Glycosyltransferases in Cancer: Prognostic Biomarkers of Survival in Patient Cohorts and Impact on Malignancy in Experimental Models
Abstract
:Simple Summary
Abstract
1. Introduction
2. Glycosyltransferase Genes Associated with Prognosis in TCGA Cohorts
3. Glycosyltransferase Genes Playing a Consistent Association in a Large Number of Cohorts
4. Glycosyltransferases with Very High Prognostic Value (VHPV)
5. Role of Glycosyltransferases in Experimental Systems
5.1. Initiating Glycosyltransferases
5.1.1. Glycosyltransferases Initiating N-Glycosylation
5.1.2. Glycosyltransferases Initiating O-Glycosylation
5.1.3. Glycosyltransferases Initiating Gangliosides
5.2. Extending Glycosyltransferases
5.2.1. Polylactosaminic Chains
5.2.2. LARGE
5.3. Capping Glycosyltransferases
5.3.1. Sialyltransferases
5.3.2. Fucosyltransferases
6. Mechanistic Aspects of Glycosyltransferase Expression
7. Discussion
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BPA | bad prognosis-associated |
| circRNA | circular RNA |
| GPA | good prognosis-associated |
| lncRNA | long non-coding RNA |
| PD-1 | programmed death 1 |
| PD-L1 | programmed death ligand 1 |
| VHPV | very high prognostic value |
| BLCA | bladder urothelial carcinoma |
| BRCA | breast invasive carcinoma |
| CESC | cervical squamous cell carcinoma and endocervical adenocarcinoma |
| COAD | colon adenocarcinoma |
| ESCA | esophageal carcinoma |
| GBM | glioblastoma multiforme |
| HNSC | head and neck squamous cell carcinoma |
| KIRC | kidney renal clear cell carcinoma |
| KIRP | kidney renal papillary carcinoma |
| LAML | acute myeloid leukemia |
| LGG | brain lower grade glioma |
| LIHC | liver hepatocellular carcinoma |
| LUAD | lung adenocarcinoma |
| LUSC | lung squamous cell carcinoma |
| OV | ovary serous cystadenocarcinoma |
| PAAD | pancreatic adenocarcinoma |
| READ | rectum adenocarcinoma |
| SARC | sarcoma |
| SKCM | skin cutaneous melanoma |
| STAD | stomach adenocarcinoma |
| TCGA | the cancer genome atlas |
| UCEC | uterine corpus endometrial carcinoma |
References
- Varki, A. Biological roles of glycans. Glycobiology 2017, 27, 3–49. [Google Scholar] [CrossRef] [Green Version]
- Dall’Olio, F.; Malagolini, N.; Trinchera, M.; Chiricolo, M. Mechanisms of cancer-associated glycosylation changes. Front. Biosci. 2012, 17, 670–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinho, S.S.; Reis, C.A. Glycosylation in cancer: Mechanisms and clinical implications. Nat. Rev. Cancer 2015, 15, 540–555. [Google Scholar] [CrossRef] [PubMed]
- Schjoldager, K.T.; Narimatsu, Y.; Joshi, H.J.; Clausen, H. Global view of human protein glycosylation pathways and functions. Nat. Rev. Mol. Cell Biol. 2020, 21, 729–749. [Google Scholar] [CrossRef] [PubMed]
- Ke, S.B.; Qiu, H.; Chen, J.M.; Shi, W.; Han, C.; Gong, Y.; Chen, Y.S. ALG3 contributes to the malignancy of non-small cell lung cancer and is negatively regulated by MiR-98-5p. Pathol.-Res. Pract. 2020, 216, 152761. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.; Wei, C.; Wang, Y. ALG3 contributes to the malignant properties of OSCC cells by regulating CDK-Cyclin pathway. Oral Dis. 2021, 27, 1426–1434. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.Z.; Jiang, Y.Y.; Hao, J.J.; Zhang, Y.; Zhang, T.T.; Shang, L.; Liu, S.G.; Shi, F.; Wang, M.R. Identification of putative target genes for amplification within 11q13.2 and 3q27.1 in esophageal squamous cell carcinoma. Clin. Transl. Oncol. 2014, 16, 606–615. [Google Scholar] [CrossRef]
- Choi, Y.W.; Bae, S.M.; Kim, Y.W.; Lee, H.N.; Kim, Y.W.; Park, T.C.; Ro, D.Y.; Shin, J.C.; Shin, S.J.; Seo, J.S.; et al. Gene expression profiles in squamous cell cervical carcinoma using array-based comparative genomic hybridization analysis. Int. J. Gynecol. Cancer 2007, 17, 687–696. [Google Scholar] [CrossRef]
- Zhou, W.; Li, J.; Lu, X.; Liu, F.; An, T.; Xiao, X.; Kuo, Z.C.; Wu, W.; He, Y. Derivation and Validation of a Prognostic Model for Cancer Dependency Genes Based on CRISPR-Cas9 in Gastric Adenocarcinoma. Front. Oncol. 2021, 11, 617289. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, D.; Chen, Y.; Lu, Y.; Zhou, F.; Li, C.; Zeng, Z.; Cai, W.; Lin, L.; Li, Q.; et al. Robust Glycogene-Based Prognostic Signature for Proficient Mismatch Repair Colorectal Adenocarcinoma. Front. Oncol. 2021, 11, 727752. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Hansen, L.; Clausen, H. Polypeptide N-acetylgalactosaminyltransferase-Associated Phenotypes in Mammals. Molecules 2021, 26, 5504. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.C.; Huang, M.J.; Liu, C.H.; Yang, T.L.; Huang, M.C. GALNT2 enhances migration and invasion of oral squamous cell carcinoma by regulating EGFR glycosylation and activity. Oral Oncol. 2014, 50, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Xue, H.; Wei, Y.; Wang, C.; Yu, R.; Wang, C.; Wang, S.; Xu, J.; Qian, M.; Meng, Q.; et al. Mucin O-glycosylating enzyme GALNT2 facilitates the malignant character of glioma by activating the EGFR/PI3K/Akt/mTOR axis. Clin. Sci. 2019, 133, 1167–1184. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Xu, Y.; Yin, D.; Zhao, F.; Hao, Z.; Zhong, Y.; Zhang, J.; Zhang, B.; Yin, X. Type 2 diabetes mellitus facilitates endometrial hyperplasia progression by activating the proliferative function of mucin O-glycosylating enzyme GALNT2. Biomed. Pharmacother. 2020, 131, 110764. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Sun, R.; Zeng, L.; Chen, Y.; Zhang, N.; Cao, S.; Deng, S.; Meng, X.; Yang, S. GALNT2 promotes cell proliferation, migration, and invasion by activating the Notch/Hes1-PTEN-PI3K/Akt signaling pathway in lung adenocarcinoma. Life Sci. 2021, 276, 119439. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, Z.; Zheng, Q.; Li, J. GALNT2/14 overexpression correlate with prognosis and methylation: Potential therapeutic targets for lung adenocarcinoma. Gene 2021, 790, 145689. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.M.; Liu, C.H.; Hu, R.H.; Huang, M.J.; Lee, J.J.; Chen, C.H.; Huang, J.; Lai, H.S.; Lee, P.H.; Hsu, W.M.; et al. Mucin Glycosylating Enzyme GALNT2 Regulates the Malignant Character of Hepatocellular Carcinoma by Modifying the EGF Receptor. Cancer Res. 2011, 71, 7270–7279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.Y.; Shun, C.T.; Hung, K.Y.; Juan, H.F.; Hsu, C.L.; Huang, M.C.; Lai, I.R. Mucin glycosylating enzyme GALNT2 suppresses malignancy in gastric adenocarcinoma by reducing MET phosphorylation. Oncotarget 2016, 7, 11251–11262. [Google Scholar] [CrossRef]
- Zhang, G.; Lu, J.; Yang, M.; Wang, Y.; Liu, H.; Xu, C. Elevated GALNT10 expression identifies immunosuppressive microenvironment and dismal prognosis of patients with high grade serous ovarian cancer. Cancer Immunol. Immunother. 2020, 69, 175–187. [Google Scholar] [CrossRef]
- Liu, Z.; Pan, L.; Yan, X.; Duan, X. The long noncoding RNA DLGAP1-AS2 facilitates cholangiocarcinoma progression via miR-505 and GALNT10. FEBS Open Bio 2021, 11, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liu, H.O.; Liu, Y.D.; Liu, W.S.; Pan, D.; Zhang, W.J.; Yang, L.; Fu, Q.; Xu, J.J.; Gu, J.X. Decreased Expression of Hepatocyte Nuclear Factor 4α (Hnf4α)/MicroRNA-122 (miR-122) Axis in Hepatitis B Virus-associated Hepatocellular Carcinoma Enhances Potential Oncogenic GALNT10 Protein Activity. J. Biol. Chem. 2015, 290, 1170–1185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.B.; Pyo, K.H.; Kim, H.R. Role and Function of O-GlcNAcylation in Cancer. Cancers 2021, 13, 5365. [Google Scholar] [CrossRef] [PubMed]
- Rozanski, W.; Krzeslak, A.; Forma, E.; Brys, M.; Blewniewski, M.; Wozniak, P.; Lipinski, M. Prediction of bladder cancer based on urinary content of MGEA5 and OGT mRNA level. Clin. Lab. 2012, 58, 579–583. [Google Scholar] [PubMed]
- Wang, L.; Chen, S.; Zhang, Z.; Zhang, J.; Mao, S.; Zheng, J.; Xuan, Y.; Liu, M.; Cai, K.; Zhang, W.; et al. Suppressed OGT expression inhibits cell proliferation while inducing cell apoptosis in bladder cancer. BMC Cancer 2018, 18, 1141. [Google Scholar] [CrossRef] [Green Version]
- Holdener, B.C.; Haltiwanger, R.S. Protein O-fucosylation: Structure and function. Curr. Opin. Struct. Biol. 2019, 56, 78–86. [Google Scholar] [CrossRef]
- Chabanais, J.; Labrousse, F.; Chaunavel, A.; Germot, A.; Maftah, A. POFUT1 as a Promising Novel Biomarker of Colorectal Cancer. Cancers 2018, 10, 411. [Google Scholar] [CrossRef] [Green Version]
- Komor, M.A.; de Wit, M.; van den Berg, J.; Martens de Kemp, S.R.; Delis-van Diemen, P.M.; Bolijn, A.S.; Tijssen, M.; Schelfhorst, T.; Piersma, S.R.; Chiasserini, D.; et al. Molecular characterization of colorectal adenomas reveals POFUT1 as a candidate driver of tumor progression. Int. J. Cancer 2020, 146, 1979–1992. [Google Scholar] [CrossRef]
- Du, Y.; Li, D.; Li, N.; Su, C.; Yang, C.; Lin, C.; Chen, M.; Wu, R.; Li, X.; Hu, G. POFUT1 promotes colorectal cancer development through the activation of Notch1 signaling. Cell Death Dis. 2018, 9, 995. [Google Scholar] [CrossRef]
- Li, D.; Lin, C.; Li, N.; Du, Y.; Yang, C.; Bai, Y.; Feng, Z.; Su, C.; Wu, R.; Song, S.; et al. PLAGL2 and POFUT1 are regulated by an evolutionarily conserved bidirectional promoter and are collaboratively involved in colorectal cancer by maintaining stemness. EBioMedicine 2019, 45, 124–138. [Google Scholar] [CrossRef] [Green Version]
- Deschuyter, M.; Pennarubia, F.; Pinault, E.; Legardinier, S.; Maftah, A. Functional Characterization of POFUT1 Variants Associated with Colorectal Cancer. Cancers 2020, 12, 1430. [Google Scholar] [CrossRef]
- Annani-Akollor, M.E.; Wang, S.; Fan, J.; Liu, L.; Padhiar, A.A.; Zhang, J. Downregulated protein O-fucosyl transferase 1 (Pofut1) expression exerts antiproliferative and antiadhesive effects on hepatocytes by inhibiting Notch signalling. Biomed. Pharmacother. 2014, 68, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Dong, P.; Liu, L.; Gao, Q.; Duan, M.; Zhang, S.; Chen, S.; Xue, R.; Wang, X. Overexpression of protein O-fucosyltransferase 1 accelerates hepatocellular carcinoma progression via the Notch signaling pathway. Biochem. Biophys. Res. Commun. 2016, 473, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Huang, H.; Zhang, J.; Wu, Q.; Chen, X.; Huang, T.; Li, W.; Liu, Y.; Zhang, J. Caveolin-1 promotes invasion and metastasis by upregulating Pofut1 expression in mouse hepatocellular carcinoma. Cell Death Dis. 2019, 10, 477. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, J.; Ma, X.; Wang, M.; Zhou, L. POFUT1 acts as a tumor promoter in glioblastoma by enhancing the activation of Notch signaling. J. Bioenerg. Biomembr. 2021, 53, 621–632. [Google Scholar] [CrossRef]
- Leng, Q.; Tsou, J.H.; Zhan, M.; Jiang, F. Fucosylation genes as circulating biomarkers for lung cancer. J. Cancer Res. Clin. Oncol. 2018, 144, 2109–2115. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Wang, Z.; Huang, B.; Zhang, J.; Ge, Y.; Fan, Q.; Wang, Z. Bioinformatics insight into glycosyltransferase gene expression in gastric cancer: POFUT1 is a potential biomarker. Biochem. Biophys. Res. Commun. 2017, 483, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Sadeghzadeh, Z.; Khosravi, A.; Jazi, M.S.; Asadi, J. Upregulation of Fucosyltransferase 3, 8 and protein O-Fucosyltransferase 1, 2 genes in esophageal cancer stem-like cells (CSLCs). Glycoconj. J. 2020, 37, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Wan, G.; Tian, L.; Yu, Y.; Li, F.; Wang, X.; Li, C.; Deng, S.; Yu, X.; Cai, X.; Zuo, Z.; et al. Overexpression of Pofut1 and activated Notch1 may be associated with poor prognosis in breast cancer. Biochem. Biophys. Res. Commun. 2017, 491, 104–111. [Google Scholar] [CrossRef]
- Yokota, S.; Ogawara, K.; Kimura, R.; Shimizu, F.; Baba, T.; Minakawa, Y.; Higo, M.; Kasamatsu, A.; Endo-Sakamoto, Y.; Shiiba, M.; et al. Protein O-fucosyltransferase 1: A potential diagnostic marker and therapeutic target for human oral cancer. Int. J. Oncol. 2013, 43, 1864–1870. [Google Scholar] [CrossRef] [Green Version]
- Wahby, S.; Jarczyk, J.; Fierek, A.; Heinkele, J.; Weis, C.A.; Eckstein, M.; Martini, T.; Porubsky, S.; Hafner, M.; Erben, P. POFUT1 mRNA expression as an independent prognostic parameter in muscle-invasive bladder cancer. Transl. Oncol. 2021, 14, 100900. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, H. UDP-Glucose Ceramide Glycosyltransferase Contributes to the Proliferation and Glycolysis of Cervical Cancer Cells by Regulating the PI3K/AKT Pathway. Ann. Clin. Lab. Sci. 2021, 51, 663–669. [Google Scholar] [PubMed]
- Tokuda, N.; Numata, S.; Li, X.; Nomura, T.; Takizawa, M.; Kondo, Y.; Yamashita, Y.; Hashimoto, N.; Kiyono, T.; Urano, T.; et al. β4GalT6 is involved in the synthesis of lactosylceramide with less intensity than β4GalT5. Glycobiology 2013, 23, 1175–1183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaji, T.; Hanada, K. Establishment of HeLa cell mutants deficient in sphingolipid-related genes using TALENs. PLoS ONE 2014, 9, e88124. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.; Li, M.; Qi, X.; Li, J. β1,4-Galactosyltransferase V Modulates Breast Cancer Stem Cells through Wnt/β-catenin Signaling Pathway. Cancer Res. Treat. 2020, 52, 1084–1102. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Ma, H.; Wei, W.; Ji, D.; Song, X.; Sun, J.; Zhang, J.; Jia, L. B4GALT family mediates the multidrug resistance of human leukemia cells by regulating the hedgehog pathway and the expression of p-glycoprotein and multidrug resistance-associated protein 1. Cell Death Dis. 2013, 4, e654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Y.J.; Ding, Y.; Levery, S.B.; Lobaton, M.; Handa, K.; Hakomori, S.I. Differential expression profiles of glycosphingolipids in human breast cancer stem cells vs. cancer non-stem cells. Proc. Natl. Acad. Sci. USA 2013, 110, 4968–4973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, T.; Wu, H.; Lin, M.; Yin, J.; Tan, L.; Ruan, Y.; Feng, M. B4GALNT1 promotes progression and metastasis in lung adenocarcinoma through JNK/c-Jun/Slug pathway. Carcinogenesis 2021, 42, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Mahata, B.; Dhir, A.; Mandal, T.K.; Biswas, K. Elevated histone H3 acetylation and loss of the Sp1-HDAC1 complex de-repress the GM2-synthase gene in renal cell carcinoma. J. Biol. Chem. 2019, 294, 1005–1018. [Google Scholar] [CrossRef] [Green Version]
- Mahata, B.; Banerjee, A.; Kundu, M.; Bandyopadhyay, U.; Biswas, K. TALEN mediated targeted editing of GM2/GD2-synthase gene modulates anchorage independent growth by reducing anoikis resistance in mouse tumor cells. Sci. Rep. 2015, 5, 9048. [Google Scholar] [CrossRef]
- Liang, Y.J.; Wang, C.Y.; Wang, I.A.; Chen, Y.W.; Li, L.T.; Lin, C.Y.; Ho, M.Y.; Chou, T.L.; Wang, Y.H.; Chiou, S.P.; et al. Interaction of glycosphingolipids GD3 and GD2 with growth factor receptors maintains breast cancer stem cell phenotype. Oncotarget 2017, 8, 47454–47473. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, H.; Koodie, L.; Jacobsen, K.; Hanzawa, K.; Miyamoto, Y.; Yamamoto, M. B4GALNT1 induces angiogenesis, anchorage independence growth and motility, and promotes tumorigenesis in melanoma by induction of ganglioside GM2/GD2. Sci. Rep. 2020, 10, 1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kundu, M.; Mahata, B.; Banerjee, A.; Chakraborty, S.; Debnath, S.; Ray, S.S.; Ghosh, Z.; Biswas, K. Ganglioside GM2 mediates migration of tumor cells by interacting with integrin and modulating the downstream signaling pathway. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 1472–1489. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.S.; Ribeiro, H.; Perez-Cabezas, B.; Cardoso, M.T.; Alegrete, N.; Gaspar, A.; Leao-Teles, E.; Macedo, M.F. The GM2 ganglioside inhibits iNKT cell responses in a CD1d-dependent manner. Mol. Genet. Metab. 2018, 125, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.Y.; Park, S.Y.; Kim, H.J.; Moon, S.; Lee, S.; Lee, S.H.; Kim, S.H. B3GNT5 is a novel marker correlated with stem-like phenotype and poor clinical outcome in human gliomas. CNS Neurosci. Ther. 2020, 26, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, A.; Marcos-Pinto, R.; Nairn, A.V.; Dela, R.M.; Ferreira, R.M.; Junqueira-Neto, S.; Freitas, D.; Gomes, J.; Oliveira, P.; Santos, M.R.; et al. Helicobacter pylori chronic infection and mucosal inflammation switches the human gastric glycosylation pathways. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2015, 1852, 1928–1939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carroll, D.J.; Burns, M.W.N.; Mottram, L.; Propheter, D.C.; Boucher, A.; Lessen, G.M.; Kumar, A.; Malaker, S.A.; Xing, C.; Hooper, L.V.; et al. Interleukin-22 regulates B3GNT7 expression to induce fucosylation of glycoproteins in intestinal epithelial cells. J. Biol. Chem. 2021, 298, 101463. [Google Scholar] [CrossRef]
- Lu, C.H.; Wu, W.Y.; Lai, Y.J.; Yang, C.M.; Yu, L.C. Suppression of B3GNT7 gene expression in colon adenocarcinoma and its potential effect in the metastasis of colon cancer cells. Glycobiology 2014, 24, 359–367. [Google Scholar] [CrossRef] [Green Version]
- Barkeer, S.; Chugh, S.; Karmakar, S.; Kaushik, G.; Rauth, S.; Rachagani, S.; Batra, S.K.; Ponnusamy, M.P. Novel role of O-glycosyltransferases GALNT3 and B3GNT3 in the self-renewal of pancreatic cancer stem cells. BMC Cancer 2018, 18, 1157. [Google Scholar] [CrossRef]
- Zhuang, H.; Zhou, Z.; Zhang, Z.; Chen, X.; Ma, Z.; Huang, S.; Gong, Y.; Zhang, C.; Hou, B. B3GNT3 overexpression promotes tumor progression and inhibits infiltration of CD8(+) T cells in pancreatic cancer. Aging 2021, 13, 2310–2329. [Google Scholar] [CrossRef]
- Zhang, W.; Hou, T.; Niu, C.; Song, L.; Zhang, Y. B3GNT3 Expression Is a Novel Marker Correlated with Pelvic Lymph Node Metastasis and Poor Clinical Outcome in Early-Stage Cervical Cancer. PLoS ONE 2015, 10, e0144360. [Google Scholar] [CrossRef]
- Wang, J.S.; Ruan, F.; Guo, L.Z.; Wang, F.G.; Wang, F.L.; An, H.M. B3GNT3 acts as a carcinogenic factor in endometrial cancer via facilitating cell growth, invasion and migration through regulating RhoA/RAC1 pathway-associated markers. Genes Genom. 2021, 43, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, T.; Xian, L.; Liu, W.; Liu, J.; Zhou, H. B3GNT3, a Direct Target of miR-149-5p, Promotes Lung Cancer Development and Indicates Poor Prognosis of Lung Cancer. Cancer Manag. Res. 2020, 12, 2381–2391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, L.; Zhang, H.; Zhang, B.; Zhu, J.; Chen, C.; Liu, W. B3GNT3 overexpression is associated with unfavourable survival in non-small cell lung cancer. J. Clin. Pathol. 2018, 71, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Kong, K.; Zhao, Y.; Xia, L.; Jiang, H.; Xu, M.; Zheng, J. B3GNT3: A prognostic biomarker associated with immune cell infiltration in pancreatic adenocarcinoma. Oncol. Lett. 2021, 21, 159. [Google Scholar] [CrossRef] [PubMed]
- Li, C.W.; Lim, S.O.; Chung, E.M.; Kim, Y.S.; Park, A.H.; Yao, J.; Cha, J.H.; Xia, W.; Chan, L.C.; Kim, T.; et al. Eradication of Triple-Negative Breast Cancer Cells by Targeting Glycosylated PD-L1. Cancer Cell 2018, 33, 187–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leng, X.; Wei, S.; Mei, J.; Deng, S.; Yang, Z.; Liu, Z.; Guo, C.; Deng, Y.; Xia, L.; Cheng, J.; et al. Identifying the prognostic significance of B3GNT3 with PD-L1 expression in lung adenocarcinoma. Transl. Lung Cancer Res. 2021, 10, 965–980. [Google Scholar] [CrossRef]
- Gupta, R.; Leon, F.; Thompson, C.M.; Nimmakayala, R.; Karmakar, S.; Nallasamy, P.; Chugh, S.; Prajapati, D.R.; Rachagani, S.; Kumar, S.; et al. Global analysis of human glycosyltransferases reveals novel targets for pancreatic cancer pathogenesis. Br. J. Cancer 2020, 122, 1661–1672. [Google Scholar] [CrossRef]
- Ho, W.L.; Che, M.I.; Chou, C.H.; Chang, H.H.; Jeng, Y.M.; Hsu, W.M.; Lin, K.H.; Huang, M.C. B3GNT3 expression suppresses cell migration and invasion and predicts favorable outcomes in neuroblastoma. Cancer Sci. 2013, 104, 1600–1608. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, F.; Wu, J.Y.; Qiu, Z.C.; Wang, Y.; Liu, F.; Ge, X.S.; Qi, X.W.; Mao, Y.; Hua, D. Clinical correlation of B7-H3 and B3GALT4 with the prognosis of colorectal cancer. World J. Gastroenterol. 2018, 24, 3538–3546. [Google Scholar] [CrossRef]
- Bi, C.; Shan, J.; Li, M.; Zhang, Q.; Li, C.; Tong, J.; Huang, Q. Long noncoding RNA differentiation antagonizing nonprotein coding RNA promotes the proliferation, invasion and migration of neuroblastoma cells via targeting β-1, 4-galactosyltransferase III by sponging miR-338-3p. Neuroreport 2021, 32, 965–974. [Google Scholar] [CrossRef]
- Chang, H.H.; Chen, C.H.; Chou, C.H.; Liao, Y.F.; Huang, M.J.; Chen, Y.H.; Wang, W.J.; Huang, J.; Hung, J.S.; Ho, W.L.; et al. β-1,4-Galactosyltransferase III Enhances Invasive Phenotypes Via β1-Integrin and Predicts Poor Prognosis in Neuroblastoma. Clin. Cancer Res. 2013, 19, 1705–1716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, T.; Li, Y.; Chen, B. B4GALT3 promotes cell proliferation and invasion in glioblastoma. Neurol. Res. 2020, 42, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yang, X.; Liu, M.; Tang, H. B4GALT3 up-regulation by miR-27a contributes to the oncogenic activity in human cervical cancer cells. Cancer Lett. 2016, 375, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Wang, S.H.; Liu, C.H.; Wu, Y.L.; Wang, W.J.; Huang, J.; Hung, J.S.; Lai, I.R.; Liang, J.T.; Huang, M.C. β-1,4-Galactosyltransferase III suppresses β1 integrin-mediated invasive phenotypes and negatively correlates with metastasis in colorectal cancer. Carcinogenesis 2014, 35, 1258–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheikh, M.O.; Halmo, S.M.; Wells, L. Recent advancements in understanding mammalian O-mannosylation. Glycobiology 2017, 27, 806–819. [Google Scholar] [CrossRef] [Green Version]
- Wells, L. The O-mannosylation pathway: Glycosyltransferases and proteins implicated in congenital muscular dystrophy. J. Biol. Chem. 2013, 288, 6930–6935. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, S.; Oliveira, T.; Bartels, M.F.; Miyoshi, E.; Pierce, M.; Taniguchi, N.; Carneiro, F.; Seruca, R.; Reis, C.A.; Strahl, S.; et al. O-mannosylation and N-glycosylation: Two coordinated mechanisms regulating the tumour suppressor functions of E-cadherin in cancer. Oncotarget 2016, 7, 65231–65246. [Google Scholar] [CrossRef] [Green Version]
- Vojta, A.; Samarzija, I.; Bockor, L.; Zoldos, V. Glyco-genes change expression in cancer through aberrant methylation. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2016, 1860, 1776–1785. [Google Scholar] [CrossRef]
- Mehta, K.A.; Patel, K.A.; Pandya, S.J.; Patel, P.S. Aberrant sialylation plays a significant role in oral squamous cell carcinoma progression. J. Oral Pathol. Med. 2020, 49, 253–259. [Google Scholar] [CrossRef]
- Sturgill, E.R.; Aoki, K.; Lopez, P.H.; Colacurcio, D.; Vajn, K.; Lorenzini, I.; Majic, S.; Yang, W.H.; Heffer, M.; Tiemeyer, M.; et al. Biosynthesis of the major brain gangliosides GD1a and GT1b. Glycobiology 2012, 22, 1289–1301. [Google Scholar] [CrossRef] [Green Version]
- Aloia, A.; Petrova, E.; Tomiuk, S.; Bissels, U.; Deas, O.; Saini, M.; Zickgraf, F.M.; Wagner, S.; Spaich, S.; Sutterlin, M.; et al. The sialyl-glycolipid stage-specific embryonic antigen 4 marks a subpopulation of chemotherapy-resistant breast cancer cells with mesenchymal features. Breast Cancer Res. 2015, 17, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, Q.; Chen, X.; Han, Y.; Lei, T.; Wu, Q.; Yu, X.; Wang, L.; Fan, Z.; Wang, S. Modification of α2,6-sialylation mediates the invasiveness and tumorigenicity of non-small cell lung cancer cells in vitro and in vivo via Notch1/Hes1/MMPs pathway. Int. J. Cancer 2018, 143, 2319–2330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, X.; Jia, L.; Zhou, H.; Song, X.; Zhou, M.; Xu, J.; Zhao, L.; Feng, X.; Zhao, Y. miR-4299 mediates the invasive properties and tumorigenicity of human follicular thyroid carcinoma by targeting ST6GALNAC4. IUBMB Life 2016, 68, 136–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reticker-Flynn, N.E.; Bhatia, S.N. Aberrant Glycosylation Promotes Lung Cancer Metastasis through Adhesion to Galectins in the Metastatic Niche. Cancer Discov. 2015, 5, 168–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balmana, M.; Diniz, F.; Feijao, T.; Barrias, C.C.; Mereiter, S.; Reis, C.A. Analysis of the Effect of Increased α2,3-Sialylation on RTK Activation in MKN45 Gastric Cancer Spheroids Treated with Crizotinib. Int. J. Mol. Sci. 2020, 21, 722. [Google Scholar] [CrossRef] [Green Version]
- Dai, Y.; Cheng, Z.; Pang, Y.; Jiao, Y.; Qian, T.; Quan, L.; Cui, L.; Liu, Y.; Si, C.; Chen, J.; et al. Prognostic value of the FUT family in acute myeloid leukemia. Cancer Gene Ther. 2020, 27, 70–80. [Google Scholar] [CrossRef]
- Jassam, S.A.; Maherally, Z.; Ashkan, K.; Pilkington, G.J.; Fillmore, H.L. Fucosyltransferase 4 and 7 mediates adhesion of non-small cell lung cancer cells to brain-derived endothelial cells and results in modification of the blood-brain-barrier: In vitro investigation of CD15 and CD15s in lung-to-brain metastasis. J. Neuro-Oncol. 2019, 143, 405–415. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.X.; Gao, W.; Cai, L. Fucosyltransferase VII promotes proliferation via the EGFR/AKT/mTOR pathway in A549 cells. OncoTargets Ther. 2017, 10, 3971–3978. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Sun, H.; Bai, G.; Wang, W.; Liu, M.; Bao, Z.; Li, J.; Liu, H. α-1,3-Fucosyltransferase-VII siRNA inhibits the expression of SLex and hepatocarcinoma cell proliferation. Int. J. Mol. Med. 2018, 42, 2700–2708. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Zheng, Q.; Chen, S.; Liu, J.; Li, S. FUT7 Promotes the Epithelial-Mesenchymal Transition and Immune Infiltration in Bladder Urothelial Carcinoma. J. Inflamm. Res. 2021, 14, 1069–1084. [Google Scholar] [CrossRef]
- Qin, H.; Liu, J.; Yu, M.; Wang, H.; Thomas, A.M.; Li, S.; Yan, Q.; Wang, L. FUT7 promotes the malignant transformation of follicular thyroid carcinoma through alpha1,3-fucosylation of EGF receptor. Exp. Cell Res. 2020, 393, 112095. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Liu, J.; Xia, Y.; Wang, Z.; Li, X.; Gao, Q. Integrated analysis reveals the participation of IL4I1, ITGB7, and FUT7 in reshaping the TNBC immune microenvironment by targeting glycolysis. Ann. Med. 2021, 53, 916–928. [Google Scholar] [CrossRef] [PubMed]
- Gomes Ferreira, I.; Pucci, M.; Venturi, G.; Malagolini, N.; Chiricolo, M.; Dall’Olio, F. Glycosylation as a Main Regulator of Growth and Death Factor Receptors Signaling. Int. J. Mol. Sci. 2018, 19, 580. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; He, Z.; Guo, L.; Wang, C.; Lin, C.; Ye, L.; Wang, X.; Li, Y.; Yang, M.; Liu, S.; et al. ALG3 contributes to stemness and radioresistance through regulating glycosylation of TGF-beta receptor II in breast cancer. J. Exp. Clin. Cancer Res. 2021, 40, 149. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhou, Y.; Xiong, X.; Huang, M.; Ying, X.; Wang, M. ALG3 Is Activated by Heat Shock Factor 2 and Promotes Breast Cancer Growth. Med. Sci. Monit. 2018, 24, 3479–3487. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, B.; Xu, G.; Han, C.; Xing, G. lncRNA MIR44352HG promotes the progression of liver cancer by upregulating B3GNT5 expression. Mol. Med. Rep. 2022, 25, 38. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhao, B.; Chen, X.; Geng, X.; Zhang, Z. Circ_0009910 sponges miR-491-5p to promote acute myeloid leukemia progression through modulating B4GALT5 expression and PI3K/AKT signaling pathway. Int. J. Lab. Hematol. 2022, 44, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.L.; Chou, C.H.; Jeng, Y.M.; Lu, M.Y.; Yang, Y.L.; Jou, S.T.; Lin, D.T.; Chang, H.H.; Lin, K.H.; Hsu, W.M.; et al. GALNT2 suppresses malignant phenotypes through IGF-1 receptor and predicts favorable prognosis in neuroblastoma. Oncotarget 2014, 5, 12247–12259. [Google Scholar] [CrossRef] [Green Version]
- Zhao, F.Y.; Zhang, Q.; Wang, J.M.; Jiang, J.Y.; Huyan, L.Y.; Liu, B.Q.; Yan, J.; Li, C.; Wang, H.Q. BAG3 epigenetically regulates GALNT10 expression via WDR5 and facilitates the stem cell-like properties of platin-resistant ovarian cancer cells. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2021, 1868, 119077. [Google Scholar] [CrossRef]
- Gomes, C.; Osorio, H.; Pinto, M.T.; Campos, D.; Oliveira, M.J.; Reis, C.A. Expression of ST3GAL4 leads to SLex expression and induces c-Met activation and an invasive phenotype in gastric carcinoma cells. PLoS ONE 2013, 8, e66737. [Google Scholar] [CrossRef]
- Mereiter, S.; Magalhaes, A.; Adamczyk, B.; Jin, C.; Almeida, A.; Drici, L.; Ibanez-Vea, M.; Gomes, C.; Ferreira, J.A.; Afonso, L.P.; et al. Glycomic analysis of gastric carcinoma cells discloses glycans as modulators of RON receptor tyrosine kinase activation in cancer. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2016, 1860, 1795–1808. [Google Scholar] [CrossRef] [PubMed]
- Greville, G.; Llop, E.; Huang, C.; Creagh-Flynn, J.; Pfister, S.; O’Flaherty, R.; Madden, S.F.; Peracaula, R.; Rudd, P.M.; McCann, A.; et al. Hypoxia Alters Epigenetic and N-Glycosylation Profiles of Ovarian and Breast Cancer Cell Lines in-vitro. Front. Oncol. 2020, 10, 1218. [Google Scholar] [CrossRef]
- Greville, G.; Llop, E.; Howard, J.; Madden, S.F.; Perry, A.S.; Peracaula, R.; Rudd, P.M.; McCann, A.; Saldova, R. 5-AZA-dC induces epigenetic changes associated with modified glycosylation of secreted glycoproteins and increased EMT and migration in chemo-sensitive cancer cells. Clin. Epigenetics 2021, 13, 34. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Shao, J.; Wang, Y.; Shen, H.; Yu, S.; Zhang, J.; Yin, L. Hsa-miR-370 inhibited P-selectin-induced cell adhesion in human colon adenocarcinoma cells. Mol. Cell. Biochem. 2019, 450, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Pucci, M.; Gomes Ferreira, I.; Orlandani, M.; Malagolini, N.; Ferracin, M.; Dall’Olio, F. High Expression of the Sda Synthase B4GALNT2 Associates with Good Prognosis and Attenuates Stemness in Colon Cancer. Cells 2020, 9, 948. [Google Scholar] [CrossRef] [Green Version]
- Pucci, M.; Ferreira, I.G.; Malagolini, N.; Ferracin, M.; Dall’Olio, F. The Sda Synthase B4GALNT2 Reduces Malignancy and Stemness in Colon Cancer Cell Lines Independently of Sialyl Lewis X Inhibition. Int. J. Mol. Sci. 2020, 21, 6558. [Google Scholar] [CrossRef]
- Pucci, M.; Malagolini, N.; Dall’Olio, F. Glycosyltransferase B4GALNT2 as a Predictor of Good Prognosis in Colon Cancer: Lessons from Databases. Int. J. Mol. Sci. 2021, 22, 4331. [Google Scholar] [CrossRef]
- Yu, P.; Zhu, L.; Cui, K.; Du, Y.; Zhang, C.; Ma, W.; Guo, J. B4GALNT2 Gene Promotes Proliferation, and Invasiveness and Migration Abilities of Model Triple Negative Breast Cancer (TNBC) Cells by Interacting With HLA-B Protein. Front. Oncol. 2021, 11, 722828. [Google Scholar] [CrossRef]
- Dall’Olio, F.; Chiricolo, M. Sialyltransferases in cancer. Glycoconj. J. 2001, 18, 841–850. [Google Scholar] [CrossRef]
- Dall’Olio, F.; Malagolini, N.; Trinchera, M.; Chiricolo, M. Sialosignaling: Sialyltransferases as engines of self-fueling loops in cancer progression. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2014, 1840, 2752–2764. [Google Scholar] [CrossRef] [Green Version]
- Shan, M.; Yang, D.; Dou, H.; Zhang, L. Fucosylation in cancer biology and its clinical applications. Prog. Mol. Biol. Transl. Sci. 2019, 162, 93–119. [Google Scholar] [CrossRef] [PubMed]
- Granovsky, M.; Fata, J.; Pawling, J.; Muller, W.J.; Khokha, R.; Dennis, J.W. Suppression of tumor growth and metastasis in Mgat5-deficient mice. Nat. Med. 2000, 6, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Bastian, K.; Scott, E.; Elliott, D.J.; Munkley, J. FUT8 α-1,6-Fucosyltransferase in Cancer. Int. J. Mol. Sci. 2021, 22, 455. [Google Scholar] [CrossRef] [PubMed]
- Pinho, S.; Marcos, N.T.; Ferreira, B.; Carvalho, A.S.; Oliveira, M.J.; Santos-Silva, F.; Harduin-Lepers, A.; Reis, C.A. Biological significance of cancer-associated sialyl-Tn antigen: Modulation of malignant phenotype in gastric carcinoma cells. Cancer Lett. 2007, 249, 157–170. [Google Scholar] [CrossRef]
- Julien, S.; Adriaenssens, E.; Ottenberg, K.; Furlan, A.; Courtand, G.; Vercoutter-Edouart, A.-S.; Hanisch, F.-G.; Delannoy, P.; Le Bourhis, X. ST6GalNAc I expression in MDA-MB-231 breast cancer cells greatly modifies their O-glycosylation pattern and enhances their tumourigenicity. Glycobiology 2006, 16, 54–64. [Google Scholar] [CrossRef] [Green Version]
- Cazet, A.; Lefebvre, J.; Adriaenssens, E.; Julien, S.; Bobowski, M.; Grigoriadis, A.; Tutt, A.; Tulasne, D.; Le Bourhis, X.; Delannoy, P. GD3 synthase expression enhances proliferation and tumor growth of MDA-MB-231 breast cancer cells through c-Met activation. Mol. Cancer Res. 2010, 8, 1526–1535. [Google Scholar] [CrossRef] [Green Version]
- Pinho, S.S.; Oliveira, P.; Cabral, J.; Carvalho, S.; Huntsman, D.; Gartner, F.; Seruca, R.; Reis, C.A.; Oliveira, C. Loss and recovery of Mgat3 and GnT-III Mediated E-cadherin N-glycosylation is a mechanism involved in epithelial-mesenchymal-epithelial transitions. PLoS ONE 2012, 7, e33191. [Google Scholar] [CrossRef] [Green Version]
- Pinho, S.S.; Reis, C.A.; Paredes, J.; Magalhaes, A.M.; Ferreira, A.C.; Figueiredo, J.; Xiaogang, W.; Carneiro, F.; Gartner, F.; Seruca, R. The role of N-acetylglucosaminyltransferase III and V in the post-transcriptional modifications of E-cadherin. Hum. Mol. Genet. 2009, 18, 2599–2608. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.; Sun, R.; Oh, W.; Kim, A.M.J.; Schwarz, J.R.; Lim, S.O. Saccharide analog, 2-deoxy-d-glucose enhances 4-1BB-mediated antitumor immunity via PD-L1 deglycosylation. Mol. Carcinog. 2020, 59, 691–700. [Google Scholar] [CrossRef]
- Lee, H.H.; Wang, Y.N.; Xia, W.; Chen, C.H.; Rau, K.M.; Ye, L.; Wei, Y.; Chou, C.K.; Wang, S.C.; Yan, M.; et al. Removal of N-Linked Glycosylation Enhances PD-L1 Detection and Predicts Anti-PD-1/PD-L1 Therapeutic Efficacy. Cancer Cell 2019, 36, 168–178. [Google Scholar] [CrossRef]
- Repas, J.; Zupin, M.; Vodlan, M.; Veranic, P.; Gole, B.; Potocnik, U.; Pavlin, M. Dual Effect of Combined Metformin and 2-Deoxy-D-Glucose Treatment on Mitochondrial Biogenesis and PD-L1 Expression in Triple-Negative Breast Cancer Cells. Cancers 2022, 14, 1343. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.; Li, C.W.; Lim, S.O.; Sun, L.; Lai, Y.J.; Hou, J.; Liu, C.; Chang, C.W.; Qiu, Y.; Hsu, J.M.; et al. Deglycosylation of PD-L1 by 2-deoxyglucose reverses PARP inhibitor-induced immunosuppression in triple-negative breast cancer. Am. J. Cancer Res. 2018, 8, 1837–1846. [Google Scholar]
- Sun, L.; Li, C.W.; Chung, E.M.; Yang, R.; Kim, Y.S.; Park, A.H.; Lai, Y.J.; Yang, Y.; Wang, Y.H.; Liu, J.; et al. Targeting Glycosylated PD-1 Induces Potent Antitumor Immunity. Cancer Res. 2020, 80, 2298–2310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.N.; Lee, H.H.; Hsu, J.L.; Yu, D.; Hung, M.C. The impact of PD-L1 N-linked glycosylation on cancer therapy and clinical diagnosis. J. Biomed. Sci. 2020, 27, 77. [Google Scholar] [CrossRef] [PubMed]
- Ashkani, J.; Naidoo, K.J. Glycosyltransferase Gene Expression Profiles Classify Cancer Types and Propose Prognostic Subtypes. Sci. Rep. 2016, 6, 26451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhat, S.A.; Mir, M.U.R.; Majid, S.; Hassan, T.; Rehman, M.U.; Kuchy, S. Diagnostic utility of glycosyltransferase mRNA expression in gastric cancer. Hematol./Oncol. Stem Cell Ther. 2018, 11, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Mohamed Abd-El-Halim, Y.; El, K.A.; Silvy, F.; Rubis, M.; Bigonnet, M.; Roques, J.; Cros, J.; Nicolle, R.; Iovanna, J.; Dusetti, N.; et al. A glycosyltransferase gene signature to detect pancreatic ductal adenocarcinoma patients with poor prognosis. EBioMedicine 2021, 71, 103541. [Google Scholar] [CrossRef]
- Noda, M.; Okayama, H.; Tachibana, K.; Sakamoto, W.; Saito, K.; Thar Min, A.K.; Ashizawa, M.; Nakajima, T.; Aoto, K.; Momma, T.; et al. Glycosyltransferase gene expression identifies a poor prognostic colorectal cancer subtype associated with mismatch repair deficiency and incomplete glycan synthesis. Clin. Cancer Res. 2018, 24, 4468–4481. [Google Scholar] [CrossRef] [Green Version]


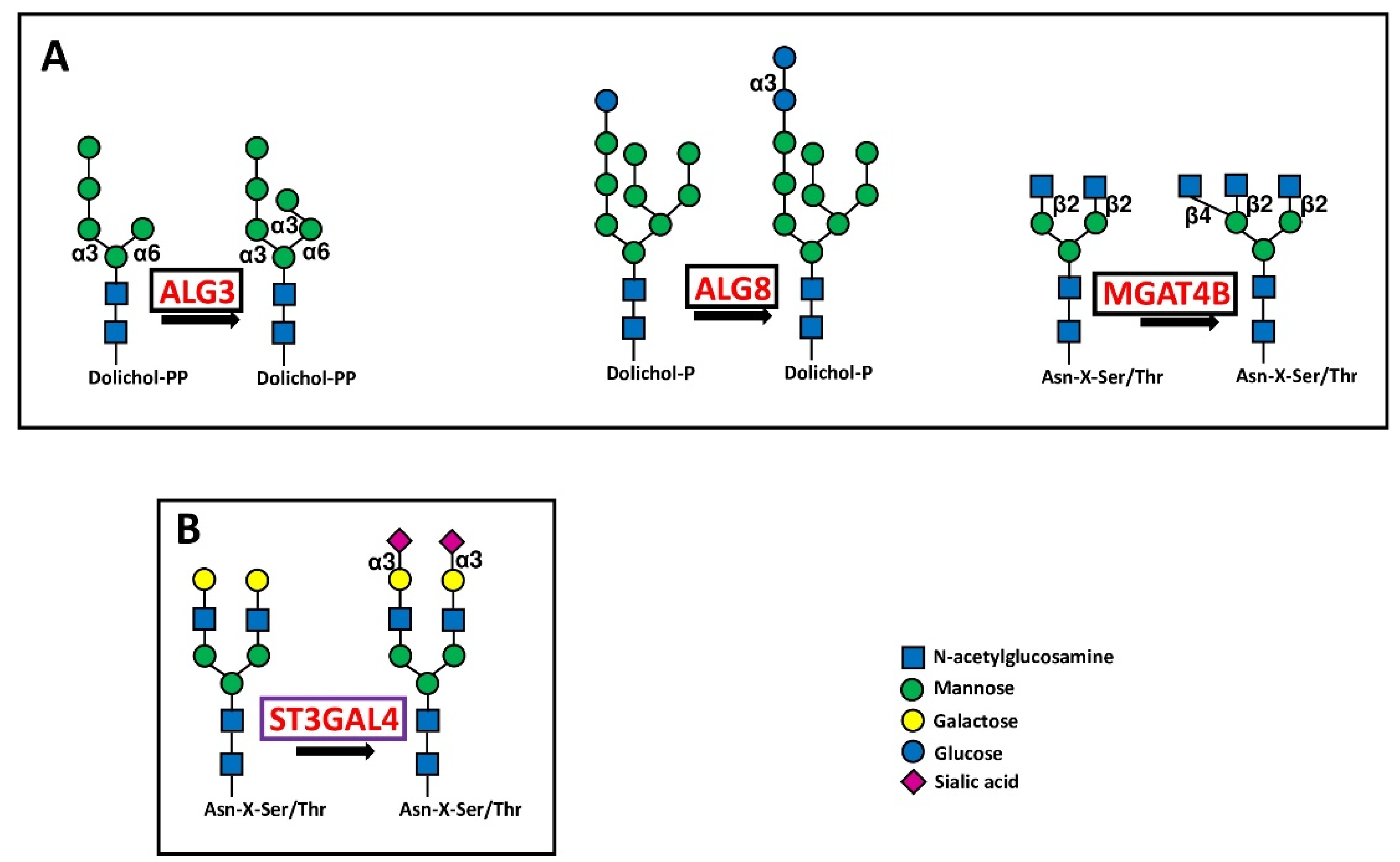
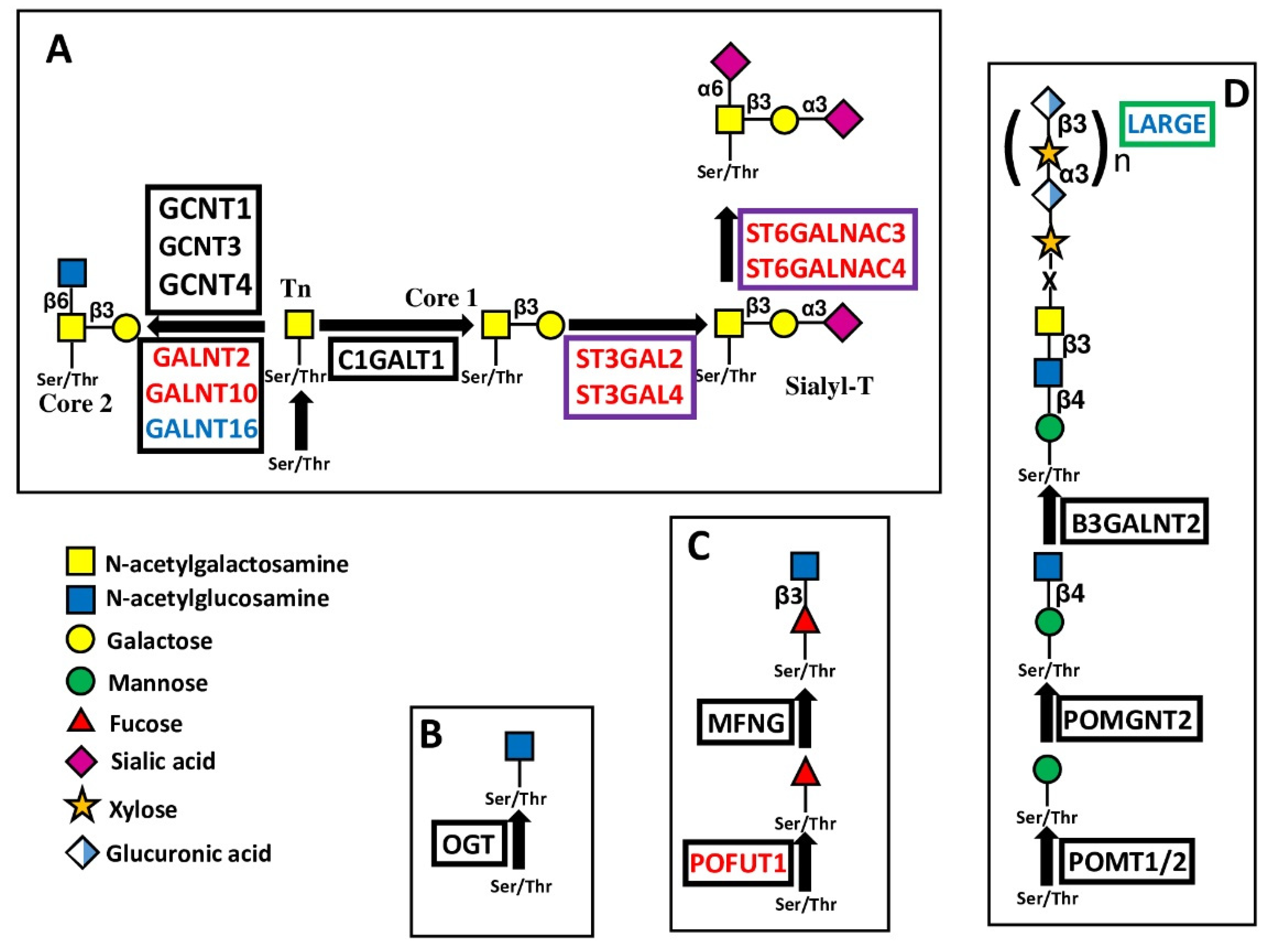
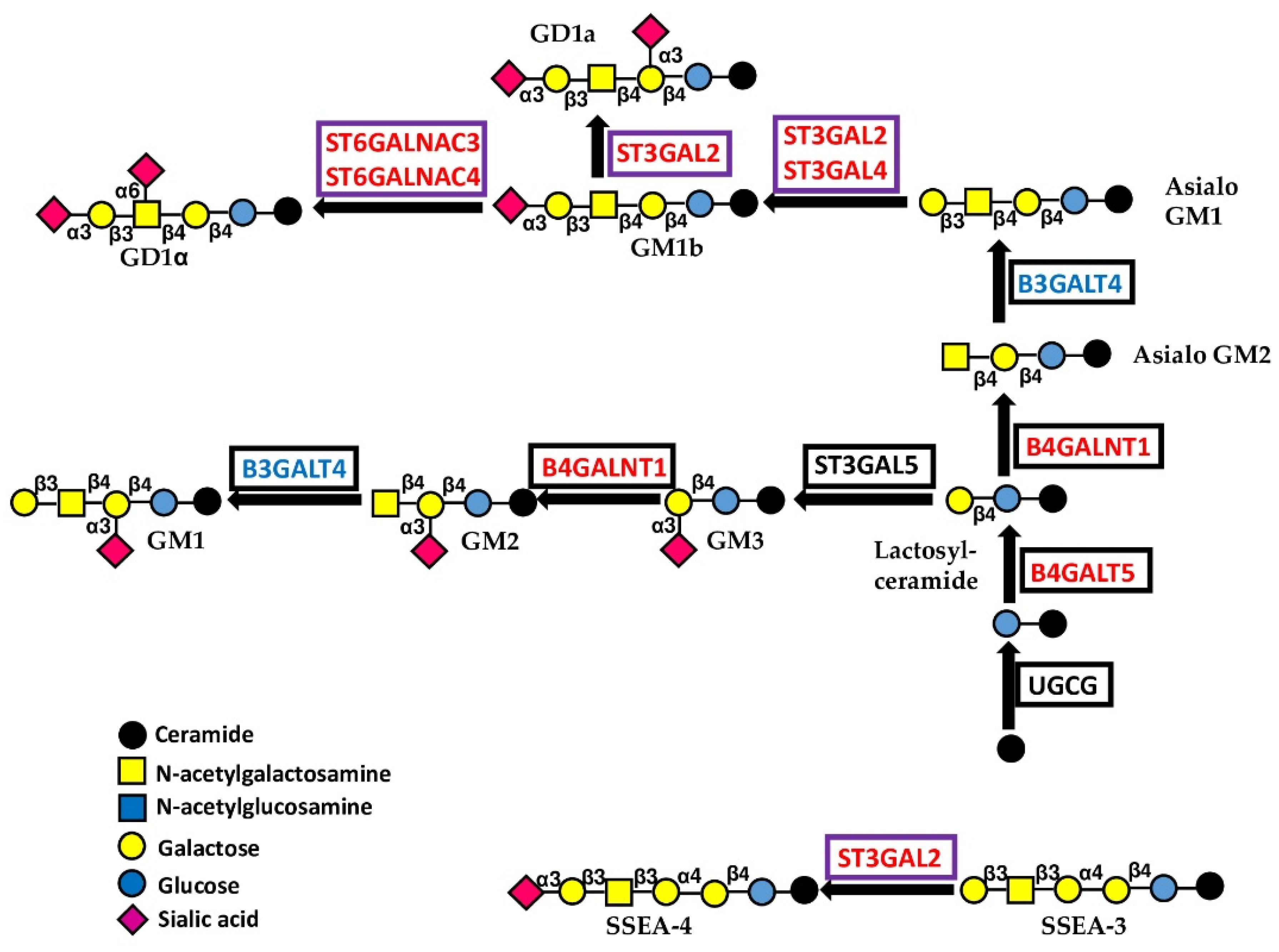
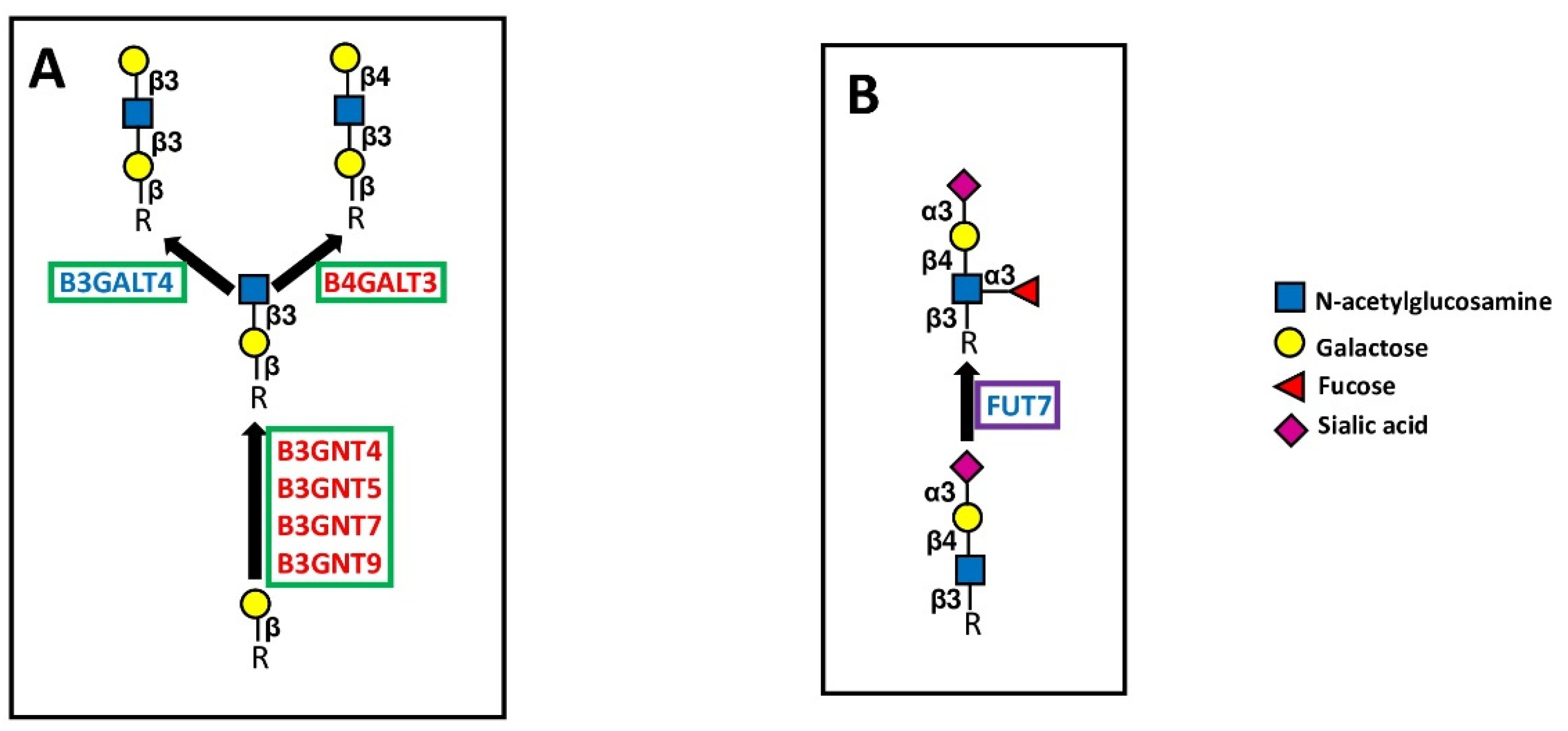
| ALG3 | ALG8 | B3GALT4 | B3GNT4 | B3GNT5 | B3GNT7 | B3GNT9 | B4GALNT1 | B4GALT3 | B4GALT5 | FUT7 | GALNT2 | GALNT10 | GALNT16 | LARGE | MGAT4B | POFUT1 | ST3GAL2 | ST3GAL4 | ST6GALNAC3 | ST6GALNAC4 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BRCA | |||||||||||||||||||||
| HNSC | |||||||||||||||||||||
| ESCA | |||||||||||||||||||||
| STAD | |||||||||||||||||||||
| COAD | |||||||||||||||||||||
| READ | |||||||||||||||||||||
| LIHC | |||||||||||||||||||||
| PAAD | |||||||||||||||||||||
| KIRC | |||||||||||||||||||||
| KIRP | |||||||||||||||||||||
| BLCA | |||||||||||||||||||||
| CESC | |||||||||||||||||||||
| UCEC | |||||||||||||||||||||
| OV | |||||||||||||||||||||
| LUAD | |||||||||||||||||||||
| LUSC | |||||||||||||||||||||
| GBM | |||||||||||||||||||||
| LGG | |||||||||||||||||||||
| SKCM | |||||||||||||||||||||
| SARC | |||||||||||||||||||||
| LAML |
| Pathway | Enzyme | Activity | Product | Score |
|---|---|---|---|---|
| Core N-glycosylation | ALG3 | α1,3-mannosyltransferase | Mannosylated precursor | 9 |
| ALG8 | α1,3-glucosyltransferase | Glucosylated precursor | 7 | |
| MGAT4B | β1,4 GlcNAc transferase B | β1,4-branched N-glycans | 6 | |
| Core O-glycosylation (mucin type) | GALNT2 | Protein:O-GalNAC transferase 2 | Tn-antigen | 8 |
| GALNT10 | Protein:O-GalNAC transferase 10 | Tn-antigen | 5 | |
| GALNT16 | Protein:O-GalNAC transferase 16 | Tn-antigen | −6 | |
| O-fucosylation | POFUT1 | Protein O-fucosyltransferase 1 | O-fucosylated NOTCH | 7 |
| Core of Glycolipids | B4GALT5 | β1,4-Galactosyltransferase 5 | Lactosylceramide | 8 |
| B4GALNT1 | β1,4-GalNAc transferase 1 | Ganglioside GM2, asialo GM2 | 8 | |
| Chain extension | B3GNT4 | β1,3-GlcNAc transferase 4 | Type 2 polylactosaminic chains | 5 |
| B3GNT5 | β1,3-GlcNAc transferase 5 | Lactotriaosylceramide | 7 | |
| B3GNT7 | β1,3-GlcNAc transferase 7 | Type 2 polylactosaminic chains | 6 | |
| B3GNT9 | β1,3-GlcNAc transferase 9 | Polylactosamines O-linked | 5 | |
| B4GALT3 | β1,4-Galactosyltransferase 3 | Type 2 lactosaminic chains | 7 | |
| B3GALT4 | β1,3-Galactosyltransferase 4 | Type 1 lactosaminic chains | −5 | |
| O-mannosylation | LARGE | Xylosyltransferase and β1,3-glucuronyltransferase | Elongated O-mannosyl glycans | −6 |
| Capping | ST3GAL2 | α2,3 to Gal sialyltransferase 2 | Sialyl-T; Gangliosides GD1a, GM1b, GT1b | 6 |
| ST3GAL4 | α2,3 to Gal sialyltransferase 4 | Sialyl-T; N-glycans; Gangliosides GD1a, GM1b | 6 | |
| ST6GALNAC3 | α2,6 to GalNAc sialyltransferase 3 | Di-sialyl T; Gangliosides GD1α, GM1b | 6 | |
| ST6GALNAC4 | α2,6 to GalNAc sialyltransferase 4 | Di-sialyl T; Ganglioside GD1α | 5 | |
| FUT7 | α1,3/6 fucosyltransferase 7 | Sialyl Lewis X | −6 |
| Enzyme | Upstream Regulator(s) | Downstream Pathways | Cancer Tissue/Cell Line | Effect * | |
|---|---|---|---|---|---|
| ALG3 | TGF-β receptor 2 | Breast | Stemness, radioresistance | [94] | |
| Heat shock factor 2 | Breast | Progression | [95] | ||
| miR-98-5p | Non-small cell lung | Progression | [5] | ||
| B3GNT3 | RhoA/RAC1 | Endometrial | Progression | [61] | |
| miR-149-5p | Lung | Progression | [62] | ||
| EGFR/PD-L1 | Lung | Immune escape | [66] | ||
| EGF/PD1-PD-L1 | Breast | Immune escape | [65] | ||
| B3GNT5 | lncRNA MIR44352HG/miR1365p | Liver | Progression | [96] | |
| B3GNT7 | Promoter methylation | Colorectal | Inhibition ** | [57] | |
| B4GALT3 | lncRNA DANCR/miR-338-3p | Neuroblastoma | Progression | [70] | |
| β1-integrins | Neuroblastoma | Progression | [71] | ||
| miR-27a | β1-integrins | Cervical | Progression | [73] | |
| β1-integrins | Colorectal | Inhibition | [74] | ||
| B4GALT5 | Wnt/β-catenin | Breast | Stemness | [44] | |
| Circ_0009910/miR-491-5p | PI3K/AKT | Acute myeloid leukemia | Progression | [97] | |
| B4GALNT1 | JNK/c-Jun/Slug | Lung | Progression | [47] | |
| EGFR | breast | Stemness | [50] | ||
| β1 integrins/FAK/SRC/ERK | Glioblastoma, lung, kidney | Progression | [52] | ||
| GM2/GD2 | Melanoma | Angiogenesis, progression | [51] | ||
| GALNT2 | IGF-R1 | Neuroblastoma | Inhibition | [98] | |
| Met | Gastric | Inhibition | [18] | ||
| EGFR | Liver | Inhibition | [17] | ||
| EGFR/AKT | Oral squamous | Progression | [12] | ||
| EGFR/PI3K/AKT/mTOR | Glioma | Progression | [13] | ||
| Notch/Hes1-PTEN-PI3K/AKT | Lung | Progression | [15] | ||
| GALNT10 | DLGAP1-AS2/miR-505 | Bile ducts | Progression | [20] | |
| HNF4/miR-122 | Liver | Progression | [21] | ||
| Histone methylation/ZBTB2 | Ovary | Stemness | [99] | ||
| FUT7 | EGFR/AKT/mTOR | Lung | Progression | [88] | |
| Promoter methylation | Bladder | Progression | [90] | ||
| EGFR | Thyroid | Progression | [91] | ||
| POFUT1 | Notch | Liver | Progression | [31,32] | |
| Colorectal | [28,29] | ||||
| Glioblastoma | [34] | ||||
| ST3GAL4 | Met, RON | Gastric | Progression | [85,100,101] | |
| Promoter methylation/GATA2 | Ovary, Breast | Progression | [102,103] | ||
| miR-370 | Colorectal | Adhesion | [104] | ||
| ST6GALNAC4 | miR-429 | Thyroid | Progression | [83] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pucci, M.; Duca, M.; Malagolini, N.; Dall’Olio, F. Glycosyltransferases in Cancer: Prognostic Biomarkers of Survival in Patient Cohorts and Impact on Malignancy in Experimental Models. Cancers 2022, 14, 2128. https://doi.org/10.3390/cancers14092128
Pucci M, Duca M, Malagolini N, Dall’Olio F. Glycosyltransferases in Cancer: Prognostic Biomarkers of Survival in Patient Cohorts and Impact on Malignancy in Experimental Models. Cancers. 2022; 14(9):2128. https://doi.org/10.3390/cancers14092128
Chicago/Turabian StylePucci, Michela, Martina Duca, Nadia Malagolini, and Fabio Dall’Olio. 2022. "Glycosyltransferases in Cancer: Prognostic Biomarkers of Survival in Patient Cohorts and Impact on Malignancy in Experimental Models" Cancers 14, no. 9: 2128. https://doi.org/10.3390/cancers14092128






