Ability of Delta Radiomics to Predict a Complete Pathological Response in Patients with Loco-Regional Rectal Cancer Addressed to Neoadjuvant Chemo-Radiation and Surgery
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Series
2.2. Standard Chemo-Radiation Therapy Protocol
2.3. Magnetic Resonance Imaging
2.4. Surgery and Histopathological Assessment
2.5. Feature Extraction and TA
2.6. Long-Term Follow-Up
2.7. Variables’ Selection
2.8. Endpoints and Statistical Analysis
2.9. Training Dataset
2.10. Dataset Validation
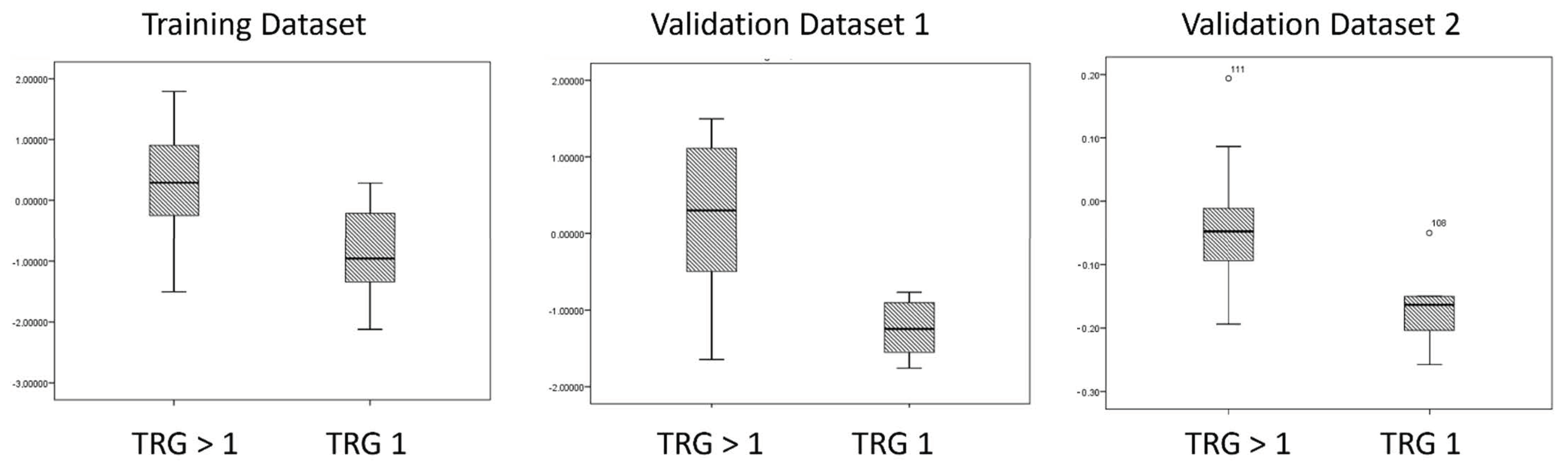
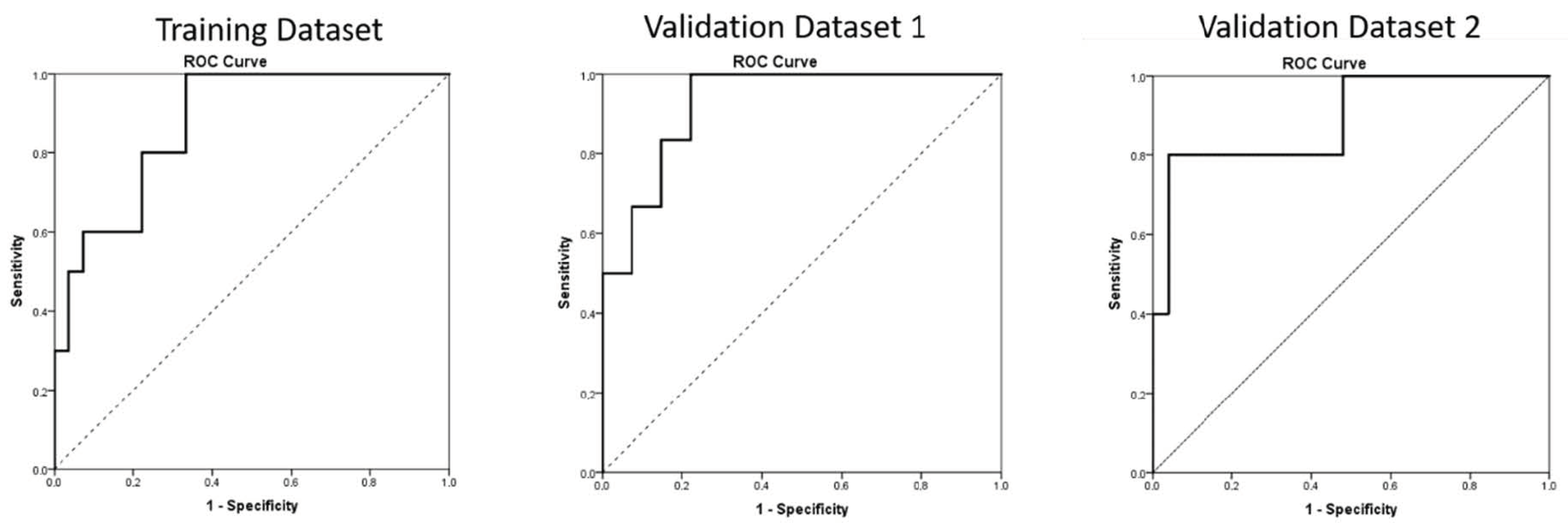
3. Results
3.1. Patients’ Features
3.2. Factors Predicting ePD
3.3. Calculation of Cut-Of
4. Discussion
Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Gijn, W.; Marijnen, C.A.; Nagtegaal, I.D.; Kranenbarg, E.M.; Putter, H.; Wiggers, T.; Rutten, H.J.; Pahlman, L.; Glimelius, B.; van de Velde, C.J.; et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011, 12, 575–582. [Google Scholar] [CrossRef]
- Sauer, R.; Liersch, T.; Merkel, S.; Fietkau, R.; Hohenberger, W.; Hess, C.; Becker, H.; Raab, H.R.; Villanueva, M.T.; Witzigmann, H.; et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: Results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J. Clin. Oncol. 2012, 30, 1926–1933. [Google Scholar] [CrossRef] [PubMed]
- Sebag-Montefiore, D.; Stephens, R.J.; Steele, R.; Monson, J.; Grieve, R.; Khanna, S.; Quirke, P.; Couture, J.; de Metz, C.; Myint, A.S.; et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): A multicentre, randomised trial. Lancet 2009, 373, 811–820. [Google Scholar] [CrossRef] [Green Version]
- Granata, V.; Faggioni, L.; Grassi, R.; Fusco, R.; Reginelli, A.; Rega, D.; Maggialetti, N.; Buccicardi, D.; Frittoli, B.; Rengo, M.; et al. Structured reporting of computed tomography in the staging of colon cancer: A Delphi consensus proposal. Radiol. Med. 2022, 127, 21–29. [Google Scholar] [CrossRef]
- Link, K.H.; Kornmann, M.; Staib, L.; Kreuser, E.D.; Gaus, W.; Röttinger, E.; Suhr, P.; Maulbecker-Armstrong, C.; Danenberg, P.; Danenberg, K.; et al. Patient-centered developments in colon- and rectal cancer with a multidisciplinary international team: From translational research to national guidelines. World J. Gastrointest. Surg. 2021, 13, 1597–1614. [Google Scholar] [CrossRef] [PubMed]
- Milone, M.; Adamina, M.; Arezzo, A.; Bejinariu, N.; Boni, L.; Bouvy, N.; de Lacy, F.B.; Dresen, R.; Ferentinos, K.; Francis, N.K.; et al. UEG and EAES rapid guideline: Systematic review, meta-analysis, GRADE assessment and evidence-informed European recommendations on TaTME for rectal cancer. Surg. Endosc. 2022, 36, 2221–2232. [Google Scholar] [CrossRef]
- van de Velde, C.J.; Boelens, P.G.; Borras, J.M.; Coebergh, J.W.; Cervantes, A.; Blomqvist, L.; Beets-Tan, R.G.; van den Broek, C.B.; Brown, G.; Van Cutsem, E.; et al. EURECCA colorectal: Multidisciplinary management: European consensus conference colon & rectum. Eur. J. Cancer 2014, 50, 1.e1–1.e34. [Google Scholar] [CrossRef]
- Kapiteijn, E.; Marijnen, C.A.; Nagtegaal, I.D.; Putter, H.; Steup, W.H.; Wiggers, T.; Rutten, H.J.; Pahlman, L.; Glimelius, B.; van Krieken, J.H.; et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N. Engl. J. Med. 2001, 345, 638–646. [Google Scholar] [CrossRef] [Green Version]
- Vendrely, V.; Rivin Del Campo, E.; Modesto, A.; Jolnerowski, M.; Meillan, N.; Chiavassa, S.; Serre, A.A.; Gérard, J.P.; Créhanges, G.; Huguet, F.; et al. Rectal cancer radiotherapy. Cancer Radiothérapie 2022, 26, 272–278. [Google Scholar] [CrossRef]
- Maas, M.; Nelemans, P.J.; Valentini, V.; Das, P.; Rodel, C.; Kuo, L.J.; Calvo, F.A.; Garcia-Aguilar, J.; Glynne-Jones, R.; Haustermans, K.; et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: A pooled analysis of individual patient data. Lancet Oncol. 2010, 11, 835–844. [Google Scholar] [CrossRef]
- Kennedy, E.; Zwaal, C.; Asmis, T.; Cho, C.; Galica, J.; Ginty, A.; Govindarajan, A. An Evidence-Based Guideline for Surveillance of Patients after Curative Treatment for Colon and Rectal Cancer. Curr. Oncol. 2022, 29, 724–740. [Google Scholar] [CrossRef] [PubMed]
- Bertocchi, E.; Barugola, G.; Nicosia, L.; Mazzola, R.; Ricchetti, F.; Dell’Abate, P.; Alongi, F.; Ruffo, G. A comparative analysis between radiation dose intensification and conventional fractionation in neoadjuvant locally advanced rectal cancer: A monocentric prospective observational study. Radiol. Med. 2020, 125, 990–998. [Google Scholar] [CrossRef] [PubMed]
- Munk, N.E.; Bondeven, P.; Pedersen, B.G. Diagnostic performance of MRI and endoscopy for assessing complete response in rectal cancer after neoadjuvant chemoradiotherapy: A systematic review of the literature. Acta Radiol. 2021, 2841851211065925. [Google Scholar] [CrossRef] [PubMed]
- Petralia, G.; Zugni, F.; Summers, P.E.; Colombo, A.; Pricolo, P.; Grazioli, L.; Colagrande, S.; Giovagnoni, A.; Padhani, A.R. Whole-body magnetic resonance imaging (WB-MRI) for cancer screening: Recommendations for use. Radiol. Med. 2021, 126, 1434–1450. [Google Scholar] [CrossRef]
- Santone, A.; Brunese, M.C.; Donnarumma, F.; Guerriero, P.; Mercaldo, F.; Reginelli, A.; Miele, V.; Giovagnoni, A.; Brunese, L. Radiomic features for prostate cancer grade detection through formal verification. Radiol. Med. 2021, 126, 688–697. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, Y.; Zhang, K.; Liu, Y.; Cui, J.; Tao, J.; Wang, Y.; Wang, S. Invasive ductal breast cancer: Preoperative predict Ki-67 index based on radiomics of ADC maps. Radiol. Med. 2020, 125, 109–116. [Google Scholar] [CrossRef]
- Srisajjakul, S.; Prapaisilp, P.; Bangchokdee, S. CT and MR features that can help to differentiate between focal chronic pancreatitis and pancreatic cancer. Radiol. Med. 2020, 125, 356–364. [Google Scholar] [CrossRef]
- Zhang, A.; Song, J.; Ma, Z. Combined dynamic contrast-enhanced magnetic resonance imaging and diffusion-weighted imaging to predict neoadjuvant chemotherapy effect in FIGO stage IB2-IIA2 cervical cancers. Radiol. Med. 2020, 125, 1233–1242. [Google Scholar] [CrossRef]
- Chen, Y.; Li, B.; Jiang, Z.; Li, H.; Dang, Y.; Tang, C.; Xia, Y.; Zhang, H.; Song, B.; Long, L. Multi-parameter diffusion and perfusion magnetic resonance imaging and radiomics nomogram for preoperative evaluation of aquaporin-1 expression in rectal cancer. Abdom. Radiol. 2022, 47, 1276–1290. [Google Scholar] [CrossRef]
- Zhang, L.; Kang, L.; Li, G.; Zhang, X.; Ren, J.; Shi, Z.; Li, J.; Yu, S. Computed tomography-based radiomics model for discriminating the risk stratification of gastrointestinal stromal tumors. Radiol. Med. 2020, 125, 465–473. [Google Scholar] [CrossRef]
- Liu, G.C.; Zhang, X.; Xie, E.; An, X.; Cai, P.Q.; Zhu, Y.; Tang, J.H.; Kong, L.H.; Lin, J.Z.; Pan, Z.Z.; et al. The Value of Restaging With Chest and Abdominal CT/MRI Scan After Neoadjuvant Chemoradiotherapy for Locally Advanced Rectal Cancer. Medicine 2015, 94, e2074. [Google Scholar] [CrossRef] [PubMed]
- Birlik, B.; Obuz, F.; Elibol, F.D.; Celik, A.O.; Sokmen, S.; Terzi, C.; Sagol, O.; Sarioglu, S.; Gorken, I.; Oztop, I. Diffusion-weighted MRI and MR- volumetry--in the evaluation of tumor response after preoperative chemoradiotherapy in patients with locally advanced rectal cancer. Magn. Reson. Imaging 2015, 33, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Reginelli, A.; Silvestro, G.; Fontanella, G.; Sangiovanni, A.; Conte, M.; Nuzzo, I.; Calvanese, M.; Traettino, M.; Ferraioli, P.; Grassi, R.; et al. Validation of DWI in assessment of radiotreated bone metastases in elderly patients. Int. J. Surg. 2016, 33 (Suppl. S1), S148–S153. [Google Scholar] [CrossRef] [PubMed]
- Scialpi, M.; Reginelli, A.; D’Andrea, A.; Gravante, S.; Falcone, G.; Baccari, P.; Manganaro, L.; Palumbo, B.; Cappabianca, S. Pancreatic tumors imaging: An update. Int. J. Surg. 2016, 28 (Suppl. S1), S142–S155. [Google Scholar] [CrossRef]
- De Piano, F.; Buscarino, V.; Maresca, D.; Maisonneuve, P.; Aletti, G.; Lazzari, R.; Vavassori, A.; Bellomi, M.; Rizzo, S. Do DWI and quantitative DCE perfusion MR have a prognostic value in high-grade serous ovarian cancer? Cancers 2019, 124, 1315–1323. [Google Scholar] [CrossRef]
- Fusco, R.; Granata, V.; Sansone, M.; Rega, D.; Delrio, P.; Tatangelo, F.; Romano, C.; Avallone, A.; Pupo, D.; Giordano, M.; et al. Validation of the standardized index of shape tool to analyze DCE-MRI data in the assessment of neo-adjuvant therapy in locally advanced rectal cancer. Radiol. Med. 2021, 126, 1044–1054. [Google Scholar] [CrossRef]
- Chiloiro, G.; Cusumano, D.; de Franco, P.; Lenkowicz, J.; Boldrini, L.; Carano, D.; Barbaro, B.; Corvari, B.; Dinapoli, N.; Giraffa, M.; et al. Does restaging MRI radiomics analysis improve pathological complete response prediction in rectal cancer patients? A prognostic model development. Radiol. Med. 2022, 127, 11–20. [Google Scholar] [CrossRef]
- Cusumano, D.; Meijer, G.; Lenkowicz, J.; Chiloiro, G.; Boldrini, L.; Masciocchi, C.; Dinapoli, N.; Gatta, R.; Casà, C.; Damiani, A.; et al. A field strength independent MR radiomics model to predict pathological complete response in locally advanced rectal cancer. Radiol. Med. 2021, 126, 421–429. [Google Scholar] [CrossRef]
- Bordron, A.; Rio, E.; Badic, B.; Miranda, O.; Pradier, O.; Hatt, M.; Visvikis, D.; Lucia, F.; Schick, U.; Bourbonne, V. External Validation of a Radiomics Model for the Prediction of Complete Response to Neoadjuvant Chemoradiotherapy in Rectal Cancer. Cancers 2022, 14, 1079. [Google Scholar] [CrossRef]
- Taylor, F.G.; Quirke, P.; Heald, R.J.; Moran, B.J.; Blomqvist, L.; Swift, I.R.; Sebag-Montefiore, D.; Tekkis, P.; Brown, G.; Magnetic Resonance Imaging in Rectal Cancer European Equivalence Study Study Group. Preoperative magnetic resonance imaging assessment of circumferential resection margin predicts disease-free survival and local recurrence: 5-year follow-up results of the MERCURY study. J. Clin. Oncol. 2014, 32, 34–43. [Google Scholar] [CrossRef]
- Memon, S.; Lynch, A.C.; Bressel, M.; Wise, A.G.; Heriot, A.G. Systematic review and meta-analysis of the accuracy of MRI and endorectal ultrasound in the restaging and response assessment of rectal cancer following neoadjuvant therapy. Colorectal Dis. 2015, 17, 748–761. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Caruso, D. Structured Reporting of Rectal Cancer Staging and Restaging: A Consensus Proposal. Cancers 2021, 13, 2135. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.N.; Ge, X.L.; Liu, X.S.; Xu, L.L. Histogram analysis of DCE-MRI for chemoradiotherapy response evaluation in locally advanced esophageal squamous cell carcinoma. Radiol. Med. 2020, 125, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Fornell-Perez, R.; Vivas-Escalona, V.; Aranda-Sanchez, J.; Gonzalez-Dominguez, M.C.; Rubio-Garcia, J.; Aleman-Flores, P.; Lozano-Rodriguez, A.; Porcel-de-Peralta, G.; Loro-Ferrer, J.F. Primary and post-chemoradiotherapy MRI detection of extramural venous invasion in rectal cancer: The role of diffusion-weighted imaging. Radiol. Med. 2020, 125, 522–530. [Google Scholar] [CrossRef]
- Davnall, F.; Yip, C.S.P.; Ljungqvist, G.; Selmi, M.; Ng, F.; Bal, S.; Miles, K.A.; Cook, G.J.; Goh, V. Assessment of tumor heterogeneity: An emerging imaging tool for clinical practice? Insights Imaging 2012, 3, 573–589. [Google Scholar] [CrossRef] [Green Version]
- Scapicchio, C.; Gabelloni, M.; Barucci, A.; Cioni, D.; Saba, L.; Neri, E. A deep look into radiomics. Radiol. Med. 2021, 126, 1296–1311. [Google Scholar] [CrossRef]
- Nazari, M.; Shiri, I.; Hajianfar, G.; Oveisi, N.; Abdollahi, H.; Deevband, M.R.; Oveisi, M.; Zaidi, H. Noninvasive Fuhrman grading of clear cell renal cell carcinoma using computed tomography radiomic features and machine learning. Radiol. Med. 2020, 125, 754–762. [Google Scholar] [CrossRef] [Green Version]
- Alobaidli, S.; McQuaid, S.; South, C.; Prakash, V.; Evans, P.; Nisbet, A. The role of texture analysis in imaging as an outcome predictor and potential tool in radiotherapy treatment planning. Br. J. Radiol. 2014, 87, 20140369. [Google Scholar] [CrossRef] [Green Version]
- Mattonen, S.A.; Tetar, S.; Palma, D.A.; Louie, A.V.; Senan, S.; Ward, A.D. Imaging texture analysis for automated prediction of lung cancer recurrence after stereotactic radiotherapy. J. Med. Imaging 2015, 2, 041010. [Google Scholar] [CrossRef]
- Mattonen, S.A.; Palma, D.A.; Haasbeek, C.J.; Senan, S.; Ward, A.D. Early prediction of tumor recurrence based on CT texture changes after stereotactic ablative radiotherapy (SABR) for lung cancer. Med. Phys. 2014, 41, 033502. [Google Scholar] [CrossRef]
- Coroller, T.P.; Agrawal, V.; Narayan, V.; Hou, Y.; Grossmann, P.; Lee, S.W.; Mak, R.H.; Aerts, H.J. Radiomic phenotype features predict pathological response in non-small cell lung cancer. Radiother. Oncol. 2016, 119, 480–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nardone, V.; Tini, P.; Carbone, S.F.; Grassi, A.; Biondi, M.; Sebaste, L.; Carfagno, T.; Vanzi, E.; De Otto, G.; Battaglia, G.; et al. Bone texture analysis using CT-simulation scans to individuate risk parameters for radiation-induced insufficiency fractures. Osteoporos. Int. 2017, 28, 1915–1923. [Google Scholar] [CrossRef] [PubMed]
- Reginelli, A.; Belfiore, M.P.; Monti, R.; Cozzolino, I.; Costa, M.; Vicidomini, G.; Grassi, R.; Morgillo, F.; Urraro, F.; Nardone, V.; et al. The texture analysis as a predictive method in the assessment of the cytological specimen of CT-guided FNAC of the lung cancer. Med. Oncol. 2020, 37, 54. [Google Scholar] [CrossRef] [PubMed]
- Nardone, V.; Tini, P.; Croci, S.; Carbone, S.F.; Sebaste, L.; Carfagno, T.; Battaglia, G.; Pastina, P.; Rubino, G.; Mazzei, M.A.; et al. 3D bone texture analysis as a potential predictor of radiation-induced insufficiency fractures. Quant. Imaging Med. Surg. 2018, 8, 14–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunese, L.; Mercaldo, F. Radiomics for Gleason Score Detection through Deep Learning. Sensors 2020, 20, 5411. [Google Scholar] [CrossRef]
- Kirienko, M.; Ninatti, G. Computed tomography (CT)-derived radiomic features differentiate prevascular mediastinum masses as thymic neoplasms versus lymphomas. Radiol. Med. 2020, 125, 951–960. [Google Scholar] [CrossRef]
- Hu, H.T.; Shan, Q.Y.; Chen, S.l.; Li, B.; Feng, S.T.; Xu, E.J.; Li, X.; Long, J.Y.; Xie, X.Y.; Lu, M.D.; et al. CT-based radiomics for preoperative prediction of early recurrent hepatocellular carcinoma: Technical reproducibility of acquisition and scanners. Radiol. Med. 2020, 125, 697–705. [Google Scholar] [CrossRef]
- Gatti, M.; Calandri, M.; Bergamasco, L.; Darvizeh, F.; Grazioli, L.; Inchingolo, R.; Ippolito, D.; Rousset, S.; Veltri, A.; Fonio, P.; et al. Characterization of the arterial enhancement pattern of focal liver lesions by multiple arterial phase magnetic resonance imaging: Comparison between hepatocellular carcinoma and focal nodular hyperplasia. Radiol. Med. 2020, 125, 348–355. [Google Scholar] [CrossRef]
- Chianca, V.; Albano, D.; Messina, C.; Vincenzo, G.; Rizzo, S.; Del Grande, F.; Sconfienza, L.M. An update in musculoskeletal tumors: From quantitative imaging to radiomics. Radiol. Med. 2021, 126, 1095–1105. [Google Scholar] [CrossRef]
- Dinapoli, N.; Barbaro, B.; Gatta, R.; Chiloiro, G.; Casa, C.; Masciocchi, C.; Damiani, A.; Boldrini, L.; Gambacorta, M.A.; Dezio, M.; et al. Magnetic Resonance, Vendor-independent, Intensity Histogram Analysis Predicting Pathologic Complete Response After Radiochemotherapy of Rectal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 765–774. [Google Scholar] [CrossRef]
- Crimì, F.; Capelli, G.; Spolverato, G.; Bao, Q.R.; Florio, A.; Milite Rossi, S.; Cecchin, D.; Albertoni, L.; Campi, C.; Pucciarelli, S.; et al. MRI T2-weighted sequences-based texture analysis (TA) as a predictor of response to neoadjuvant chemo-radiotherapy (nCRT) in patients with locally advanced rectal cancer (LARC). Radiol. Med. 2020, 125, 1216–1224. [Google Scholar] [CrossRef]
- Chuanji, Z.; Zheng, W.; Shaolv, L.; Linghou, M.; Yixin, L.; Xinhui, L.; Ling, L.; Yunjing, T.; Shilai, Z.; Shaozhou, M.; et al. Comparative study of radiomics, tumor morphology, and clinicopathological factors in predicting overall survival of patients with rectal cancer before surgery. Transl. Oncol. 2022, 18, 101352. [Google Scholar] [CrossRef]
- Antunes, J.T.; Ofshteyn, A.; Bera, K.; Wang, E.Y.; Brady, J.T.; Willis, J.E.; Friedman, K.A.; Marderstein, E.L.; Kalady, M.F.; Stein, S.L.; et al. Radiomic Features of Primary Rectal Cancers on Baseline T(2) -Weighted MRI Are Associated With Pathologic Complete Response to Neoadjuvant Chemoradiation: A Multisite Study. J. Magn. Reson. Imaging 2020, 52, 1531–1541. [Google Scholar] [CrossRef] [PubMed]
- Nardone, V.; Reginelli, A.; Grassi, R.; Boldrini, L.; Vacca, G.; D’Ippolito, E.; Annunziata, S.; Farchione, A.; Belfiore, M.P.; Desideri, I.; et al. Delta radiomics: A systematic review. Radiol. Med. 2021, 126, 1571–1583. [Google Scholar] [CrossRef] [PubMed]
- Bera, K.; Velcheti, V.; Madabhushi, A. Novel Quantitative Imaging for Predicting Response to Therapy: Techniques and Clinical Applications. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 1008–1018. [Google Scholar] [CrossRef]
- Lee, G.; Bak, S.H.; Lee, H.Y.; Choi, J.Y.; Park, H.; Lee, S.H.; Ohno, Y.; Nishino, M.; van Beek, E.J.R.; Lee, K.S. Measurement Variability in Treatment Response Determination for Non-Small Cell Lung Cancer: Improvements Using Radiomics. J. Thorac. Imaging 2019, 34, 103–115. [Google Scholar] [CrossRef] [Green Version]
- Mazzei, M.A.; Nardone, V.; Di Giacomo, L.; Bagnacci, G.; Gentili, F.; Tini, P.; Marrelli, D.; Volterrani, L. The role of delta radiomics in gastric cancer. Quant. Imaging Med. Surg. 2018, 8, 719–721. [Google Scholar] [CrossRef]
- van Dijk, L.V.; Langendijk, J.A.; Zhai, T.T.; Vedelaar, T.A.; Noordzij, W.; Steenbakkers, R.; Sijtsema, N.M. Delta-radiomics features during radiotherapy improve the prediction of late xerostomia. Sci. Rep. 2019, 9, 12483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzei, M.A.; Giacomo, L.D.; Bagnacci, G.; Nardone, V.; Gentili, F.; Lucii, G.; Tini, P.; Marrelli, D.; Morgagni, P.; Mura, G.; et al. Delta-radiomics and response to neoadjuvant treatment in locally advanced gastric cancer—a multicenter study of GIRCG (Italian Research Group for Gastric Cancer). Quant. Imaging Med. Surg. 2021, 11, 2376–2387. [Google Scholar] [CrossRef]
- Boldrini, L.; Cusumano, D.; Chiloiro, G.; Casa, C.; Masciocchi, C.; Lenkowicz, J.; Cellini, F.; Dinapoli, N.; Azario, L.; Teodoli, S.; et al. Delta radiomics for rectal cancer response prediction with hybrid 0.35 T magnetic resonance-guided radiotherapy (MRgRT): A hypothesis-generating study for an innovative personalized medicine approach. Radiol. Med. 2019, 124, 145–153. [Google Scholar] [CrossRef] [Green Version]
- Nardone, V.; Reginelli, A.; Guida, C.; Belfiore, M.P.; Biondi, M.; Mormile, M.; BanciBuonamici, F.; Di Giorgio, E.; Spadafora, M.; Tini, P.; et al. Delta-radiomics increases multicentre reproducibility: A phantom study. Med. Oncol. 2020, 37, 38. [Google Scholar] [CrossRef] [PubMed]
- Cusumano, D.; Boldrini, L.; Yadav, P.; Yu, G.; Musurunu, B.; Chiloiro, G.; Piras, A.; Lenkowicz, J.; Placidi, L.; Romano, A.; et al. Delta radiomics for rectal cancer response prediction using low field magnetic resonance guided radiotherapy: An external validation. Phys. Med. 2021, 84, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Chiloiro, G.; Rodriguez-Carnero, P.; Lenkowicz, J.; Casà, C.; Masciocchi, C.; Boldrini, L.; Cusumano, D.; Dinapoli, N.; Meldolesi, E.; Carano, D.; et al. Delta Radiomics Can Predict Distant Metastasis in Locally Advanced Rectal Cancer: The Challenge to Personalize the Cure. Front. Oncol. 2020, 10, 595012. [Google Scholar] [CrossRef] [PubMed]
- Shayesteh, S.; Nazari, M.; Salahshour, A.; Sandoughdaran, S.; Hajianfar, G.; Khateri, M.; YaghobiJoybari, A.; Jozian, F.; FatehiFeyzabad, S.H.; Arabi, H.; et al. Treatment response prediction using MRI-based pre-, post-, and delta-radiomic features and machine learning algorithms in colorectal cancer. Med. Phys. 2021, 48, 3691–3701. [Google Scholar] [CrossRef]
- Chen, H.; Shi, L.; Nguyen, K.N.B.; Monjazeb, A.M.; Matsukuma, K.E.; Loehfelm, T.W.; Huang, H.; Qiu, J.; Rong, Y. MRI Radiomics for Prediction of Tumor Response and Downstaging in Rectal Cancer Patients after Preoperative Chemoradiation. Adv. Radiat. Oncol. 2020, 5, 1286–1295. [Google Scholar] [CrossRef]
- Wan, L.; Peng, W.; Zou, S.; Ye, F.; Geng, Y.; Ouyang, H.; Zhao, X.; Zhang, H. MRI-based delta-radiomics are predictive of pathological complete response after neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Acad. Radiol. 2020, 28, S95–S104. [Google Scholar] [CrossRef]
- Jeon, S.H.; Song, C.; Chie, E.K.; Kim, B.; Kim, Y.H.; Chang, W.; Lee, Y.J.; Chung, J.H.; Chung, J.B.; Lee, K.W.; et al. Delta-radiomics signature predicts treatment outcomes after preoperative chemoradiotherapy and surgery in rectal cancer. Radiat. Oncol. 2019, 14, 43. [Google Scholar] [CrossRef] [Green Version]
- Valentini, V.; Gambacorta, M.A.; Barbaro, B.; Chiloiro, G.; Coco, C.; Das, P.; Fanfani, F.; Joye, I.; Kachnic, L.; Maingon, P.; et al. International consensus guidelines on Clinical Target Volume delineation in rectal cancer. Radiother. Oncol. 2016, 120, 195–201. [Google Scholar] [CrossRef] [Green Version]
- Brown, G.; Richards, C.J.; Newcombe, R.G.; Dallimore, N.S.; Radcliffe, A.G.; Carey, D.P.; Bourne, M.W.; Williams, G.T. Rectal carcinoma: Thin-section MR imaging for staging in 28 patients. Radiology 1999, 211, 215–222. [Google Scholar] [CrossRef]
- Vecchio, F.M.; Valentini, V.; Minsky, B.D.; Padula, G.D.; Venkatraman, E.S.; Balducci, M.; Micciche, F.; Ricci, R.; Morganti, A.G.; Gambacorta, M.A.; et al. The relationship of pathologic tumor regression grade (TRG) and outcomes after preoperative therapy in rectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 752–760. [Google Scholar] [CrossRef]
- Mandard, A.M.; Dalibard, F.; Mandard, J.C.; Marnay, J.; Henry-Amar, M.; Petiot, J.F.; Roussel, A.; Jacob, J.H.; Segol, P.; Samama, G.; et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clin. Correl. Cancer 1994, 73, 2680–2686. [Google Scholar]
- Nioche, C.; Orlhac, F.; Boughdad, S.; Reuze, S.; Goya-Outi, J.; Robert, C.; Pellot-Barakat, C.; Soussan, M.; Frouin, F.; Buvat, I. LIFEx: A Freeware for Radiomic Feature Calculation in Multimodality Imaging to Accelerate Advances in the Characterization of Tumor Heterogeneity. Cancer Res. 2018, 78, 4786–4789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Schaaf, A.; Xu, C.J.; van Luijk, P.; Van’t Veld, A.A.; Langendijk, J.A.; Schilstra, C. Multivariate modeling of complications with data driven variable selection: Guarding against overfitting and effects of data set size. Radiother. Oncol. 2012, 105, 115–121. [Google Scholar] [CrossRef]
- Nardone, V.; Reginelli, A.; Scala, F. Magnetic-Resonance-Imaging Texture Analysis Predicts Early Progression in Rectal Cancer Patients Undergoing Neoadjuvant Chemoradiation. Gastroenterol. Res. Pract. 2019, 2019, 8505798. [Google Scholar] [CrossRef]
- Nardone, V.; Tini, P.; Pastina, P.; Botta, C.; Reginelli, A.; Carbone, S.F.; Giannicola, R.; Calabrese, G.; Tebala, C.; Guida, C.; et al. Radiomics predicts survival of patients with advanced non-small cell lung cancer undergoing PD-1 blockade using Nivolumab. Oncol. Lett. 2020, 19, 1559–1566. [Google Scholar] [CrossRef] [PubMed]
- De Cecco, C.N.; Ciolina, M.; Caruso, D.; Rengo, M.; Ganeshan, B.; Meinel, F.G.; Musio, D.; De Felice, F.; Tombolini, V.; Laghi, A. Performance of diffusion-weighted imaging, perfusion imaging, and texture analysis in predicting tumoral response to neoadjuvant chemoradiotherapy in rectal cancer patients studied with 3T MR: Initial experience. Abdom. Radiol. 2016, 41, 1728–1735. [Google Scholar] [CrossRef] [PubMed]
- De Cecco, C.N.; Ganeshan, B.; Ciolina, M.; Rengo, M.; Meinel, F.G.; Musio, D.; De Felice, F.; Raffetto, N.; Tombolini, V.; Laghi, A. Texture analysis as imaging biomarker of tumoral response to neoadjuvant chemoradiotherapy in rectal cancer patients studied with 3-T magnetic resonance. Investig. Radiol. 2015, 50, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Rosa, C.; Caravatta, L.; Di Tommaso, M.; Fasciolo, D.; Gasparini, L.; Di Guglielmo, F.C.; Augurio, A.; Vinciguerra, A.; Vecchi, C.; Genovesi, D. Cone-beam computed tomography for organ motion evaluation in locally advanced rectal cancer patients. Radiol. Med. 2021, 126, 147–154. [Google Scholar] [CrossRef]
- Petralia, G.; Summers, P.E.; Agostini, A.; Ambrosini, R.; Cianci, R.; Cristel, G.; Calistri, L.; Colagrande, S. Dynamic contrast-enhanced MRI in oncology: How we do it. Radiol. Med. 2020, 125, 1288–1300. [Google Scholar] [CrossRef]
- Benedetti, G.; Mori, M.; Panzeri, M.M.; Barbera, M.; Palumbo, D.; Sini, C.; Muffatti, F.; Andreasi, V.; Steidler, S.; Doglioni, C.; et al. CT-derived radiomic features to discriminate histologic characteristics of pancreatic neuroendocrine tumors. Radiol. Med. 2021, 126, 745–760. [Google Scholar] [CrossRef]
- Gregucci, F.; Fiorentino, A.; Mazzola, R.; Ricchetti, F.; Bonaparte, I.; Surgo, A.; Figlia, V.; Carbonara, R.; Caliandro, M.; Ciliberti, M.P.; et al. Radiomic analysis to predict local response in locally advanced pancreatic cancer treated with stereotactic body radiation therapy. Radiol. Med. 2022, 127, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Karmazanovsky, G.; Gruzdev, I.; Tikhonova, V.; Kondratyev, E.; Revishvili, A. Computed tomography-based radiomics approach in pancreatic tumors characterization. Radiol. Med. 2021, 126, 1388–1395. [Google Scholar] [CrossRef] [PubMed]
- Satake, H.; Ishigaki, S.; Ito, R.; Naganawa, S. Radiomics in breast MRI: Current progress toward clinical application in the era of artificial intelligence. Radiol. Med. 2022, 127, 39–56. [Google Scholar] [CrossRef]
- Cappabianca, S.; Granata, V.; Di Grezia, G.; Mandato, Y.; Reginelli, A.; Di Mizio, V.; Grassi, R.; Rotondo, A. The role of nasoenteric intubation in the MR study of patients with Crohn’s disease: Our experience and literature review. Radiol. Med. 2011, 116, 389–406. [Google Scholar] [CrossRef]
- Mostafaei, S.; Abdollahi, H.; KazempourDehkordi, S.; Shiri, I.; Razzaghdoust, A.; Zoljalali Moghaddam, S.H.; Saadipoor, A.; Koosha, F.; Cheraghi, S.; Mahdavi, S.R. CT imaging markers to improve radiation toxicity prediction in prostate cancer radiotherapy by stacking regression algorithm. Radiol. Med. 2020, 125, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Avesani, G.; Arshad, M.; Lu, H.; Fotopoulou, C.; Cannone, F.; Melotti, R.; Aboagye, E.; Rockall, A. Radiological assessment of Peritoneal Cancer Index on preoperative CT in ovarian cancer is related to surgical outcome and survival. Radiol. Med. 2020, 125, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Agazzi, G.M.; Ravanelli, M.; Roca, E.; Medicina, D.; Balzarini, P.; Pessina, C.; Vermi, W.; Berruti, A.; Maroldi, R.; Farina, D. CT texture analysis for prediction of EGFR mutational status and ALK rearrangement in patients with non-small cell lung cancer. Radiol. Med. 2021, 126, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Bracci, S.; Dolciami, M.; Trobiani, C.; Izzo, A.; Pernazza, A.; D’Amati, G.; Manganaro, L.; Ricci, P. Quantitative CT texture analysis in predicting PD-L1 expression in locally advanced or metastatic NSCLC patients. Radiol. Med. 2021, 126, 1425–1433. [Google Scholar] [CrossRef]
- Masci, G.M.; Iafrate, F.; Ciccarelli, F.; Pambianchi, G.; Panebianco, V.; Pasculli, P.; Ciardi, M.R.; Mastroianni, C.M.; Ricci, P.; Catalano, C.; et al. Tocilizumab effects in COVID-19 pneumonia: Role of CT texture analysis in quantitative assessment of response to therapy. Radiol. Med. 2021, 126, 1170–1180. [Google Scholar] [CrossRef]
- Caruso, D.; Pucciarelli, F.; Zerunian, M.; Ganeshan, B.; De Santis, D.; Polici, M.; Rucci, C.; Polidori, T.; Guido, G.; Bracci, B.; et al. Chest CT texture-based radiomics analysis in differentiating COVID-19 from other interstitial pneumonia. Radiol. Med. 2021, 126, 1415–1424. [Google Scholar] [CrossRef]
- Reginelli, A.; Vanzulli, A.; Sgrazzutti, C.; Caschera, L.; Serra, N.; Raucci, A.; Urraro, F.; Cappabianca, S. Vascular microinvasion from hepatocellular carcinoma: CT findings and pathologic correlation for the best therapeutic strategies. Med. Oncol. 2017, 34, 93. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Que, Q.; Lin, P.; Li, X.; Wang, X.R.; He, Y.; Chen, J.Q.; Yang, H. Magnetic resonance imaging (MRI) radiomics of papillary thyroid cancer (PTC): A comparison of predictive performance of multiple classifiers modeling to identify cervical lymph node metastases before surgery. Radiol. Med. 2021, 126, 1312–1327. [Google Scholar] [CrossRef] [PubMed]
- Gündoğdu, E.; Emekli, E.; Kebapçı, M. Evaluation of relationships between the final Gleason score, PI-RADS v2 score, ADC value, PSA level, and tumor diameter in patients that underwent radical prostatectomy due to prostate cancer. Radiol. Med. 2020, 125, 827–837. [Google Scholar] [CrossRef]
- Cellina, M.; Gibelli, D.; Martinenghi, C.; Giardini, D.; Soresina, M.; Menozzi, A.; Oliva, G.; Carrafiello, G. Non-contrast magnetic resonance lymphography (NCMRL) in cancer-related secondary lymphedema: Acquisition technique and imaging findings. Radiol. Med. 2021, 126, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Palmisano, A.; Di Chiara, A.; Esposito, A.; Rancoita, P.M.V.; Fiorino, C.; Passoni, P.; Albarello, L.; Rosati, R.; Del Maschio, A.; De Cobelli, F. MRI prediction of pathological response in locally advanced rectal cancer: When apparent diffusion coefficient radiomics meets conventional volumetry. Clin. Radiol. 2020, 75, 798.e1–798.e11. [Google Scholar] [CrossRef] [PubMed]
- Traverso, A.; Kazmierski, M.; Shi, Z.; Kalendralis, P.; Welch, M.; Nissen, H.D.; Jaffray, D.; Dekker, A.; Wee, L. Stability of radiomic features of apparent diffusion coefficient (ADC) maps for locally advanced rectal cancer in response to image pre-processing. Phys. Med. 2019, 61, 44–51. [Google Scholar] [CrossRef] [Green Version]
- Nie, K.; Shi, L.; Chen, Q.; Hu, X.; Jabbour, S.K.; Yue, N.; Niu, T.; Sun, X. Rectal Cancer: Assessment of Neoadjuvant Chemoradiation Outcome based on Radiomics of Multiparametric MRI. Clin. Cancer Res. 2016, 22, 5256–5264. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Lv, H.; Liu, L.H.; Yang, Z.H.; Jin, E.H.; Wang, Z.C. Locally advanced rectal cancer: Predicting non-responders to neoadjuvant chemoradiotherapy using apparent diffusion coefficient textures. Int. J. Colorectal Dis. 2017, 32, 1009–1012. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, L.; Xia, K.; Jiang, H.; Weng, X.; Jiang, J.; Wu, M. Prediction of Clinical Pathologic Prognostic Factors for Rectal Adenocarcinoma: Volumetric Texture Analysis Based on Apparent Diffusion Coefficient Maps. J. Med. Syst. 2019, 43, 331. [Google Scholar] [CrossRef]
- Yin, J.D.; Song, L.R.; Lu, H.C.; Zheng, X. Prediction of different stages of rectal cancer: Texture analysis based on diffusion-weighted images and apparent diffusion coefficient maps. World J. Gastroenterol. 2020, 26, 2082–2096. [Google Scholar] [CrossRef]
- Chan, A.K.; Wong, A.; Jenken, D.; Heine, J.; Buie, D.; Johnson, D. Posttreatment TNM staging is a prognostic indicator of survival and recurrence in tethered or fixed rectal carcinoma after preoperative chemotherapy and radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2005, 61, 665–677. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Training Dataset | Validation Dataset One | Validation Dataset Two |
|---|---|---|---|
| Vendor | Signa Excite HD, GE Healthcare 1.5 T | Signa Voyager HD, GE Healthcare 1.5 T | 1.5T system, Achieva XR, Software release 5.3.1, Philips, Amsterdam, The Netherlands |
| Sequences | FSE T2 (axial, coronal, sagittal), T1 (axial pre and post c.e.), DWI and ADC | FSE T2 (axial, coronal, sagittal), T1 (axial pre and post c.e.), DWI and ADC | FSE T2 (axial, coronal, sagittal), T1 (axial pre and post c.e.), DWI and ADC |
| DWI | B 0. 500. 800 s/mm2 | B 0. 500. 1000 s/mm2 | B 0. 600. 1000 s/mm2 |
| Characteristic | Training Dataset | Validation Dataset 1 | Validation Dataset 2 | Chi-Square Test |
|---|---|---|---|---|
| Sex | p:0.597 | |||
| Males | 26 (70%) | 21 (64%) | 17 (57%) | |
| Females | 11 (30%) | 12 (36%) | 13 (43%) | |
| Age | ||||
| <70 years | 23 (62%) | 22 (64%) | 16 (53%) | p:0.841 |
| >70 years | 14 (38%) | 11 (36%) | 14 (47%) | |
| Stage (T) | p:0.340 | |||
| cT2 | 8 (22%) | 6 (18%) | 3 (10%) | |
| cT3 | 25 (67%) | 18 (55%) | 20 (66%) | |
| cT4 | 4 (11%) | 9 (27%) | 7 (24%) | |
| Stage (N) | p:0.323 | |||
| cN0 | 5 (14%) | 4 (12%) | 2 (7%) | |
| cN1/2 | 32 (86%) | 29 (88%) | 28 (93%) | |
| Grading | p:0.743 | |||
| G1 | 2 (5%) | 3 (9%) | 2 (7%) | |
| G2 | 30 (81%) | 23 (70%) | 20 (67%) | |
| G3 | 5 (14%) | 7 (21%) | 8 (26%) | |
| TRG | p:0.180 | |||
| 1 | 10 (27%) | 6 (18%) | 5 (17%) | |
| 2 | 13 (35%) | 19 (57%) | 13 (43%) | |
| 3 | 13 (35%) | 8 (24%) | 8 (26%) | |
| 4 | 1 (3%) | 0 (0%) | 4 (14%) |
| MRI Sequence | TA Parameter | Univariate Analysis (Chi-Square) | Bonferroni Correction (Number: 27) |
|---|---|---|---|
| T2-MRI | Volume.ml | 0.49 | NS |
| Skewness | 0.68 | NS | |
| Sphericity | 0.82 | NS | |
| Compacity | 0.21 | NS | |
| GLCM.homogeneity | 0.17 | NS | |
| GLCM.entropy | 0.97 | NS | |
| GLCM.dissimilarity | 0.62 | NS | |
| DWI-MRI | Volume.ml | 0.00018 | 0.0486 |
| Skewness | 0.03 | NS | |
| Kurtosis | 0.20 | NS | |
| Entropy | 0.25 | NS | |
| Compacity | 0.71 | NS | |
| GLCM.homogeneity | 0.45 | NS | |
| GLCM.contrast | 0.37 | NS | |
| GLCM.correlation | 0.72 | NS | |
| GLCM.entropy | 0.0017 | 0.0459 | |
| GLCM.dissimilarity | 0.32 | NS | |
| ADC-MRI | Volume.ml | 0.54 | NS |
| Skewness | 0.98 | NS | |
| Kurtosis | 0.90 | NS | |
| Entropy | 0.42 | NS | |
| Energy | 0.59 | NS | |
| Sphericity | 0.78 | NS | |
| Compacity | 0.11 | NS | |
| GLCM.homogeneity | 0.03 | NS | |
| GLCM.contrast | 0.40 | NS | |
| GLCM.entropy | 0.00017 | 0.00459 | |
| GLCM.dissimilarity | 0.60 | NS | |
| Clinical Parameters | Sex | 0.32 | NS |
| Age | 0.25 | NS | |
| Stage | 0.24 | NS | |
| Grading | 0.28 | NS |
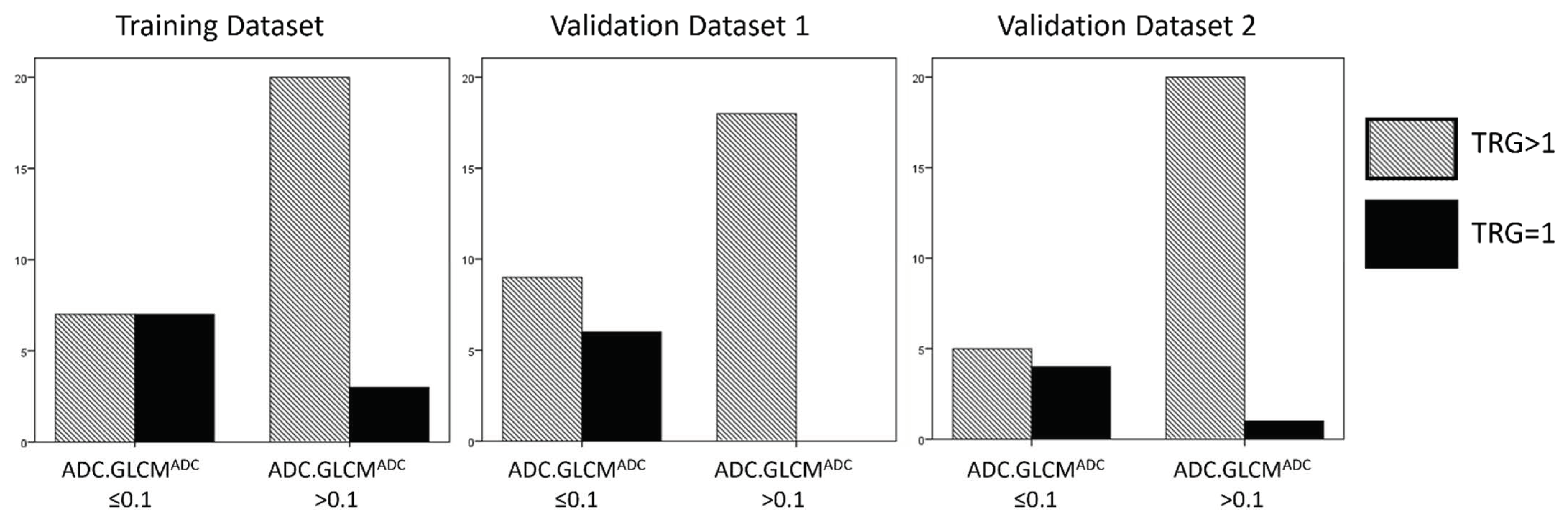
| Model | Count | TRG > 0 | TRG0 | Total |
|---|---|---|---|---|
| Training Dataset | GLCM.EntropyADC < 0.10 | 7 | 7 | 14 |
| GLCM.EntropyADC > 0.10 | 20 | 3 | 23 | |
| Validation Dataset 1 | GLCM.EntropyADC < 0.10 | 9 | 6 | 15 |
| GLCM.EntropyADC > 0.10 | 18 | 0 | 18 | |
| Validation Dataset 2 | GLCM.EntropyADC < 0.10 | 5 | 4 | 9 |
| GLCM.EntropyADC > 0.10 | 20 | 1 | 21 | |
| Legend | GLCM.EntropyADC < 0.10 | False Positive | True Positive | |
| GLCM.EntropyADC > 0.10 | True Negative | False Negative |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nardone, V.; Reginelli, A.; Grassi, R.; Vacca, G.; Giacobbe, G.; Angrisani, A.; Clemente, A.; Danti, G.; Correale, P.; Carbone, S.F.; et al. Ability of Delta Radiomics to Predict a Complete Pathological Response in Patients with Loco-Regional Rectal Cancer Addressed to Neoadjuvant Chemo-Radiation and Surgery. Cancers 2022, 14, 3004. https://doi.org/10.3390/cancers14123004
Nardone V, Reginelli A, Grassi R, Vacca G, Giacobbe G, Angrisani A, Clemente A, Danti G, Correale P, Carbone SF, et al. Ability of Delta Radiomics to Predict a Complete Pathological Response in Patients with Loco-Regional Rectal Cancer Addressed to Neoadjuvant Chemo-Radiation and Surgery. Cancers. 2022; 14(12):3004. https://doi.org/10.3390/cancers14123004
Chicago/Turabian StyleNardone, Valerio, Alfonso Reginelli, Roberta Grassi, Giovanna Vacca, Giuliana Giacobbe, Antonio Angrisani, Alfredo Clemente, Ginevra Danti, Pierpaolo Correale, Salvatore Francesco Carbone, and et al. 2022. "Ability of Delta Radiomics to Predict a Complete Pathological Response in Patients with Loco-Regional Rectal Cancer Addressed to Neoadjuvant Chemo-Radiation and Surgery" Cancers 14, no. 12: 3004. https://doi.org/10.3390/cancers14123004
APA StyleNardone, V., Reginelli, A., Grassi, R., Vacca, G., Giacobbe, G., Angrisani, A., Clemente, A., Danti, G., Correale, P., Carbone, S. F., Pirtoli, L., Bianchi, L., Vanzulli, A., Guida, C., Grassi, R., & Cappabianca, S. (2022). Ability of Delta Radiomics to Predict a Complete Pathological Response in Patients with Loco-Regional Rectal Cancer Addressed to Neoadjuvant Chemo-Radiation and Surgery. Cancers, 14(12), 3004. https://doi.org/10.3390/cancers14123004








