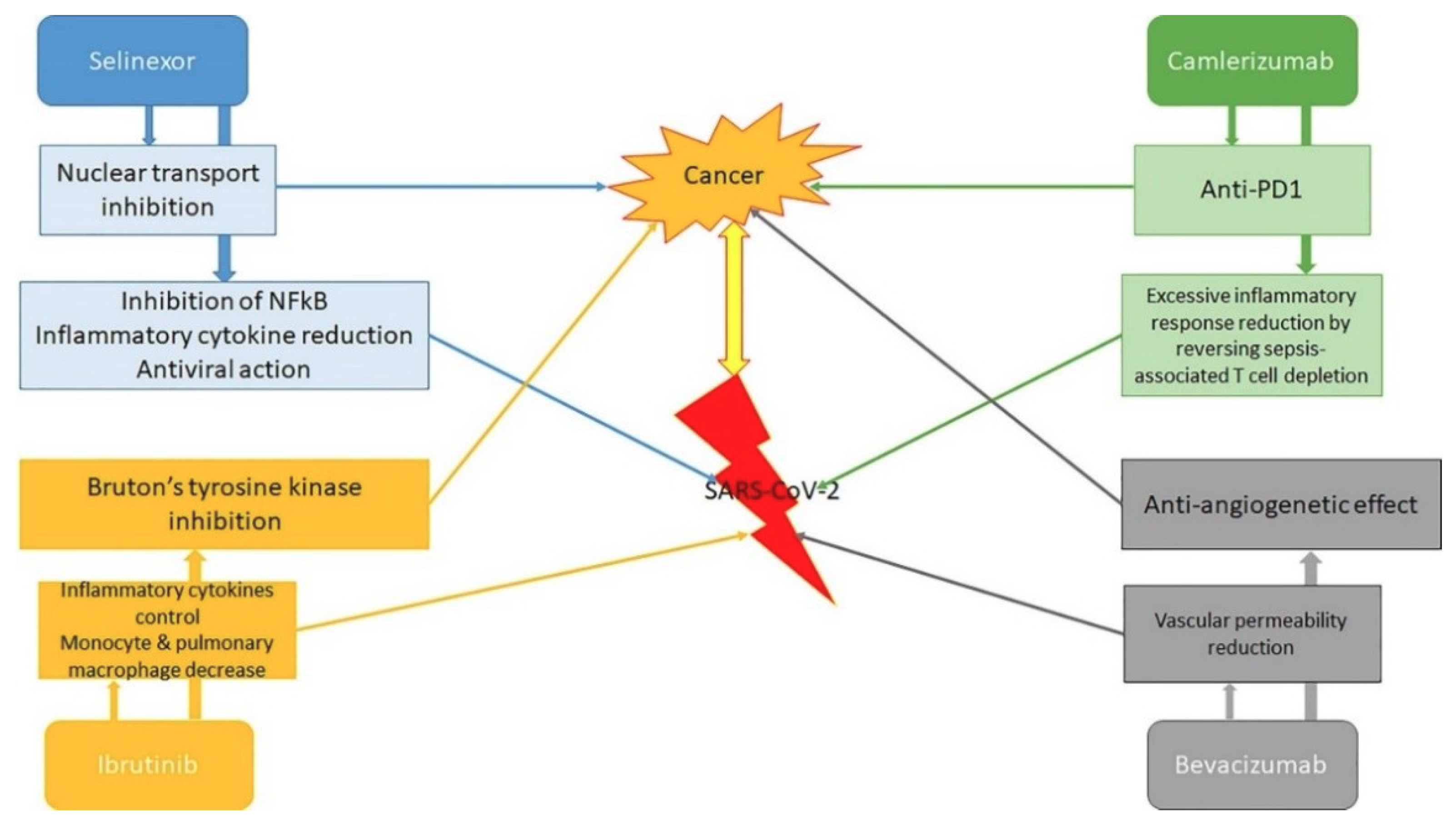
4. Antineoplastic and Antiviral Therapy in Infected Patients with Neoplasia
5. The Management of Cancer Patients with SARS-CoV2 Infection
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). 16–24 February 2020. Available online: https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf (accessed on 7 April 2020).
- Chen, W.; Zheng, R.; Baade, P.D.; Zhang, S.; Zeng, H.; Bray, F.; Jemal, A.; Yu, X.Q.; He, J. Cancer statistics in China, 2015. CA Cancer J. Clin. 2016, 66, 115–132. [Google Scholar] [CrossRef] [Green Version]
- Kamboj, M.; Sepkowitz, K.A. Nosocomial infections in patients with cancer. Lancet Oncol. 2009, 10, 589–597. [Google Scholar] [CrossRef]
- Ganatra, S.; Hammond, S.P.; Nohria, A. The novel coronavirus disease (COVID-19) threat for patients with cardiovascular disease and cancer. JACC CardioOncol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Allegra, A.; Musolino, C.; Tonacci, A.; Pioggia, G.; Gangemi, S. Interactions between the microRNAs and microbiota in cancer development: Roles and therapeutic opportunities. Cancers 2020, 12, 805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allegra, A.; Innao, V.; Allegra, A.G.; Ettari, R.; Pugliese, M.; Pulvirenti, N.; Musolino, C. Role of the microbiota in hematologic malignancies. Neth. J. Med. 2019, 77, 67–80. [Google Scholar]
- Jazieh, A.-R.; Alenazi, T.H.; Alhejazi, A.; Al Safi, F.; Al Olayan, A. Outcome of oncology patients infected with coronavirus. JCO Glob. Oncol. 2020, 6, 471–475. [Google Scholar] [CrossRef]
- Chowell, G.; Ayala, A.; Berisha, V.; Viboud, C.; Schumacher, M. Risk factors for mortality among 2009 A/H1N1 INflUENZA HOSPITALIZATIONs in Maricopa County, Arizona, April 2009 to March 2010. Comput. Math. Methods Med. 2012, 2012, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Lai, A.G.; Pasea, L.; Banerjee, A.; Denaxas, S.; Katsoulis, M.; Chang, W.H.; Williams, B.; Pillay, D.; Noursadeghi, M.; Linch, D.; et al. Estimating excess mortality in people with cancer and multimorbidity in the COVID-19 emergency. medRxiv 2020. [Google Scholar] [CrossRef]
- Liang, W.; Guan, W.; Chen, R.; Wang, W.; Li, J.; Xu, K.; Li, C.; Ai, Q.; Lu, W.; Liang, H.; et al. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol. 2020, 21, 335–337. [Google Scholar] [CrossRef]
- WorldOMeter. COVID-19 Coronavirus Pandemic. 2020. Available online: www.worldometers.info/coronavirus/ (accessed on 21 March 2010).
- Hrusak, O.; Kalina, T.; Wolf, J.; Balduzzi, A.; Provenzi, M.; Rizzari, C.; Rives, S.; del Pozo Carlavilla, M.; Alonso, V.M.E.; Domínguez Pinilla, N.; et al. Flash survey on SARS-CoV-2 infections in pediatric patients on anticancer treatment. Eur. J. Cancer 2020. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Ouyang, W.; Chua, M.L.K.; Xie, C. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. 2020, e200980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Deng, G.; Yin, M.; Chen, X.; Zeng, F. Clinical determinants for fatality of 44,672 patients with COVID-19. Crit. Care. 2020, 24, 179. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Chen, L.; Chen, L.; Yuan, G.; Fang, Y.; Chen, W.; Wu, D.; Liang, B.; Lu, X.; Ma, Y.; et al. COVID-19 in persons with haematological cancers. Leukemia 2020, 1–9. [Google Scholar] [CrossRef]
- Palmieri, L.; Andrianou, X.; Barbariol, P.; Bella, A.; Bellino, S.; Benelli, E.; Members of the COVID-19 Surveillance Group. Characteristics of COVID-19 Patients Dying in Italy. Report Based on Available Data on March 20th. 2020. Available online: https://www.epicentro.iss.it/coronavirus/bollettino/Report-COVID-2019_20_marzo_eng.pdf (accessed on 20 March 2020).
- Onder, G.; Rezza, G.; Brusaferro, S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA 2020. [Google Scholar] [CrossRef] [PubMed]
- Balduzzi, A.; Brivio, E.; Rovelli, A.; Rizzari, C.; Gasperini, S.; Melzi, M.L.; Conter, V.; Biondi, A. Lessons after the early management of the COVID-19 outbreak in a paediatric transplant and haemato-oncology centre embedded within a COVID-19 dedicated hospital in Lombardia, Italy. Bone Marrow Transplant. 2020, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Miyashita, H.; Mikami, T.; Chopra, N.; Yamada, T.; Chernyavsk, S.; Dahlia Rizk, D.; Cruz, C. Do patients with cancer have a poorer prognosis of COVID-19? An experience in New York City. Ann. Oncol. 2020. [Google Scholar] [CrossRef]
- Feng, R.-M.; Zong, Y.-N.; Cao, S.-M.; Xu, R.-H. Current cancer situation in China: Good or bad news from the 2018 global cancer statistics? Cancer Commun. 2019, 39, 22. [Google Scholar] [CrossRef] [Green Version]
- Dai, M.; Liu, D.; Liu, M.; Zhou, F.; Li, G.; Chen, Z.; Zhang, Z.; You, H.; Wu, M.; Zheng, Q.; et al. Patients with cancer appear more vulnerable to SARS-COV-2: A multi-center study during the COVID-19 outbreak. Cancer Discov. 2020. CD-20-0422. [Google Scholar] [CrossRef]
- Bhatraju, P.K.; Ghassemieh, B.J.; Nichols, M.; Kim, R.; Jerome, K.R.; Nalla, A.K.; Greninger, A.L.; Pipavath, S.; Wurfel, M.M.; Evans, L.; et al. Covid-19 in critically ill patients in the Seattle region—Case series. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- Seneff, M.G.; Zimmerman, J.E.; Knaus, W.A.; Wagner, D.P.; Draper, E.A. Predicting the duration of mechanical ventilation. The importance of disease and patient characteristics. Chest 1996, 110, 469–479. [Google Scholar] [CrossRef] [PubMed]
- ICNARC COVID-19 Study Case Mix Programme Database. ICNARC Report on COVID-19 in Critical Care. 2020. Available online: https://www.icnarc.org/About/Latest-News/2020/04/04/Report-On2249-Patients-Critically-Ill-With-Covid-19 (accessed on 27 March 2020).
- Janka, G.E.; Lehmberg, K. Hemophagocytic syndromes—An update. Blood Rev. 2014, 28, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Song, C.; Xu, C.; Jin, G.; Chen, Y.; Xu, X.; Ma, H.; Chen, W.; Lin, Y.; Zheng, Y.; et al. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci. China Life Sci. 2020, 63, 706–711. [Google Scholar] [CrossRef] [Green Version]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, X.; Yin, C.; Lu, S.; Chen, Y.; Liu, Q.; Bai, J.; Lu, Y. Two things about COVID-19 might need attention. Preprints 2020, 2020020315. [Google Scholar] [CrossRef]
- Yang, J.; Li, H.; Hu, S.; Zhou, Y. ACE2 correlated with immune infiltration serves as a prognostic biomarker in endometrial carcinoma and renal papillary cell carcinoma: Implication for COVID-19. Aging (Albany NY) 2020, 12, 6518–6535. [Google Scholar] [CrossRef]
- Zhang, J.J.; Dong, X.; Cao, Y.Y.; Yuan, Y.-D.; Yang, Y.-B.; Yan, Y.-Q.; Akdis, C.A.; Gao, Y.-D. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 2020. [Google Scholar] [CrossRef]
- Bao, L.; Deng, W.; Huang, B.; Gao, H.; Liu, J.; Ren, L.; Wei, Q.; Yu, P.; Xu, Y.; Qi, F.; et al. The pathogenicity of 2019 novel coronavirus in hACE2 transgenic mice. Nature 2020. [Google Scholar] [CrossRef]
- Yang, X.H.; Deng, W.; Tong, Z.; Liu, Y.-X.; Zhang, L.-F.; Zhu, H.; Gao, H.; Huang, L.; Liu, Y.-L.; Ma, C.-M.; et al. Mice transgenic for human angiotensin-converting enzyme 2 provide a model for SARS coronavirus infection. Comp. Med. 2007, 57, 450–459. [Google Scholar]
- Kong, Q.; Xiang, Z.; Wu, Y.; Gu, Y.; Guo, J.; Geng, F. Analysis of the susceptibility of lung cancer patients to SARS-CoV-2 infection. Mol. Cancer. 2020, 19, 80. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Cai, G. Bulk and single-cell transcriptomics identify tobacco-use disparity in lung gene expression of ACE2, the receptor of 2019-nCov. medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Guan, W.-J.; Ni, Z.-Y.; Hu, Y.; Liang, W.-H.; Ou, C.-Q.; He, J.-X.; Liu, L.; Shan, H.; Lei, C.-L.; Hui, D.S.C.; et al. China medical treatment expert group for Covid-19. Clinical characteristics of 2019 novel coronavirus infection in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Xia, Y.; Jin, R.; Zhao, J.; Li, W.; Shen, H. Risk of COVID-19 for patients with cancer. Lancet Oncol. 2020, 21, e180. [Google Scholar] [CrossRef]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science 2011, 331, 1565–1570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryzhakov, G.; Lai, C.C.; Blazek, K.; To, K.W.; Hussell, T.; Udalova, I. IL-17 boosts proinflammatory outcome of antiviral response in human cells. J. Immunol. 2011, 187, 5357–5362. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Jin, Y.-H.; Kang, H.S.; Kim, B.S. Interleukin-6 (IL-6) and IL-17 synergistically promote viral persistence by inhibiting cellular apoptosis and cytotoxic T cell function. J. Virol. 2014, 88, 8479–8489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patera, A.C.; Pesnicak, L.; Bertin, J.; Cohen, J.I. Interleukin 17 modulates the immune response to vaccinia virus infection. Virology 2002, 299, 56–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.T.; Melton, A.C.; Su, G.; Hamm, D.E.; LaFemina, M.; Howard, J.; Fang, X.; Bhat, S.; Huynh, K.M.; O’Kane, C.M.; et al. Unexpected role for adaptive αβTh17 cells in acute respiratory distress syndrome. J. Immunol. 2015, 195, 87–95. [Google Scholar] [CrossRef]
- Ding, P.; Zhang, S.; Yu, M.; Feng, Y.; Long, Q.; Yang, H.; Li, J.; Wang, M. IL-17A promotes the formation of deep vein thrombosis in a mouse model. Int. Immunopharmacol. 2018, 57, 132–138. [Google Scholar] [CrossRef]
- Pan, B.; Che, D.; Cao, J.; Shen, J.; Jin, S.; Zhou, Y.; Liu, F.; Gu, K.; Man, Y.; Shang, L.; et al. Interleukin-17 levels correlate with poor prognosis and vascular endothelial growth factor concentration in the serum of patients with non-small cell lung cancer. Biomarkers 2015, 20, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Shen, J.; Cao, J.; Zhou, Y.; Shang, L.; Jin, S.; Cao, S.; Che, D.; Liu, F.; Yu, Y. Interleukin-17 promotes angiogenesis by stimulating VEGF production of cancer cells via the STAT3/GIV signaling pathway in non-small-cell lung cancer. Sci. Rep. 2015, 5, 16053–16066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Yang, P.; Sun, Y.; Li, T.; Wang, C.; Wang, Z.; Zou, Z.; Yan, Y.; Wang, W.; Wang, C.; et al. IL-17 response mediates acute lung induced by the 2009 pandemic influenza A (H1N1) virus. Cell Res. 2012, 22, 528–538. [Google Scholar] [CrossRef] [Green Version]
- Fabre, J.; Giustiniani, J.; Garbar, C.; Antonicelli, F.; Merrouche, Y.; Bensussan, A.; Bagot, M.; Al-Dacak, R. Targeting the tumor microenvironment: The protumor effect of IL-17 related to cancer type. J. Mol. Sci. 2016, 17, 1433. [Google Scholar] [CrossRef] [Green Version]
- Righetti, R.F.; Dos Santos, T.M.; Camargo, L.D.N.; Aristóteles, L.R.C.R.B.; Fukuzaki, S.; de Souza, F.C.R.; Santana, F.P.R.; de Agrela, M.V.R.; Cruz, M.M.; Alonso-Vale, M.I.C.; et al. Protective effects of anti-IL17 on acute lung injury induced by LPS in mice. Front. Pharmacol. 2018, 9, 1021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mi, S.; Li, Z.; Yang, H.Z.; Liu, H.; Wang, J.P.; Ma, Y.G.; Wang, X.X.; Liu, H.Z.; Sun, W.; Hu, Z.W. Blocking IL-17A promotes the resolution of pulmonary inflammation and fibrosis via TGFbeta1-dependent and -independent mechanisms. J. Immunol. 2011, 187, 3003–3014. [Google Scholar] [CrossRef]
- Cafarotti, S. SARS-CoV2 infection and lung cancer patients: The potential role of IL17 target therapy. J. Thorac. Oncol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Yang, R.; Song, L.; Kamel, I.R. Atypical lung feature on chest CT in a lung adenocarcinoma cancer patient infected with COVID-19. Ann. Oncol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.J.; Wang, J.; He, X.H.; Qin, Y.; Yang, S.; Hu, X.S.; Wang, H.Y.; Huang, J.; Zhou, A.P.; Ma, F.; et al. The differential diagnosis of pulmonary infiltrates in cancer patients during the outbreak of the 2019 novel coronavirus disease. Zhonghua Zhong Liu Za Zhi 2020, 42, 305–311. [Google Scholar] [PubMed]
- Wei, X.; Su, J.; Yang, K.; Wei, J.; Wan, H.; Cao, X.; Tan, W.; Wang, H. Elevations of serum cancer biomarkers correlate with severity of COVID-19. J. Med. Virol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Stockley, R.A.; Shaw, J.; Whitfield, A.G.; Clarke, C.A.; Burnett, D. Effect of cigarette smoking, pulmonary inflammation, and lung disease on concentrations of carcinoembryonic antigen in serum and secretions. Thorax 1986, 41, 17–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirakata, Y.; Kobayashi, J.; Sugama, Y.; Kitamura, S. Elevation of tumour markers in serum and bronchoalveolar lavage fluid in pulmonary alveolar proteinosis. Eur. Respir. J. 1995, 8, 689–696. [Google Scholar] [PubMed]
- Barouchos, N.; Papazafiropoulou, A.; Iacovidou, N.; Vrachnis, N.; Barouchos, N.; Armeniakou, E.; Dionyssopoulou, V.; Mathioudakis, A.G.; Christopoulou, E.; Koltsida, S.; et al. Comparison of tumor markers and inflammatory biomarkers in chronic obstructive pulmonary disease (COPD) exacerbations. Scand. J. Clin. Lab. Invest. 2015, 75, 126132. [Google Scholar] [CrossRef]
- Nagy, B., Jr.; Bene, Z.; Fejes, Z.; Heltshe, S.; Reid, D.; Ronan, N.; McCarthy, Y.; Smith, D.; Nagy, A.; Joseloff, E.; et al. Human epididymis protein 4 (he4) levels inversely correlate with lung function improvement (delta fev1) in cystic fibrosis patients receiving ivacaftor treatment. J. Cystic Fibros. 2019, 18, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Akil, E.; Bulut, A.; Kaplan, I.; Özdemir, H.H.; Arslan, D.; Aluçlu, M.U. The increase of carcinoembryonic antigen (CEA), high-sensitivity c-reactive protein, and neutrophil/lymphocyte ratio in Parkinson’s disease. Neurol. Sci. 2015, 36, 423–428. [Google Scholar] [CrossRef]
- Bagaria, B.; Bagaria, A.; Sharma, R.; Sood, S.; Lalwani, S. Significance of correlation between levels of carcinoembryonic antigen and carbohydrate antigen 19-9, carcinoembryonic antigen and c-reactive protein, carcinoembryonic antigen and alpha-1 antitrypsin in gastric and colon cancer patients. Clin. Cancer Investig. J. 2014, 3, 291–298. [Google Scholar] [CrossRef]
- American Association for Cancer Research. Cancer Labs Pivot to Battle COVID-19. Cancer Discov. 2020, 10, 634. [Google Scholar] [CrossRef]
- Kourie, H.R.; Awada, G.; Awada, A.H. Learning from the ’tsunami’ of immune checkpoint inhibitors in 2015. Crit. Rev. Oncol. Hematol. 2016, 101, 213–220. [Google Scholar] [CrossRef]
- Atkins, M.B.; Plimack, E.R.; Puzanov, I.; Fishman, M.N.; McDermott, D.F.; Cho, D.C.; Vaishampayan, U.; George, S.; Olencki, T.E.; Tarazi, J.C.; et al. Axitinib in combination with pembrolizumab in patients with advanced renal cell cancer: A non-randomised, open-label, dose-finding, and dose-expansion phase 1b trial. Lancet Oncol. 2018, 19, 405–415. [Google Scholar] [CrossRef]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R.; et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N. Engl. J. Med. 2015, 373, 1803–1813. [Google Scholar] [CrossRef]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Hegg, R.; Im, S.-A.; Wright, G.S.; et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef] [PubMed]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.-Y.; Choo, S.-P.; Trojan, J.; Welling Rd, T.H.; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef]
- Ostios-Garcia, L.; Faig, J.; Leonardi, G.C.; Adeni, A.E.; Subegdjo, S.J.; Lydon, C.A.; Rangachari, D.; Huberman, M.S.; Sehgal, K.; Shea, M.; et al. Safety and efficacy of PD-1 inhibitors among HIV-positive patients with non–small cell lung cancer. J. Thorac. Oncol. 2018, 13, 1037–1042. [Google Scholar] [CrossRef] [Green Version]
- Picchi, H.; Mateus, C.; Chouaid, C.; Besse, B.; Marabelle, A.; Michot, J.M.; Champiat, S.; Voisin, A.L.; Lambotte, O. Infectious complications associated with the use of immune checkpoint inhibitors in oncology: Reactivation of tuberculosis after anti PD-1 treatment. Clin. Microbiol. Infect. 2018, 24, 216–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, D.B.; McDonnell, W.J.; Gonzalez-Ericsson, P.I.; Al-Rohil, R.; Mobley, B.C.; Salem, J.-E.; Wang, D.Y.; Sanchez, V.; Wang, Y.; Chastain, C.A.; et al. A case report of clonal EBV-like memory CD4+ T cell activation in fatal checkpoint inhibitor-induced encephalitis. Nat. Med. 2019, 25, 1243–1250. [Google Scholar] [CrossRef]
- Rassy, E.E.; Assi, T.; Rizkallah, J.; Kattan, J. Diffuse edema suggestive of cytokine release syndrome in a metastatic lung carcinoma patient treated with pembrolizumab. Immunotherapy 2017, 9, 309–311. [Google Scholar] [CrossRef]
- Bersanelli, M. Controversies about COVID-19 and anticancer treatment with immune checkpoint inhibitors. Immunotherapy 2020. [Google Scholar] [CrossRef] [Green Version]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- NHS. Clinical Guide for the Management of Cancer Patients During the Coronavirus Pandemic. 2020. Available online: www.england.nhs.uk/coronavirus/publication/specialty-guides/ (accessed on 17 March 2020).
- Kattan, J.; Kattan, C.; Assi, T. Do checkpoint inhibitors compromise the cancer patients’ immunity and increase the vulnerability to COVID-19 infection? Immunotherapy 2020, 12, 351–354. [Google Scholar] [CrossRef] [Green Version]
- Davis, A.P.; Boyer, M.; Lee, J.H.; Kao, S.C. COVID-19: The use of immunotherapy in metastatic lung cancer. Immunotherapy 2020. [Google Scholar] [CrossRef]
- Florence, J.M.; Krupa, A.; Booshehri, L.M.; Davis, S.A.; Matthay, M.A.; Kurdowska, A.K. Inhibiting Bruton’s tyrosine kinase rescues mice from lethal influenza induced acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 315, L52–L58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeffries, C.A.; Doyle, S.; Brunner, C.; Dunne, A.; Brint, E.; Wietek, C.; Walch, E.; Wirth, T.; O’Neill, L.A.J. Bruton’s tyrosine kinase is a Toll/interleukin-1 receptor domain-binding protein that participates in nuclear factor κB activation by Toll-like receptor 4. J. Biol. Chem. 2003, 278, 26258–26264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, G.; Zhou, Y.; Liu, X.; Xu, L.; Cao, Y.; Manning, R.J.; Patterson, C.J.; Buhrlage, S.J.; Gray, N.; Tai, Y.-T.; et al. A mutation in MYD88 (L265P) supports the survival of lymphoplasmacytic cells by activation of Bruton tyrosine kinase in Waldenström macroglobulinemia. Blood 2013, 122, 1222–1232. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Buhrlage, S.; Tan, L.; Liu, X.; Chen, J.; Xu, L.; Tsakmaklis, N.; Chen, J.G.; Patterson, C.J.; Brown, J.R.; et al. HCK is a survival determinant transactivated by mutated MYD88, and a direct target of ibrutinib. Blood 2016, 127, 3237–3252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Liu, L. The membrane protein of severe acute respiratory syndrome coronavirus functions as a novel cytosolic pathogen-associated molecular pattern to promote beta interferon induction via a toll-like-receptor-related TRAF3-independent mechanism. mBio 2016, 7, e01872-15. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.G.; Liu, X.; Munshi, M.; Xu, L.; Tsakmaklis, N.; Demos, M.G.; Kofides, A.; Guerrera, M.L.; Chan, G.G.; Patterson, C.J.; et al. BTKCys481Ser drives ibrutinib resistance via ERK1/2 and protects BTKwild-type MYD88-mutated cells by a paracrine mechanism. Blood 2018, 131, 2047–2059. [Google Scholar] [CrossRef] [Green Version]
- Ernst, M.; Inglese, M.; Scholz, G.M.; Harder, K.W.; Clay, F.J.; Bozinovski, S.; Waring, P.; Darwiche, R.; Kay, T.; Sly, P.; et al. Constitutive activation of the SRC family kinase HCK results in spontaneous pulmonary inflammation and an enhanced innate immune response. J. Exp. Med. 2002, 196, 589–604. [Google Scholar] [CrossRef]
- Yao, X.H.; Li, T.Y.; He, Z.C.; Ping, Y.F.; Liu, H.W.; Yu, S.C.; Mou, H.M.; Wang, L.H.; Zhang, H.R.; Fu, W.J.; et al. A pathological report of three COVID-19 cases by minimally invasive autopsies. Zhonghua Bing Li Xue Za Zhi 2020, 49, E009. [Google Scholar]
- Treon, S.P.; Castillo, J.; Skarbnik, A.P.; Soumerai, J.D.; Ghobrial, I.M.; Guerrera, M.L.; Meid, K.E.; Yang, G. The BTK-inhibitor ibrutinib may protect against pulmonary injury in COVID-19 infected patients. Blood 2020. [Google Scholar] [CrossRef] [Green Version]
- Allegra, A.; Innao, V.; Allegra, A.G.; Leanza, R.; Musolino, C. Selective inhibitors of nuclear export in the treatment of hematologic malignancies. Clin. Lymphoma Myeloma Leuk. 2019, 19, 689–698. [Google Scholar] [CrossRef]
- Karyopharm Therapeutics Inc. Karyopharm to Evaluate Low Dose Selinexor as a Potential Treatment for Hospitalized Patients with COVID-19; Karyopharm Therapeutics Inc.: Newton, MA, USA, 2020; Available online: https://bit.ly/2VftoVw (accessed on 7 April 2020).
- Ueda, M.; Martins, R.; Hendrie, P.C.; McDonnell, T.; Crews, J.R.; Wong, T.L.; McCreery, B.; Jagels, B.; Crane, A.; Byrd, D.R.; et al. Managing cancer care during the COVID-19 pandemic: Agility and collaboration toward a common goal. J. Natl. Compr. Cancer Netw. 2020, 18, 366–369. [Google Scholar] [CrossRef] [Green Version]
- UK Coronavirus Cancer Monitoring Project Team. The UK Coronavirus cancer monitoring project: Protecting patients with cancer in the era of COVID-19. Lancet Oncol. 2020, 21, 622–624. [Google Scholar] [CrossRef]
- Kutikov, A.; Weinberg, D.S.; Edelman, M.J.; Horwitz, E.M.; Uzzo, R.G.; Fisher, R.I. A war on two fronts: Cancer care in the time of COVID-19. Ann. Intern. Med. 2020, M20-1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Z.; Yang, C.; Li, C. Strategies for patient with cancer during COVID-19 pandemic. Asia Pac. J. Clin. Oncol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Jiang, F.; Su, W.; Chen, C.; Chen, J.; Mei, W.; Zhan, L.Y.; Jia, Y.; Zhang, L.; Liu, D.; et al. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. EClinicalMedicine 2020, 100331. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Liu, Y.; Tian, D.; Wang, C.; Wang, S.; Cheng, J.; Hu, M.; Fang, M.; Gao, Y. Potential benefits of precise corticosteroids therapy for severe 2019-nCoV pneumonia. Signal. Transduc. Target. Ther. 2020, 5, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emanuel, E.J.; Persad, G.; Upshur, R.; Thome, B.; Parker, M.; Glickman, A.; Zhang, C.; Boyle, C.; Smith, M.; Phillips, J.P. Fair allocation of scarce medical resources in the time of COVID-19. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- COVID-19 Rapid Guideline: Delivery of Systemic Anticancer Treatments. NICE Guideline. Available online: https://www.nice.org.uk/guidance/ng161/chapter/6-Prioritising-systemic-anticancer-treatments (accessed on 9 June 2020).
- Tabrizi, S.; Trippa, L.; Cagney, D.; Tanguturi, S.; Ventz, S.; Fell, G.; Wen, P.Y.; Alexander, B.M.; Rahman, R. A quantitative framework for modeling COVID-19 risk during adjuvant therapy using published randomized trials of glioblastoma in the elderly. Neuro-Oncology 2020. [Google Scholar] [CrossRef] [PubMed]
- Blandino, G. Cancer at the time of the COVID-19 hurricane. J. Exp. Clin. Cancer Res. 2020, 39, 74. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, M.A.; Hosseini, M.S. Implications for cancer care in Iran during COVID-19 pandemic. Radiother. Oncol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Mooney, M.; McCaskill-Stevens, W. Memorandum: Interim Guidance for Patients on Clinical Trials Supported by the NCI Cancer Therapy Evaluation Program and the NCI Community Oncology Research Program (NCORP); Public Health Service, U.S. Department of Health and Human Services: Washington, DC, USA, 13 March 2020; Available online: www.ncicirb.org/system/files/Interim_Guidance_Clinical_Trial_Activities_Affected_by_Novel_Coronavirus_3-13-2020_0.pdf (accessed on 18 March 2020).

| Study | Subjects | Findings |
|---|---|---|
| All/various countries/continents | ||
| Worldometer (2020) [11] | All confirmed worldwide cases (>5 million) | Critical events in 48–54% of cancer patients vs. 16% in general population; death in 5.6–29% vs. 3.4% |
| Hrusak et al. (2020) Various countries [12] | 10,000, of which 2000 children analyzed | 4.5% with tumor, of which 88.9% asymptomatic or mildly symptomatic |
| Asia | ||
| Liang et al. (2020) China [10] | 2007 | Tumor history in SARS-CoV vs. general population: 1% vs. 0.29% Subjects with tumor worsened more quickly (13 vs. 43 days until critical events) and were more critical (39% vs. 8%) |
| Yu et al. (2020) China [13] | 1524 | 0.79% with tumor in SARS-CoV 25% of those with tumor had severe respiratory syndrome, 8.3% were admitted to intensive care unit (ICU) |
| Chen et al. (2020) China [14] | 99 | Tumor history in SARS-CoV vs. general population: 1% vs. 0.2% |
| Deng et al. (2020) China [15] | 44,672 | Relative Risk=2.926 for tumors as risk factors for fatality of patients with COVID-19 |
| WHO (2020) China [1] | 75,465 | 7.6% of mortality for cancer patients, fifth highest after cardiovascular disease (13.2%), diabetes (9.2%), hypertension (8.4%), chronic respiratory disease (8.0%) |
| He et al. (2020) China [16] | 354 | 10% with tumor in SARS-CoV-2 in general public (7% in healthcare providers) More severe SARS-CoV in patients with haematological diseases |
| Europe | ||
| Palmieri et al. (2020) Italy [17] | 37,860 | 20% with tumor in SARS-CoV-2 |
| Onder et al. (2020) Italy [18] | 355 | 20.3% with tumor in SARS-CoV-2 Mortality = 20% of subjects with tumor older than 80 |
| Balduzzi et al. (2020) Italy [19] | 5 children | 100% survival (60% were paucisymptomatic) |
| Americas | ||
| Miyashita et al. (2020) USA [20] | 5688 | 6% with tumor in SARS-CoV Higher complications, higher mortality in younger patients with cancer |
| Common Features of SARS-CoV-2 and Tumor non-SARS-CoV-2 | Common Markers of SARS-CoV-2 and Tumor non-SARS-CoV-2 | Other Clinical Characteristics Common in SARS-CoV-2 and Tumor non-SARS-CoV-2 |
|---|---|---|
| Superior vena cava obstruction Lung metastasis Upper airway tumors Breathlessness Distress Fever Fatigue Dyspnea | Carbohydrate antigens Carcinoembryonic antigen Human epididymis protein 4 Cytokeratin-19 fragment CYFRA21-1 Squamous cell carcinoma antigen (only critical cases) | Hematologic malignancies |
| Priority Level | Treatment |
|---|---|
| 1 | Curative treatment with high chance of success (>50%) Adjuvant/neoadjuvant treatment which adds at least 50% chance of cure to surgery/radiotherapy alone/treatment given at relapse |
| 2 | Curative treatment with an intermediate chance of success (20–50%) Adjuvant/neoadjuvant treatment which adds 20–50% chance of cure to surgery/radiotherapy alone/treatment given at relapse |
| 3 | Curative treatment with a low chance of success (10–20%) Adjuvant/neoadjuvant treatment which adds 10–20% chance of cure to surgery/radiotherapy alone/treatment given at relapse Non-curative treatment with a high chance of more than 1 year extension (>50%) |
| 4 | Curative treatment with a very low chance of success (0–10%) Adjuvant/neoadjuvant treatment which adds less than 10% chance of cure to surgery/radiotherapy alone/treatment given at relapse Non-curative treatment with an intermediate chance of more than 1 year extension to life (15% to 50%) |
| 5 | Non-curative treatment with a high chance of palliation or temporary tumor control (>50%) and less than 1 year expected extension to life |
| 6 | Non-curative treatment with an intermediate chance of palliation or temporary tumor control (15–50%) and less than 1 year expected extension to life |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allegra, A.; Pioggia, G.; Tonacci, A.; Musolino, C.; Gangemi, S. Cancer and SARS-CoV-2 Infection: Diagnostic and Therapeutic Challenges. Cancers 2020, 12, 1581. https://doi.org/10.3390/cancers12061581
Allegra A, Pioggia G, Tonacci A, Musolino C, Gangemi S. Cancer and SARS-CoV-2 Infection: Diagnostic and Therapeutic Challenges. Cancers. 2020; 12(6):1581. https://doi.org/10.3390/cancers12061581
Chicago/Turabian StyleAllegra, Alessandro, Giovanni Pioggia, Alessandro Tonacci, Caterina Musolino, and Sebastiano Gangemi. 2020. "Cancer and SARS-CoV-2 Infection: Diagnostic and Therapeutic Challenges" Cancers 12, no. 6: 1581. https://doi.org/10.3390/cancers12061581







