Outcomes and Toxicities of Modern Combined Modality Therapy with Atezolizumab Plus Bevacizumab and Radiation Therapy for Hepatocellular Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
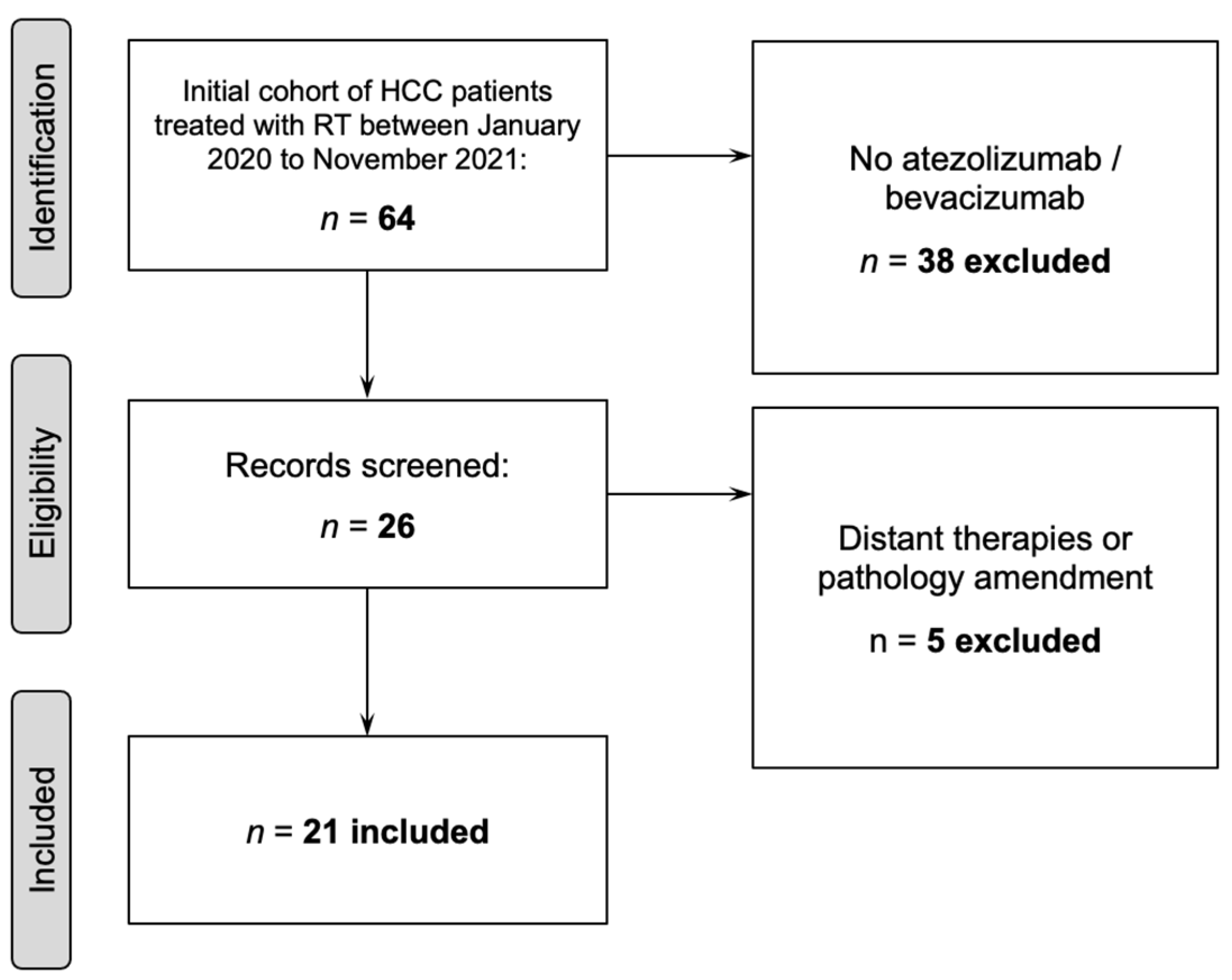
2.1. Patient Selection
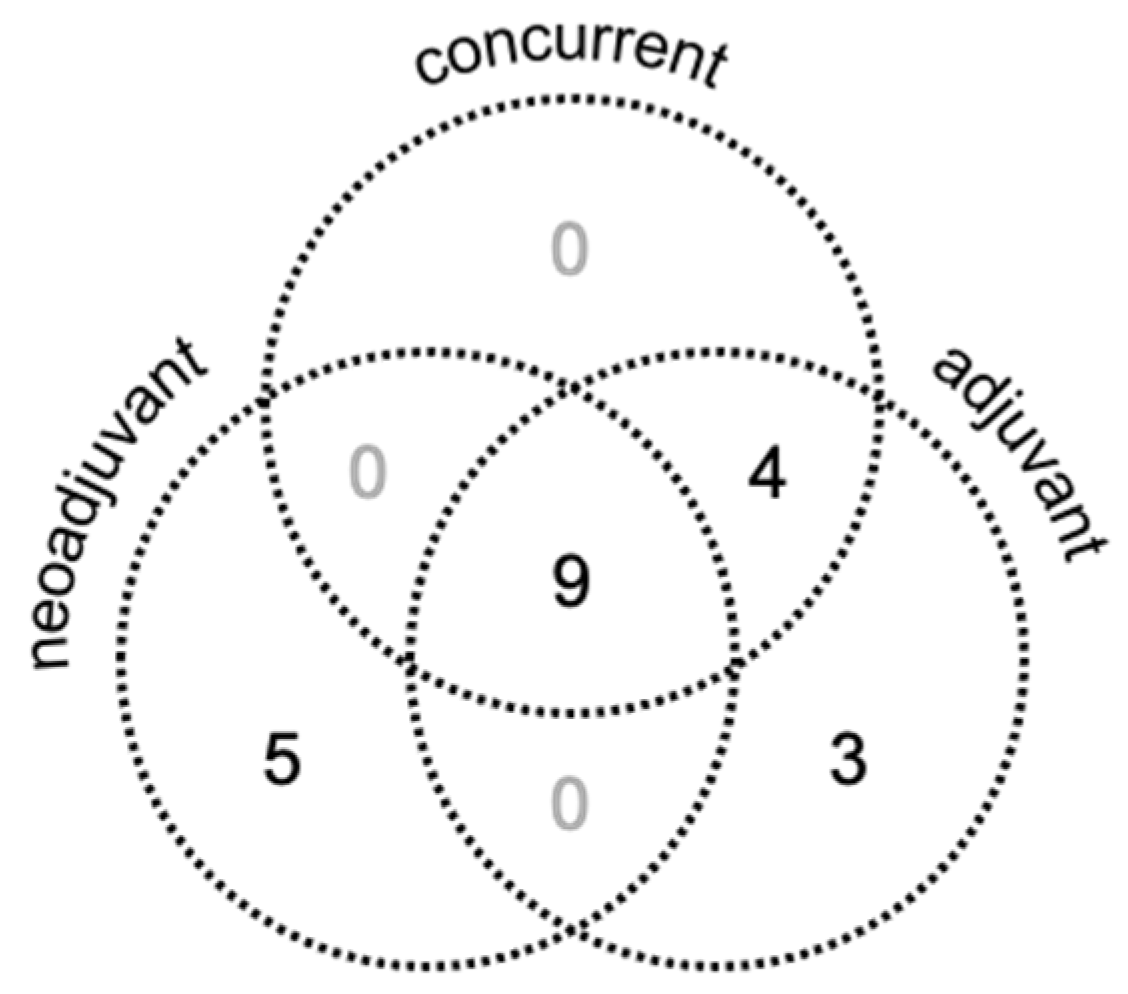
2.2. Treatment
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Patient, Tumor, and Treatment Characteristics
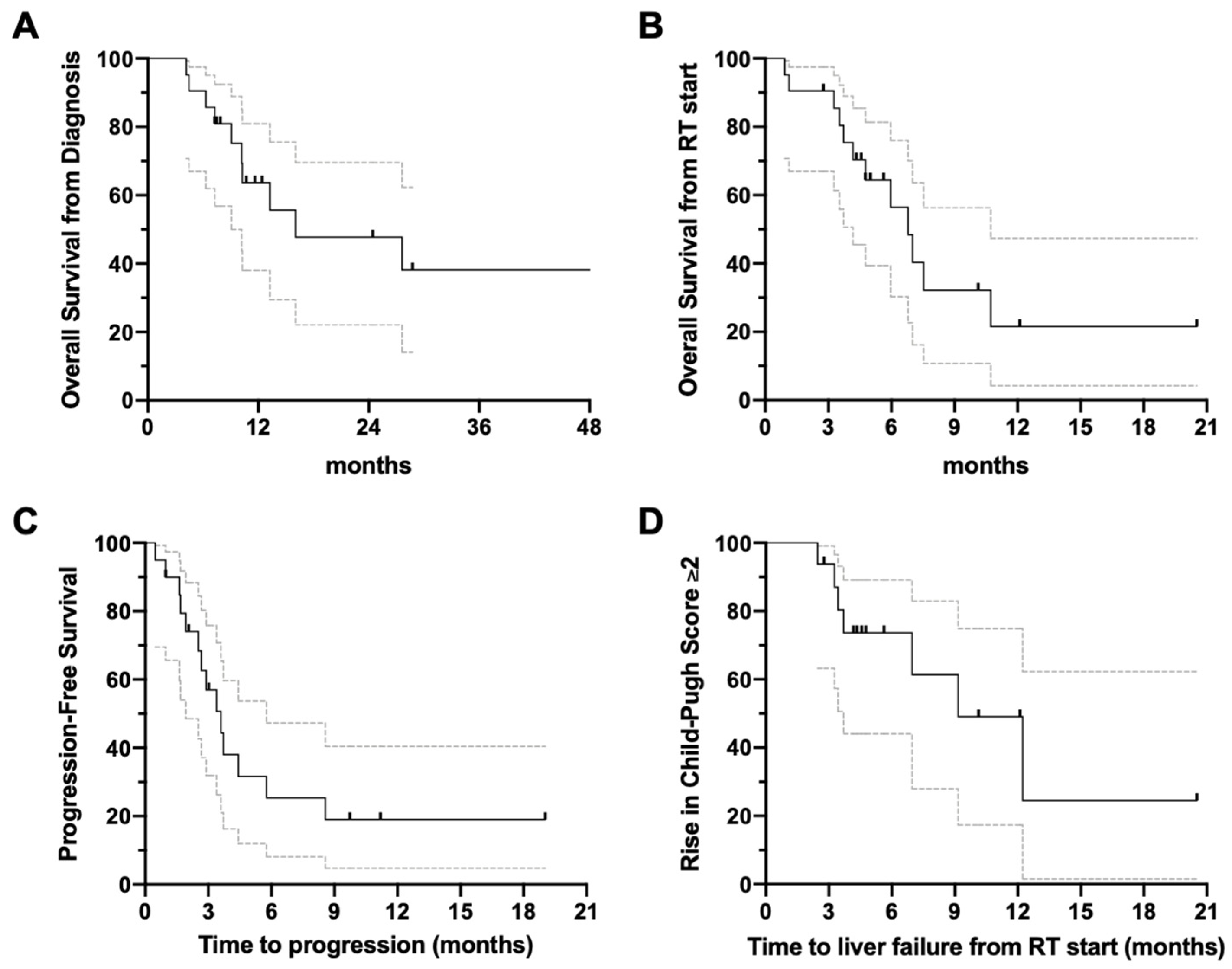
3.2. Overview of Clinical Outcomes
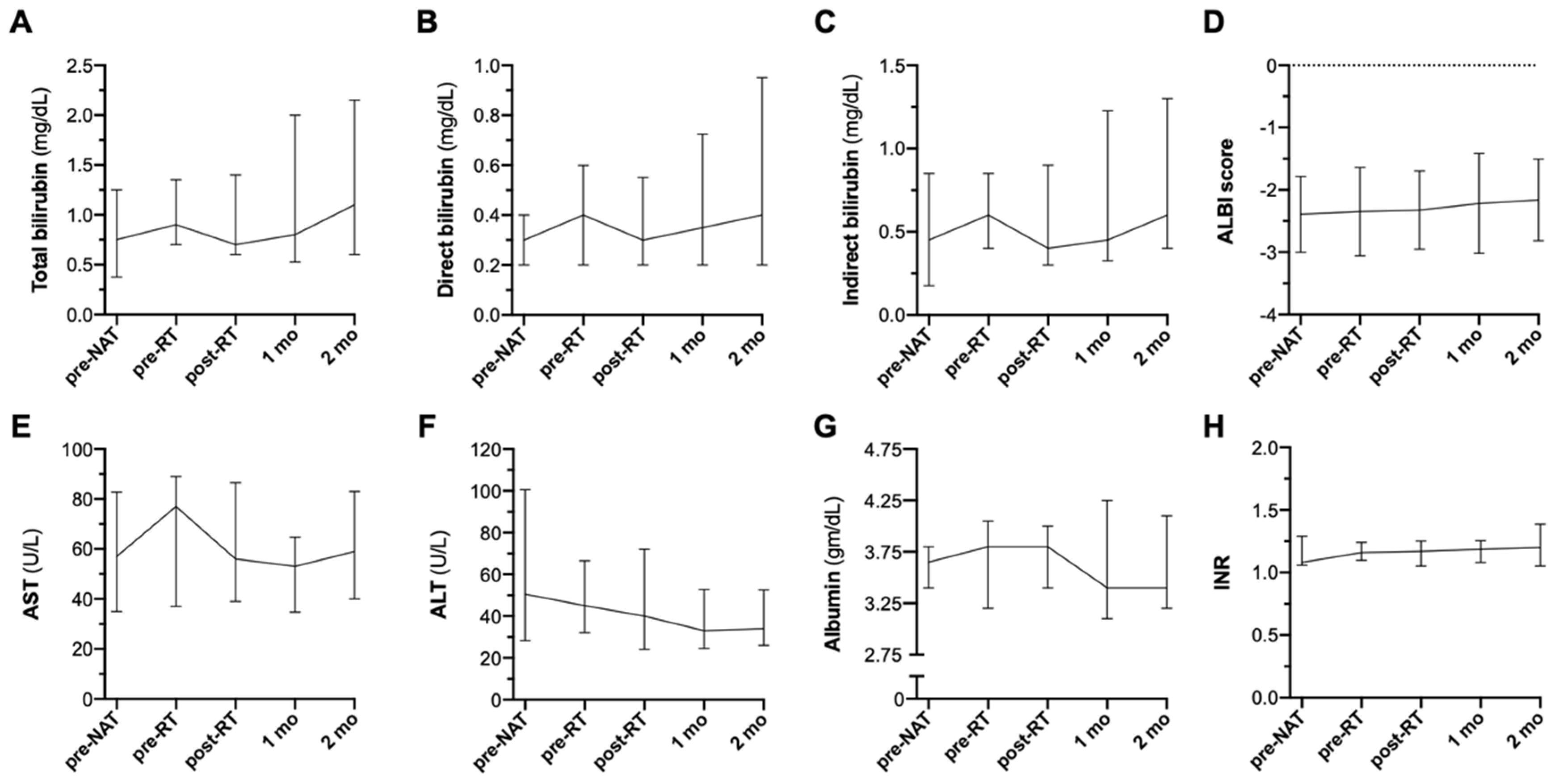
3.3. Acute Toxicity
3.4. Autoimmune Toxicity
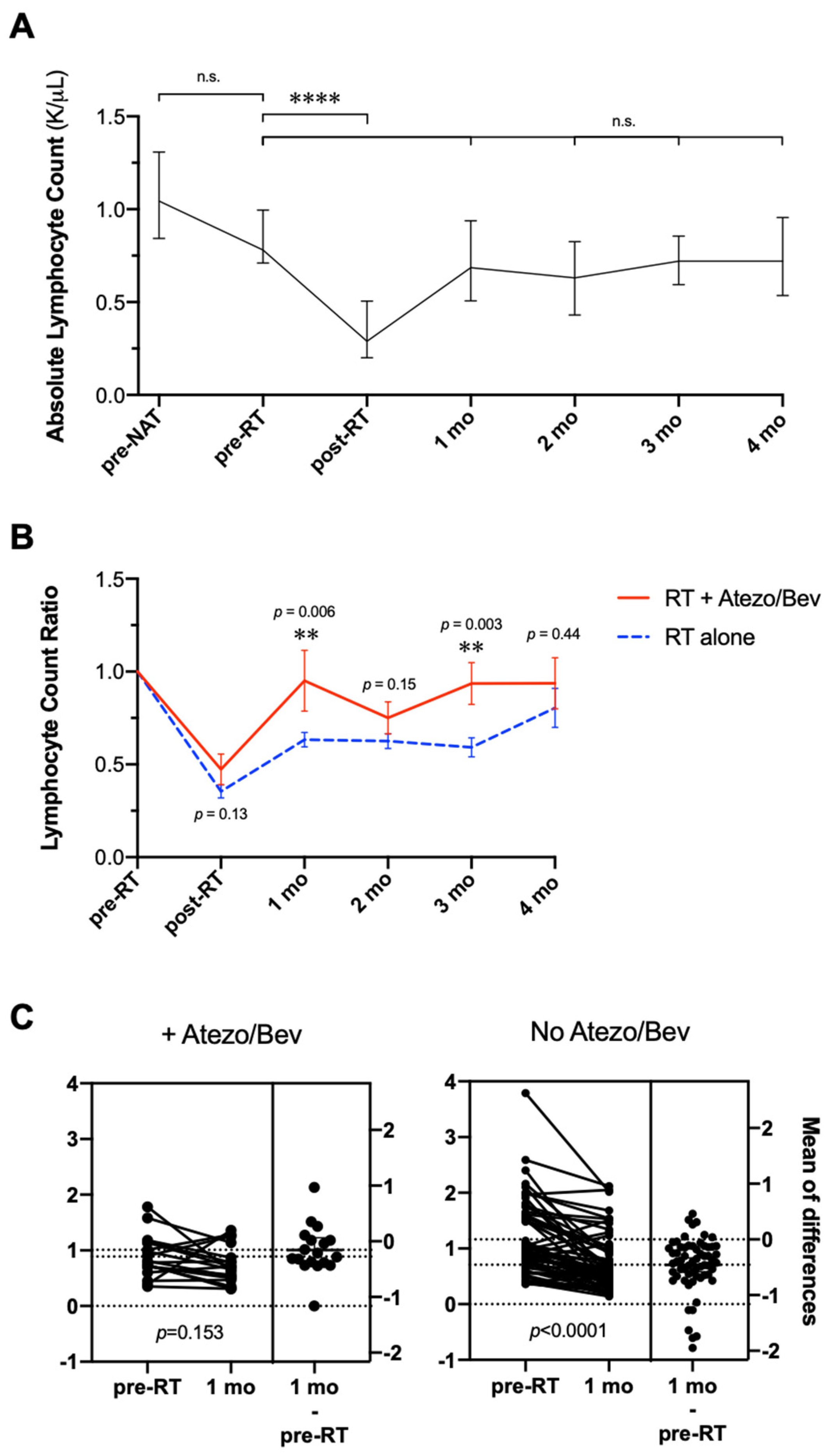
3.5. Changes in Absolute Lymphocyte Count during RT with Atezolizumab Plus Bevacizumab
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 73 (Suppl. S1), 209–249. [Google Scholar] [CrossRef] [PubMed]
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021, 73, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Sood, G.K. Hepatocellular carcinoma review: Current treatment, and evidence-based medicine. World J. Gastroenterol. 2014, 20, 4115–4127. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.-F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.-L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef] [Green Version]
- Vogel, A.; Rimassa, L.; Sun, H.C.; Abou-Alfa, G.K.; El-Khoueiry, A.; Pinato, D.J.; Sanchez-Alvarez, J.; Daigl, M.; Orfanos, P.; Leibfried, M.; et al. Comparative Efficacy of Atezolizumab plus Bevacizumab and Other Treatment Options for Patients with Unresectable Hepatocellular Carcinoma: A Network Meta-Analysis. Liver Cancer 2021, 10, 240–248. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.-H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.-W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef] [Green Version]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Hergert, J. Atezolizumab/Bevacizumab Combo Sets the Standard for New Doublets in Advanced HCC; OncLive: Cranbury, NJ, USA, 2021. [Google Scholar]
- Liu, X.; Lu, Y.; Qin, S. Atezolizumab and bevacizumab for hepatocellular carcinoma: Mechanism, pharmacokinetics and future treatment strategies. Future Oncol. 2021, 17, 2243–2256. [Google Scholar] [CrossRef]
- Rizzo, A.; Dadduzio, V.; Ricci, A.D.; Massari, F.; Di Federico, A.; Gadaleta-Caldarola, G.; Brandi, G. Lenvatinib plus pembrolizumab: The next frontier for the treatment of hepatocellular carcinoma? Expert Opin. Investig. Drugs 2022, 31, 371–378. [Google Scholar] [CrossRef]
- Rizzo, A.; Brandi, G. Biochemical predictors of response to immune checkpoint inhibitors in unresectable hepatocellular carcinoma. Cancer Treat. Res. Commun. 2021, 27, 100328. [Google Scholar] [CrossRef]
- Chen, C.P. Role of Radiotherapy in the Treatment of Hepatocellular Carcinoma. J. Clin. Transl. Hepatol. 2019, 7, 183–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holliday, E.B.; Tao, R.; Brownlee, Z.; Das, P.; Krishnan, S.; Taniguchi, C.; Minsky, B.D.; Herman, J.M.; Kaseb, A.; Raghav, K.; et al. Definitive radiation therapy for hepatocellular carcinoma with portal vein tumor thrombus. Clin. Transl. Radiat. Oncol. 2017, 4, 39–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benedict, S.H.; Yenice, K.M.; Followill, D.; Galvin, J.M.; Hinson, W.; Kavanagh, B.; Keall, P.; Lovelock, M.; Meeks, S.; Papiez, L.; et al. Stereotactic body radiation therapy: The report of AAPM Task Group 101. Med. Phys. 2010, 37, 4078–4101. [Google Scholar] [CrossRef] [Green Version]
- Crane, C.H.; Koay, E.J. Solutions that enable ablative radiotherapy for large liver tumors: Fractionated dose painting, simultaneous integrated protection, motion management, and computed tomography image guidance. Cancer 2016, 122, 1974–1986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.H.; Park, J.W.; Kim, Y.J.; Kim, B.H.; Woo, S.M.; Moon, S.H.; Kim, S.S.; Lee, W.J.L.; Kim, D.Y.K.; Kim, C.-M. Simultaneous integrated boost-intensity modulated radiation therapy for inoperable hepatocellular carcinoma. Strahlenther Onkol. 2014, 190, 882–890. [Google Scholar] [CrossRef]
- De, B.; Ng, S.P.; Liu, A.Y.; Avila, S.; Tao, R.; Holliday, E.B.; Brownlee, Z.; Kaseb, A.; Lee, S.; Raghav, K.; et al. Radiation-Associated Lymphopenia and Outcomes of Patients with Unresectable Hepatocellular Carcinoma Treated with Radiotherapy. J. Hepatocell. Carcinoma 2021, 8, 57–69. [Google Scholar] [CrossRef]
- Palaskas, N.; Lopez-Mattei, J.; Durand, J.B.; Iliescu, C.; Deswal, A. Immune Checkpoint Inhibitor Myocarditis: Pathophysiological Characteristics, Diagnosis, and Treatment. J. Am. Heart Assoc. 2020, 9, e013757. [Google Scholar] [CrossRef]
- Rajha, E.; Chaftari, P.; Kamal, M.; Maamari, J.; Chaftari, C.; Yeung, S.J. Gastrointestinal adverse events associated with immune checkpoint inhibitor therapy. Gastroenterol. Rep. 2020, 8, 25–30. [Google Scholar] [CrossRef] [Green Version]
- Reyngold, M.; Koay, E.J.; Crane, C.H. Hypofractionated ablative radiation therapy for hepatocellular carcinoma: Practical considerations and review of the literature. Hepatoma Res. 2018, 4, 49. [Google Scholar] [CrossRef] [Green Version]
- Hilal, L.; Reyngold, M.; Wu, A.J.; Araji, A.; Abou-Alfa, G.K.; Jarnagin, W.; Harding, J.J.; Gambarin, M.; El Dika, I.; Brady, P.; et al. Ablative radiation therapy for hepatocellular carcinoma is associated with reduced treatment- and tumor-related liver failure and improved survival. J. Gastrointest. Oncol. 2021, 12, 1743–1752. [Google Scholar] [CrossRef]
- Apisarnthanarax, S.; Barry, A.; Cao, M.; Czito, B.; DeMatteo, R.; Drinane, M.; Hallemeir, C.L.; Koay, E.J.; Lasley, F.; Meyer, J.; et al. External Beam Radiation Therapy for Primary Liver Cancers: An ASTRO Clinical Practice Guideline. Pract. Radiat. Oncol. 2022, 12, 28–51. [Google Scholar] [CrossRef] [PubMed]
- Siegel, A.B.; Cohen, E.I.; Ocean, A.; Lehrer, D.; Goldenberg, A.; Chen, J.J.K.; Clark-Garvey, S.; Weinberg, A.; Mandeli, J.; Christos, P.; et al. Phase II trial evaluating the clinical and biologic effects of bevacizumab in unresectable hepatocellular carcinoma. J. Clin. Oncol. 2008, 26, 2992–2998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boige, V.; Malka, D.; Bourredjem, A.; Dromain, C.; Baey, C.; Jacques, N.; Pignon, J.-P.; Vimond, N.; Bouvet-Forteau, N.; De Baere, T.; et al. Efficacy, safety, and biomarkers of single-agent bevacizumab therapy in patients with advanced hepatocellular carcinoma. Oncologist 2012, 17, 1063–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crane, C.H.; Winter, K.; Regine, W.F.; Safran, H.; Rich, T.A.; Wolff, W.C.A.; Willett, C.G. Phase II study of bevacizumab with concurrent capecitabine and radiation followed by maintenance gemcitabine and bevacizumab for locally advanced pancreatic cancer: Radiation Therapy Oncology Group RTOG 0411. J. Clin. Oncol. 2009, 27, 4096–4102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, T.M.; Liu, Y.; Wang, J.B.; Liu, L.X. Adverse Effects of Immune-Checkpoint Inhibitors in Hepatocellular Carcinoma. OncoTargets Ther. 2020, 13, 11725–11740. [Google Scholar] [CrossRef] [PubMed]
- Grossman, S.A.; Ellsworth, S.; Campian, J.; Wild, A.T.; Herman, J.M.; Laheru, D.; Brock, M.; Balmanoukian, A.; Ye, X. Survival in Patients with Severe Lymphopenia Following Treatment with Radiation and Chemotherapy for Newly Diagnosed Solid Tumors. J. Natl. Compr. Cancer Netw. 2015, 13, 1225–1231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byun, H.K.; Kim, N.; Park, S.; Seong, J. Acute severe lymphopenia by radiotherapy is associated with reduced overall survival in hepatocellular carcinoma. Strahlenther Onkol. 2019, 195, 1007–1017. [Google Scholar] [CrossRef]
- Inman, B.A.; Longo, T.A.; Ramalingam, S.; Harrison, M.R. Atezolizumab: A PD-L1-Blocking Antibody for Bladder Cancer. Clin. Cancer Res. 2017, 23, 1886–1890. [Google Scholar] [CrossRef] [Green Version]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.-Y.; Choo, S.-P.; Trojan, J.; Welling, T.H., 3rd; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef]
- Yau, T.; Kang, Y.-K.; Kim, T.-Y.; El-Khoueiry, A.B.; Santoro, A.; Sangro, B.; Melero, I.; Kudo, M.; Hou, M.-M.; Matilla, A.; et al. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients with Advanced Hepatocellular Carcinoma Previously Treated with Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020, 6, e204564. [Google Scholar] [CrossRef]
- National Library of Medicine USA. Study of Cabozantinib in Combination with Atezolizumab Versus Sorafenib in Subjects with Advanced HCC Who Have Not Received Previous Systemic Anticancer Therapy (COSMIC-312). Available online: https://clinicaltrials.gov/ct2/show/NCT03755791 (accessed on 1 May 2021).
- National Library of Medicine USA. Study of Durvalumab and Tremelimumab as First-line Treatment in Patients with Advanced Hepatocellular Carcinoma (HIMALAYA). Available online: https://clinicaltrials.gov/ct2/show/NCT03298451 (accessed on 1 May 2021).
- Finn, R.S.; Ikeda, M.; Zhu, A.X.; Sung, M.W.; Baron, A.D.; Kudo, M.; Okusaka, T.; Kobayashi, M.; Kumada, H.; Kaneko, S.; et al. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients with Unresectable Hepatocellular Carcinoma. J. Clin. Oncol. 2020, 38, 2960–2970. [Google Scholar] [CrossRef] [PubMed]
- Yau, T.; Park, J.W.; Finn, R.S.; Cheng, A.-L.; Mathurin, P.; Edeline, J.; Kudo, M.; Han, K.-H.; Harding, J.J.; Merle, P.; et al. CheckMate 459: A randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann. Oncol. 2019, 30, v874–v875. [Google Scholar] [CrossRef]
| Patient Characteristics | n | % | |
|---|---|---|---|
| Total No. | 21 | ||
| Age | |||
| Median (range), years | 68 (38–78) | ||
| Sex | |||
| Male | 17 | 81% | |
| Child–Pugh Score (CPS), pre-RT | |||
| 5 A | 10 | 47.6% | |
| 6 A | 4 | 19% | |
| 7 B | 2 | 9.5% | |
| 8 B | 2 | 9.5% | |
| 9 B | 2 | 9.5% | |
| 10 C | 1 | 4.8% | |
| BCLC | |||
| A | 1 | 4.8% | |
| B | 2 | 9.5% | |
| C | 17 | 80.9% | |
| D | 1 | 4.8% | |
| Clinical stage at diagnosis | |||
| rpIB | 1 | 4.8% | |
| II | 1 | 4.8% | |
| IIIA | 3 | 14.3% | |
| IIIB | 7 | 33.3% | |
| IVA | 3 | 14.3% | |
| IVB | 6 | 28.6% | |
| PV Tumor Thrombus (PVTT) | |||
| Yes | 15 | 71.4% | |
| No | 6 | 28.6% | |
| Etiology | |||
| Viral (HBV or HCV) | 14 | 66.6% | |
| Metabolic syndrome | 6 | 28.6% | |
| Alcoholic cirrhosis | 1 | 4.8% | |
| T-stage | |||
| rpT1 b, T2, T3 | 6 | 28.6% | |
| T4 | 15 | 71.4% | |
| Median GTV (range), cc3 | 149.4 (25.1–3426.9) | ||
| Median irradiated tumor diameter (range), cm | 9.1 (3.9–18.6) | ||
| Median of “mean liver–GTV” dose (IQR), Gy | 17.4 (12.2–19.9, max 21.9) | ||
| Volume of liver receiving ≤20 Gy (IQR), cc3 | 331.2 (175.6–509.3, max 628.7) | ||
| N-stage | |||
| N0 | 15 | 71.4% | |
| N1 | 6 | 28.6% | |
| M-stage | |||
| M0 | 15 | 71.4% | |
| M1 | 6 | 28.6% | |
| Modality | |||
| IMRT | 13 | 61.9% | |
| Proton | 7 | 33.3% | |
| 3 D | 1 | 4.8% | |
| Treatment | |||
| Median total dose (range), Gy | 60 (20–75) | ||
| Fractions (range) | 15 (5–25) | ||
| BED10 (Gy) | |||
| >70 Gy (mean 89.3 Gy) | 17 | 81% | |
| 28–56 Gy | 4 | 19% | |
| Prior Therapy (Interval between First Dose of Prior Therapy and Initiation of A/B, Months) |
|---|
| TACE with doxorubicin (6, 3) |
| Lenvatinib (4), one dose with poor tolerance |
| Nivolumab (31), lost to follow-up until reemergence |
| Lenvatinib (3) |
| Lenvatinib (7), Nivolumab (4), Ramucirumab (1) |
| Resection (50), RFA (34), sorafenib (21), nivolumab (17), regorafenib (14) |
| Lenvatinib (23), Nivolumab (16) |
| Pain | Fatigue | Nausea | Vomiting | Diarrhea | Constipation | Dermatitis | |
|---|---|---|---|---|---|---|---|
| Grade 1 | 7/21 (33.3%) | 12/21 (57.1%) | 10/21 (47.6%) | 2/21 (9.5%) | 4/21 (19.1%) | 2/21 (9.5%) | 3/21 (14.3%) |
| Grade 2 | 1/21 (4.8%) | 1/21 (4.8%) | 1/21 (4.8%) | 1/21 (4.8%) | 0/21 (0%) | 0/21 (0%) | 0/21 (0%) |
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Distant POD | ◊◊◊◊ | ⋅ | ⋅ | ◊ | ⋅ | ◊◊◊ | ◊◊◊◊ | ◊◊◊ | ⋅ | ◊ | ⋅ | ◊◊◊◊ |
| RT toxicity | ⋅ | ⋅ | ⋅ | ⋅ | ◊ | ⋅ | ⋅ | ⋅ | ⋅ | ⋅ | ⋅ | ⋅ |
| A/B toxicity | ⋅ | ⋅ | ⋅ | ⋅ | ◊ | ⋅ | ⋅ | ⋅ | ⋅ | ⋅ | ⋅ | ⋅ |
| TRLF | ⋅ | ⋅ | ⋅ | ◊◊◊◊ | ⋅ | ⋅ | ⋅ | ⋅ | ◊◊◊ | ◊◊◊ | ◊◊◊ | ⋅ |
| RT-related LF | ⋅ | ⋅ | ⋅ | ⋅ | ⋅ | ⋅ | ⋅ | ⋅ | ⋅ | ⋅ | ⋅ | ⋅ |
| Comorbidity | ⋅ | ◊◊◊ | ◊◊◊◊ | ⋅ | ⋅ | ◊ | ⋅ | ⋅ | ⋅ | ⋅ | ⋅ | ◊ |
| Emergent | ⋅ | ⋅ | ◊◊◊◊ | ⋅ | ◊◊◊◊ | ⋅ | ⋅ | ⋅ | ⋅ | ⋅ | ⋅ | ⋅ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manzar, G.S.; De, B.S.; Abana, C.O.; Lee, S.S.; Javle, M.; Kaseb, A.O.; Vauthey, J.-N.; Tran Cao, H.S.; Koong, A.C.; Smith, G.L.; et al. Outcomes and Toxicities of Modern Combined Modality Therapy with Atezolizumab Plus Bevacizumab and Radiation Therapy for Hepatocellular Carcinoma. Cancers 2022, 14, 1901. https://doi.org/10.3390/cancers14081901
Manzar GS, De BS, Abana CO, Lee SS, Javle M, Kaseb AO, Vauthey J-N, Tran Cao HS, Koong AC, Smith GL, et al. Outcomes and Toxicities of Modern Combined Modality Therapy with Atezolizumab Plus Bevacizumab and Radiation Therapy for Hepatocellular Carcinoma. Cancers. 2022; 14(8):1901. https://doi.org/10.3390/cancers14081901
Chicago/Turabian StyleManzar, Gohar Shahwar, Brian Sandeep De, Chike Osita Abana, Sunyoung S. Lee, Milind Javle, Ahmed O. Kaseb, Jean-Nicolas Vauthey, Hop Sanderson Tran Cao, Albert C. Koong, Grace Li Smith, and et al. 2022. "Outcomes and Toxicities of Modern Combined Modality Therapy with Atezolizumab Plus Bevacizumab and Radiation Therapy for Hepatocellular Carcinoma" Cancers 14, no. 8: 1901. https://doi.org/10.3390/cancers14081901






