Combination of High-Resolution Structures for the B Cell Receptor and Co-Receptors Provides an Understanding of Their Interactions with Therapeutic Antibodies
Abstract
:Simple Summary
Abstract
1. Introduction
2. The B Cell Receptor
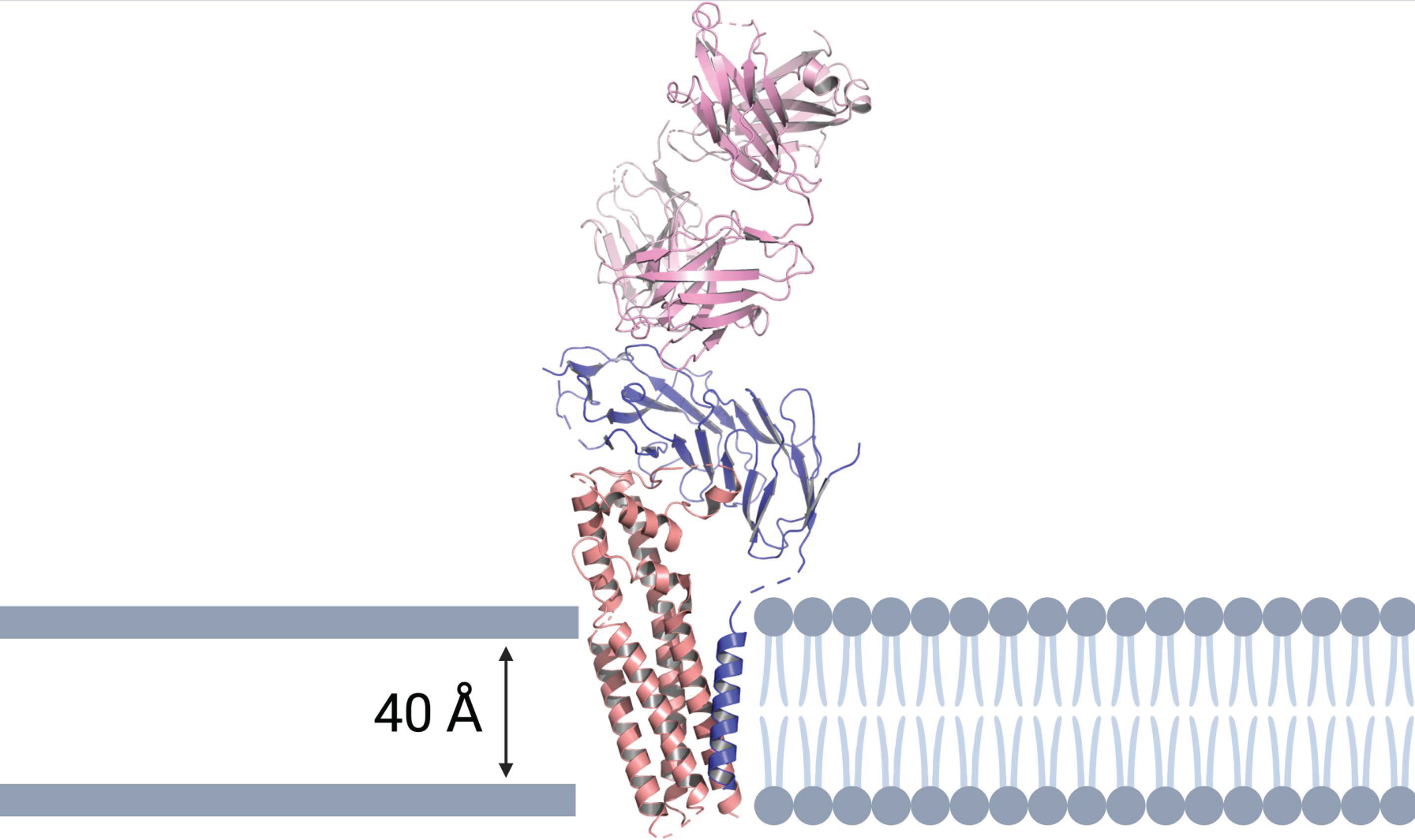
2.1. B Cell Receptor Structure
2.2. Targeting the BCR for Treating B Cell Malignancies
2.3. Polatuzumab Vedotin
2.4. Potential BCR Targets Identified by Cryo-Electron Microscopy
3. CD19, CD81 and CD21 Co-Receptor Complex
3.1. CD19 Regulation of the B Cell Receptor
3.2. Targeting CD19 and CD81 for Treating B Cell Malignancies
3.3. Coltuximab Ravtansine
3.4. Inebilizumab
3.5. Denintuzumab Mafodotin (SGN-CD19A)
3.6. Tafasitamab (XmAb5574)
3.7. Loncastuximab Tesirine
3.8. Blinatumomab
3.9. Antibodies against CD81
4. CD22 Co-Receptor
4.1. CD22 Structure
4.2. Antibodies against CD22
4.3. Epratuzumab and Radioimmunoconjugates
4.4. CD22 Antibody-Drug Conjugates
4.5. CD22 CAR-T Therapies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Winkler, T.H.; Mårtensson, I.-L. The Role of the Pre-B Cell Receptor in B Cell Development, Repertoire Selection, and Tolerance. Front. Immunol. 2018, 9, 2423. [Google Scholar] [CrossRef] [PubMed]
- Zaretsky, I.; Atrakchi, O.; Mazor, R.D.; Stoler-Barak, L.; Biram, A.; Feigelson, S.W.; Gitlin, A.D.; Engelhardt, B.; Shulman, Z. ICAMs support B cell interactions with T follicular helper cells and promote clonal selection. J. Exp. Med. 2017, 214, 3435–3448. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.A.; Wiestner, A. Targeting B cell receptor signalling in cancer: Preclinical and clinical advances. Nat. Rev. Cancer. 2018, 18, 148–167. [Google Scholar] [CrossRef]
- Li, J.; Yin, W.; Jing, Y.; Kang, D.; Yang, L.; Cheng, J.; Yu, Z.; Peng, Z.; Li, X.; Wen, Y.; et al. The Coordination Between B Cell Receptor Signaling and the Actin Cytoskeleton During B Cell Activation. Front. Immunol. 2018, 9, 3096. [Google Scholar] [CrossRef] [PubMed]
- Susa, K.J.; Seegar, T.C.; Blacklow, S.C.; Kruse, A.C. A dynamic interaction between CD19 and the tetraspanin CD81 controls B cell co-receptor trafficking. eLife 2020, 9, e52337. [Google Scholar] [CrossRef] [PubMed]
- Carter, R.H.; Fearon, D.T. CD19: Lowering the threshold for antigen receptor stimulation of B lymphocytes. Science 1992, 256, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Gasparrini, F.; Feest, C.; Bruckbauer, A.; Mattila, P.K.; Müller, J.; Nitschke, L.; Bray, D.; Batista, F.D. Nanoscale organization and dynamics of the siglec CD22 cooperate with the cytoskeleton in restraining BCR signalling. EMBO J. 2016, 35, 258–280. [Google Scholar] [CrossRef]
- Young, R.M.; Phelan, J.D.; Wilson, W.H.; Staudt, L.M. Pathogenic B-cell receptor signaling in lymphoid malignancies: New insights to improve treatment. Immunol. Rev. 2019, 291, 190–213. [Google Scholar] [CrossRef]
- Zhong, L.; Li, Y.; Xiong, L.; Wang, W.; Wu, M.; Yuan, T.; Yang, W.; Tian, C.; Miao, Z.; Wang, T.; et al. Small molecules in targeted cancer therapy: Advances, challenges, and future perspectives. Signal Transduct. Target. Ther. 2021, 6, 201. [Google Scholar] [CrossRef]
- Khongorzul, P.; Ling, C.J.; Khan, F.U.; Ihsan, A.U.; Zhang, J. Antibody-Drug Conjugates: A Comprehensive Review. Mol. Cancer Res. 2020, 18, 3–19. [Google Scholar] [CrossRef]
- Beers, S.A.; French, R.R.; Chan, H.C.; Lim, S.H.; Jarrett, T.C.; Vidal, R.M.; Wijayaweera, S.S.; Dixon, S.V.; Kim, H.; Cox, K.L.; et al. Antigenic modulation limits the efficacy of anti-CD20 antibodies: Implications for antibody selection. Blood 2010, 115, 5191–5201. [Google Scholar] [CrossRef] [PubMed]
- Huehls, A.M.; Coupet, T.A.; Sentman, C.L. Bispecific T-cell engagers for cancer immunotherapy. Immunol. Cell Biol. 2015, 93, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Moore, P.A.; Zhang, W.; Rainey, G.J.; Burke, S.; Li, H.; Huang, L.; Gorlatov, S.; Veri, M.C.; Aggarwal, S.; Yang, Y.; et al. Application of dual affinity retargeting molecules to achieve optimal redirected T-cell killing of B-cell lymphoma. Blood 2011, 117, 4542–4551. [Google Scholar] [CrossRef]
- Efremov, D.G.; Turkalj, S.; Laurenti, L. Mechanisms of B Cell Receptor Activation and Responses to B Cell Receptor Inhibitors in B Cell Malignancies. Cancers 2020, 12, 1396. [Google Scholar] [CrossRef] [PubMed]
- Casan, J.M.L.; Wong, J.; Northcott, M.J.; Opat, S. Anti-CD20 monoclonal antibodies: Reviewing a revolution. Hum. Vaccin. Immunother. 2018, 14, 2820–2841. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhu, Y.; Dong, D.; Chen, Y.; Wang, S.; Yang, D.; Ma, Z.; Zhang, A.; Zhang, F.; Guo, C.; et al. Cryo-EM structures of two human B cell receptor isotypes. Science 2022, 377, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Chen, M.; Shi, Y.; Zhang, X.; Huang, G.; Huang, B.; Liu, D.; Liu, Z.; Shi, Y. Cryo-EM structure of the human IgM B cell receptor. Science 2022, 377, 875–880. [Google Scholar] [CrossRef]
- Susa, K.J.; Rawson, S.; Kruse, A.C.; Blacklow, S.C. Cryo-EM structure of the B cell co-receptor CD19 bound to the tetraspanin CD81. Science 2021, 371, 300–305. [Google Scholar] [CrossRef]
- Dong, Y.; Pi, X.; Bartels-Burgahn, F.; Saltukoglu, D.; Liang, Z.; Yang, J.; Alt, F.W.; Reth, M.; Wu, H. Structural principles of B cell antigen receptor assembly. Nature 2022, 612, 156–161. [Google Scholar] [CrossRef]
- Ereño-Orbea, J.; Sicard, T.; Cui, H.; Mazhab-Jafari, M.T.; Benlekbir, S.; Guarné, A.; Rubinstein, J.L.; Julien, J.-P. Molecular basis of human CD22 function and therapeutic targeting. Nat. Commun. 2017, 8, 764. [Google Scholar] [CrossRef]
- Tolar, P.; Pierce, S.K. A conformation-induced oligomerization model for B cell receptor microclustering and signaling. Curr. Top. Microbiol. Immunol. 2010, 340, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.A.; Pleiman, C.M.; Pao, L.; Schneringer, J.; Hippen, K.; Cambier, J.C. Phosphorylated immunoreceptor signaling motifs (ITAMs) exhibit unique abilities to bind and activate Lyn and Syk tyrosine kinases. J. Immunol. 1995, 155, 4596–4603. [Google Scholar] [CrossRef] [PubMed]
- Grupp, S.A.; Campbell, K.; Mitchell, R.N.; Cambier, J.C.; Abbas, A.K. Signaling-defective mutants of the B lymphocyte antigen receptor fail to associate with Ig-alpha and Ig-beta/gamma. J. Biol. Chem. 1993, 268, 25776–25779. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Panana, E.M.; Bannish, G.; Karnell, F.G.; Treml, J.F.; Monroe, J.G. Analysis of the individual contributions of Igalpha (CD79a)- and Igbeta (CD79b)-mediated tonic signaling for bone marrow B cell development and peripheral B cell maturation. J. Immunol. 2006, 177, 7913–7922. [Google Scholar] [CrossRef]
- Crotty, S. Follicular helper CD4 T cells (TFH). Annu. Rev. Immunol. 2011, 29, 621–663. [Google Scholar] [CrossRef]
- Klein, U.; Dalla-Favera, R. Germinal centres: Role in B-cell physiology and malignancy. Nat. Rev. Immunol. 2008, 8, 22–33. [Google Scholar] [CrossRef]
- Ozaki, K.; Spolski, R.; Feng, C.G.; Qi, C.F.; Cheng, J.; Sher, A.; Morse, H.C., 3rd; Liu, C.; Schwartzberg, P.L.; Leonard, W.J. A critical role for IL-21 in regulating immunoglobulin production. Science 2002, 298, 1630–1634. [Google Scholar] [CrossRef]
- Srinivasan, L.; Sasaki, Y.; Calado, D.P.; Zhang, B.; Paik, J.H.; DePinho, R.A.; Kutok, J.L.; Kearney, J.F.; Otipoby, K.L.; Rajewsky, K. PI3 Kinase Signals BCR-Dependent Mature B Cell Survival. Cell 2009, 139, 573–586. [Google Scholar] [CrossRef]
- Kovács, K.G.; Mácsik-Valent, B.; Matkó, J.; Bajtay, Z.; Erdei, A. Revisiting the Coreceptor Function of Complement Receptor Type 2 (CR2, CD21); Coengagement With the B-Cell Receptor Inhibits the Activation, Proliferation, and Antibody Production of Human B Cells. Front. Immunol. 2021, 12, 620427. [Google Scholar] [CrossRef]
- Wakabayashi, C.; Adachi, T.; Wienands, J.; Tsubata, T. A distinct signaling pathway used by the IgG-containing B cell antigen receptor. Science 2002, 298, 2392–2395. [Google Scholar] [CrossRef]
- Ramesh, S.; Park, S.; Im, W.; Call, M.J.; Call, M.E. T cell and B cell antigen receptors share a conserved core transmembrane structure. PNAS. 2022, 119, e2208058119. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.; Call, M.E. Modular Activating Receptors in Innate and Adaptive Immunity. Biochemistry 2017, 56, 1383–1402. [Google Scholar] [CrossRef] [PubMed]
- Tolar, P.; Sohn, H.W.; Pierce, S.K. The initiation of antigen-induced B cell antigen receptor signaling viewed in living cells by fluorescence resonance energy transfer. Nat. Immunol. 2005, 6, 1168–1176. [Google Scholar] [CrossRef]
- Schamel, W.W.; Reth, M. Monomeric and oligomeric complexes of the B cell antigen receptor. Immunity 2000, 13, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger, L.; DeLano, W. PyMOL. 2020. Available online: https://www.pymol.org/pymol (accessed on 3 February 2023).
- Venkitaraman, A.R.; Williams, G.T.; Dariavach, P.; Neuberger, M.S. The B-cell antigen receptor of the five immunoglobulin classes. Nature 1991, 352, 777–781. [Google Scholar] [CrossRef]
- Davey, A.M.; Pierce, S.K. Intrinsic differences in the initiation of B cell receptor signaling favor responses of human IgG(+) memory B cells over IgM(+) naive B cells. J. Immunol. 2012, 188, 3332–3341. [Google Scholar] [CrossRef]
- Wan, Z.; Chen, X.; Chen, H.; Ji, Q.; Chen, Y.; Wang, J.; Cao, Y.; Wang, F.; Lou, J.; Tang, Z.; et al. The activation of IgM- or isotype-switched IgG- and IgE-BCR exhibits distinct mechanical force sensitivity and threshold. eLife 2015, 4, e06925. [Google Scholar] [CrossRef]
- Schroeder, H.W., Jr.; Cavacini, L. Structure and function of immunoglobulins. J. Allergy Clin. Immunol. 2010, 125, S41–S52. [Google Scholar] [CrossRef]
- Noviski, M.; Mueller, J.L.; Satterthwaite, A.; Garrett-Sinha, L.A.; Brombacher, F.; Zikherman, J. IgM and IgD B cell receptors differentially respond to endogenous antigens and control B cell fate. eLife 2018, 7, e35074. [Google Scholar] [CrossRef]
- Martin, A.W. Chapter 6–Immunohistology of Non-Hodgkin Lymphoma. In Diagnostic Immunohistochemistry, 3rd ed.; Dabbs, D.J., Ed.; W.B. Saunders: Philadelphia, PA, USA, 2011; pp. 156–188. [Google Scholar]
- Jahn, L.; Hombrink, P.; Hassan, C.; Kester, M.G.; van der Steen, D.M.; Hagedoorn, R.S.; Falkenburg, J.H.; van Veelen, P.A.; Heemskerk, M.H. Therapeutic targeting of the BCR-associated protein CD79b in a TCR-based approach is hampered by aberrant expression of CD79b. Blood 2015, 125, 949–958. [Google Scholar] [CrossRef]
- Hafeez, U.; Parakh, S.; Gan, H.K.; Scott, A.M. Antibody-Drug Conjugates for Cancer Therapy. Molecules 2020, 25, 4764. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, M.; Luo, Y.; Han, T.; Yoshida, M.; Seon, B.K. Three new monoclonal antibodies that define a unique antigen associated with prolymphocytic leukemia/non-Hodgkin’s lymphoma and are effectively internalized after binding to the cell surface antigen. Blood 1993, 81, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Fuji, R.N.; Elkins, K.; Yu, S.F.; Fuh, F.K.; Chuh, J.; Tan, C.; Hongo, J.A.; Raab, H.; Kozak, K.R.; et al. In vivo effects of targeting CD79b with antibodies and antibody-drug conjugates. Mol. Cancer Ther. 2009, 8, 2937–2946. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, N.; Nishito, Y.; Yoshimura, Y.; Yoshiura, S. The molecular rationale for the combination of polatuzumab vedotin plus rituximab in diffuse large B-cell lymphoma. Br. J. Haematol. 2022, 199, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, M.; Zheng, B.; Erdmann, T.; Koeppen, H.; McCord, R.; Grau, M.; Staiger, A.; Chai, A.; Sandmann, T.; Madle, H.; et al. Anti-CD22 and anti-CD79B antibody drug conjugates are active in different molecular diffuse large B-cell lymphoma subtypes. Leukemia 2015, 29, 1578–1586. [Google Scholar] [CrossRef]
- Palanca-Wessels, M.C.; Czuczman, M.; Salles, G.; Assouline, S.; Sehn, L.H.; Flinn, I.; Patel, M.R.; Sangha, R.; Hagenbeek, A.; Advani, R.; et al. Safety and activity of the anti-CD79B antibody-drug conjugate polatuzumab vedotin in relapsed or refractory B-cell non-Hodgkin lymphoma and chronic lymphocytic leukaemia: A phase 1 study. Lancet Oncol. 2015, 16, 704–715. [Google Scholar] [CrossRef]
- Hatake, K.; Kinoshita, T.; Terui, Y.; Yokoyama, M.; Maruyama, D.; Makita, S.; Yamamoto, K.; Higuchi, Y.; Murakami, S.; Uechi, A.; et al. A phase I pharmacokinetic and safety study of polatuzumab vedotin in Japanese patients with relapsed/refractory b-cell non-Hodgkin lymphoma: A comparison with non-Japanese DCS4968g study. J. Clin. Oncol. 2016, 34, e19070. [Google Scholar] [CrossRef]
- Morschhauser, F.; Flinn, I.W.; Advani, R.; Sehn, L.H.; Diefenbach, C.; Kolibaba, K.; Press, O.W.; Salles, G.; Tilly, H.; Chen, A.I.; et al. Polatuzumab vedotin or pinatuzumab vedotin plus rituximab in patients with relapsed or refractory non-Hodgkin lymphoma: Final results from a phase 2 randomised study (ROMULUS). The Lancet Haematol. 2019, 6, e254–e265. [Google Scholar] [CrossRef]
- Sehn, L.H.; Kamdar, M.; Herrera, A.F.; McMillan, A.; Flowers, C.; Kim, W.S.; Kim, T.M.; Özcan, M.; Demeter, J.; Hertzberg, M.; et al. Randomized phase 2 trial of polatuzumab vedotin (pola) with bendamustine and rituximab (BR) in relapsed/refractory (r/r) FL and DLBCL. J. Clin. Oncol. 2018, 36, 7507. [Google Scholar] [CrossRef]
- Sehn, L.H.; Hertzberg, M.; Opat, S.; Herrera, A.F.; Assouline, S.; Flowers, C.R.; Kim, T.M.; McMillan, A.; Ozcan, M.; Safar, V.; et al. Polatuzumab vedotin plus bendamustine and rituximab in relapsed/refractory DLBCL: Survival update and new extension cohort data. Blood Adv. 2022, 6, 533–543. [Google Scholar] [CrossRef]
- Tilly, H.; Morschhauser, F.; Sehn, L.H.; Friedberg, J.W.; Trněný, M.; Sharman, J.P.; Herbaux, C.; Burke, J.M.; Matasar, M.; Rai, S.; et al. Polatuzumab Vedotin in Previously Untreated Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2022, 386, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Younes, A.; Kim, S.; Romaguera, J.; Copeland, A.; Farial Sde, C.; Kwak, L.W.; Fayad, L.; Hagemeister, F.; Fanale, M.; Neelapu, S.; et al. Phase I multidose-escalation study of the anti-CD19 maytansinoid immunoconjugate SAR3419 administered by intravenous infusion every 3 weeks to patients with relapsed/refractory B-cell lymphoma. J. Clin. Oncol. 2012, 30, 2776–2782. [Google Scholar] [CrossRef] [PubMed]
- Trnĕný, M.; Verhoef, G.; Dyer, M.J.; Ben Yehuda, D.; Patti, C.; Canales, M.; Lopez, A.; Awan, F.T.; Montgomery, P.G.; Janikova, A.; et al. A phase II multicenter study of the anti-CD19 antibody drug conjugate coltuximab ravtansine (SAR3419) in patients with relapsed or refractory diffuse large B-cell lymphoma previously treated with rituximab-based immunotherapy. Haematologica 2018, 103, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Coiffier, B.; Thieblemont, C.; de Guibert, S.; Dupuis, J.; Ribrag, V.; Bouabdallah, R.; Morschhauser, F.; Navarro, R.; Le Gouill, S.; Haioun, C.; et al. A phase II, single-arm, multicentre study of coltuximab ravtansine (SAR3419) and rituximab in patients with relapsed or refractory diffuse large B-cell lymphoma. Br. J. Haematol. 2016, 173, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Ohmachi, K.; Ogura, M.; Suehiro, Y.; Ando, K.; Uchida, T.; Choi, I.; Ogawa, Y.; Kobayashi, M.; Fukino, K.; Yokoi, Y.; et al. A multicenter phase I study of inebilizumab, a humanized anti-CD19 monoclonal antibody, in Japanese patients with relapsed or refractory B-cell lymphoma and multiple myeloma. Int. J. Hematol. 2019, 109, 657–664. [Google Scholar] [CrossRef]
- Salles, G.; Duell, J.; González Barca, E.; Tournilhac, O.; Jurczak, W.; Liberati, A.M.; Nagy, Z.; Obr, A.; Gaidano, G.; André, M.; et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): A multicentre, prospective, single-arm, phase 2 study. Lancet Oncol. 2020, 21, 978–988. [Google Scholar] [CrossRef]
- Kahl, B.S.; Hamadani, M.; Caimi, P.F.; Carlo-Stella, C.; Ai, W.; Alderuccio, J.P.; Ardeshna, K.M.; Hess, B.; Radford, J.; Solh, M.; et al. ABCL-022: LOTIS-2 Follow-Up Analysis: Updated Results from a Phase 2 Study of Loncastuximab Tesirine (Lonca) in Relapsed or Refractory Diffuse Large B-Cell Lymphoma. Clin. Lymphoma Myeloma Leuk. 2021, 21, S377–S378. [Google Scholar] [CrossRef]
- Topp, M.S.; Gökbuget, N.; Stein, A.S.; Zugmaier, G.; O’Brien, S.; Bargou, R.C.; Dombret, H.; Fielding, A.K.; Heffner, L.; Larson, R.A.; et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: A multicentre, single-arm, phase 2 study. Lancet Oncol. 2015, 16, 57–66. [Google Scholar] [CrossRef]
- Kantarjian, H.; Stein, A.; Gökbuget, N.; Fielding, A.K.; Schuh, A.C.; Ribera, J.-M.; Wei, A.; Dombret, H.; Foà, R.; Bassan, R.; et al. Blinatumomab versus Chemotherapy for Advanced Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2017, 376, 836–847. [Google Scholar] [CrossRef]
- Morschhauser, F.; Kraeber-Bodéré, F.; Wegener, W.A.; Harousseau, J.L.; Petillon, M.O.; Huglo, D.; Trümper, L.H.; Meller, J.; Pfreundschuh, M.; Kirsch, C.M.; et al. High rates of durable responses with anti-CD22 fractionated radioimmunotherapy: Results of a multicenter, phase I/II study in non-Hodgkin’s lymphoma. J. Clin. Oncol. 2010, 28, 3709–3716. [Google Scholar] [CrossRef]
- Witzig, T.E.; Tomblyn, M.B.; Misleh, J.G.; Kio, E.A.; Sharkey, R.M.; Wegener, W.A.; Goldenberg, D.M. Anti-CD22 90Y-epratuzumab tetraxetan combined with anti-CD20 veltuzumab: A phase I study in patients with relapsed/refractory, aggressive non-Hodgkin lymphoma. Haematologica 2014, 99, 1738–1745. [Google Scholar] [CrossRef] [PubMed]
- Kraeber-Bodere, F.; Pallardy, A.; Maisonneuve, H.; Campion, L.; Moreau, A.; Soubeyran, I.; Le Gouill, S.; Tournilhac, O.; Daguindau, E.; Jardel, H.; et al. Consolidation anti-CD22 fractionated radioimmunotherapy with (90)Y-epratuzumab tetraxetan following R-CHOP in elderly patients with diffuse large B-cell lymphoma: A prospective, single group, phase 2 trial. Lancet Haematol. 2017, 4, e35–e45. [Google Scholar] [CrossRef] [PubMed]
- Lindén, O.; Bates, A.T.; Cunningham, D.; Hindorf, C.; Larsson, E.; Cleton, A.; Pinkert, J.; Huang, F.; Bladt, F.; Hennekes, H.; et al. (227)Th-Labeled Anti-CD22 Antibody (BAY 1862864) in Relapsed/Refractory CD22-Positive Non-Hodgkin Lymphoma: A First-in-Human, Phase I Study. Cancer Biother. Radiopharm. 2021, 36, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Kantarjian, H.M.; DeAngelo, D.J.; Stelljes, M.; Liedtke, M.; Stock, W.; Gökbuget, N.; O’Brien, S.M.; Jabbour, E.; Wang, T.; Liang White, J.; et al. Inotuzumab ozogamicin versus standard of care in relapsed or refractory acute lymphoblastic leukemia: Final report and long-term survival follow-up from the randomized, phase 3 INO-VATE study. Cancer 2019, 125, 2474–2487. [Google Scholar] [CrossRef]
- Kantarjian, H.M.; DeAngelo, D.J.; Stelljes, M.; Martinelli, G.; Liedtke, M.; Stock, W.; Gökbuget, N.; O’Brien, S.; Wang, K.; Wang, T.; et al. Inotuzumab Ozogamicin versus Standard Therapy for Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2016, 375, 740–753. [Google Scholar] [CrossRef]
- Jabbour, E.; Sasaki, K.; Ravandi, F.; Huang, X.; Short, N.J.; Khouri, M.; Kebriaei, P.; Burger, J.; Khoury, J.; Jorgensen, J.; et al. Chemoimmunotherapy with inotuzumab ozogamicin combined with mini-hyper-CVD, with or without blinatumomab, is highly effective in patients with Philadelphia chromosome-negative acute lymphoblastic leukemia in first salvage. Cancer 2018, 124, 4044–4055. [Google Scholar] [CrossRef]
- Kantarjian, H.; Ravandi, F.; Short, N.J.; Huang, X.; Jain, N.; Sasaki, K.; Daver, N.; Pemmaraju, N.; Khoury, J.D.; Jorgensen, J.; et al. Inotuzumab ozogamicin in combination with low-intensity chemotherapy for older patients with Philadelphia chromosome-negative acute lymphoblastic leukaemia: A single-arm, phase 2 study. Lancet Oncol. 2018, 19, 240–248. [Google Scholar] [CrossRef]
- Kreitman, R.J.; Dearden, C.; Zinzani, P.L.; Delgado, J.; Karlin, L.; Robak, T.; Gladstone, D.E.; le Coutre, P.; Dietrich, S.; Gotic, M.; et al. Moxetumomab pasudotox in relapsed/refractory hairy cell leukemia. Leukemia 2018, 32, 1768–1777. [Google Scholar] [CrossRef]
- Fry, T.J.; Shah, N.N.; Orentas, R.J.; Stetler-Stevenson, M.; Yuan, C.M.; Ramakrishna, S.; Wolters, P.; Martin, S.; Delbrook, C.; Yates, B.; et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat. Med. 2018, 24, 20–28. [Google Scholar] [CrossRef]
- Shah, N.N.; Highfill, S.L.; Shalabi, H.; Yates, B.; Jin, J.; Wolters, P.L.; Ombrello, A.; Steinberg, S.M.; Martin, S.; Delbrook, C.; et al. CD4/CD8 T-Cell Selection Affects Chimeric Antigen Receptor (CAR) T-Cell Potency and Toxicity: Updated Results From a Phase I Anti-CD22 CAR T-Cell Trial. J. Clin. Oncol. 2020, 38, 1938–1950. [Google Scholar] [CrossRef]
- Crook, Z.R.; Nairn, N.W.; Olson, J.M. Miniproteins as a Powerful Modality in Drug Development. Trends Biochem. Sci. 2020, 45, 332–346. [Google Scholar] [CrossRef] [PubMed]
- Bao, G.; Tang, M.; Zhao, J.; Zhu, X. Nanobody: A promising toolkit for molecular imaging and disease therapy. EJNMMI Res. 2021, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.L.; Juergens, D.; Bennett, N.R.; Trippe, B.L.; Yim, J.; Eisenach, H.E.; Ahern, W.; Borst, A.J.; Ragotte, R.J.; Milles, L.F.; et al. Broadly applicable and accurate protein design by integrating structure prediction networks and diffusion generative models. bioRxiv 2022. [Google Scholar] [CrossRef]
- Bradbury, L.E.; Kansas, G.S.; Levy, S.; Evans, R.L.; Tedder, T.F. The CD19/CD21 signal transducing complex of human B lymphocytes includes the target of antiproliferative antibody-1 and Leu-13 molecules. J. Immunol. 1992, 149, 2841–2850. [Google Scholar] [CrossRef] [PubMed]
- Buhl, A.M.; Cambier, J.C. Phosphorylation of CD19 Y484 and Y515, and linked activation of phosphatidylinositol 3-kinase, are required for B cell antigen receptor-mediated activation of Bruton’s tyrosine kinase. J. Immunol. 1999, 162, 4438–4446. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, M.; Fujimoto, Y.; Poe, J.C.; Jansen, P.J.; Lowell, C.A.; DeFranco, A.L.; Tedder, T.F. CD19 regulates Src family protein tyrosine kinase activation in B lymphocytes through processive amplification. Immunity 2000, 13, 47–57. [Google Scholar] [CrossRef]
- Dal Porto, J.M.; Gauld, S.B.; Merrell, K.T.; Mills, D.; Pugh-Bernard, A.E.; Cambier, J. B cell antigen receptor signaling 101. Mol. Immunol. 2004, 41, 599–613. [Google Scholar] [CrossRef]
- Cherukuri, A.; Carter, R.H.; Brooks, S.; Bornmann, W.; Finn, R.; Dowd, C.S.; Pierce, S.K. B Cell Signaling Is Regulated by Induced Palmitoylation of CD81*. J. Biol. Chem. 2004, 279, 31973–31982. [Google Scholar] [CrossRef]
- Matsumoto, A.K.; Kopicky-Burd, J.; Carter, R.H.; Tuveson, D.A.; Tedder, T.F.; Fearon, D.T. Intersection of the complement and immune systems: A signal transduction complex of the B lymphocyte-containing complement receptor type 2 and CD19. J. Exp. Med. 1991, 173, 55–64. [Google Scholar] [CrossRef]
- Tuveson, D.A.; Ahearn, J.M.; Matsumoto, A.K.; Fearon, D.T. Molecular interactions of complement receptors on B lymphocytes: A CR1/CR2 complex distinct from the CR2/CD19 complex. J. Exp. Med. 1991, 173, 1083–1089. [Google Scholar] [CrossRef]
- Roozendaal, R.; Carroll, M.C. Complement receptors CD21 and CD35 in humoral immunity. Immunol. Rev. 2007, 219, 157–166. [Google Scholar] [CrossRef]
- Benkerrou, M.; Jais, J.P.; Leblond, V.; Durandy, A.; Sutton, L.; Bordigoni, P.; Garnier, J.L.; Le Bidois, J.; Le Deist, F.; Blanche, S.; et al. Anti-B-cell monoclonal antibody treatment of severe posttransplant B-lymphoproliferative disorder: Prognostic factors and long-term outcome. Blood 1998, 92, 3137–3147. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, B.; Kelly, B.; McMillan, B.J.; Seegar, T.C.M.; Dror, R.O.; Kruse, A.C.; Blacklow, S.C. Crystal Structure of a Full-Length Human Tetraspanin Reveals a Cholesterol-Binding Pocket. Cell 2016, 167, 1041–1051.e1011. [Google Scholar] [CrossRef] [PubMed]
- Scheuermann, R.H.; Racila, E. CD19 antigen in leukemia and lymphoma diagnosis and immunotherapy. Leuk. Lymphoma 1995, 18, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Horna, P.; Nowakowski, G.; Endell, J.; Boxhammer, R. Comparative Assessment of Surface CD19 and CD20 Expression on B-Cell Lymphomas from Clinical Biopsies: Implications for Targeted Therapies. Blood 2019, 134, 5345. [Google Scholar] [CrossRef]
- Roßkopf, S.; Eichholz, K.M.; Winterberg, D.; Diemer, K.J.; Lutz, S.; Münnich, I.A.; Klausz, K.; Rösner, T.; Valerius, T.; Schewe, D.M.; et al. Enhancing CDC and ADCC of CD19 Antibodies by Combining Fc Protein-Engineering with Fc Glyco-Engineering. Antibodies 2020, 9, 63. [Google Scholar] [CrossRef]
- Beck, A.; Goetsch, L.; Dumontet, C.; Corvaïa, N. Strategies and challenges for the next generation of antibody–drug conjugates. Nat. Rev. Drug Discov. 2017, 16, 315–337. [Google Scholar] [CrossRef]
- Frigault, M.J.; Maus, M.V. State of the art in CAR T cell therapy for CD19+ B cell malignancies. J. Clin. Invest. 2020, 130, 1586–1594. [Google Scholar] [CrossRef]
- Goebeler, M.E.; Knop, S.; Viardot, A.; Kufer, P.; Topp, M.S.; Einsele, H.; Noppeney, R.; Hess, G.; Kallert, S.; Mackensen, A.; et al. Bispecific T-Cell Engager (BiTE) Antibody Construct Blinatumomab for the Treatment of Patients with Relapsed/Refractory Non-Hodgkin Lymphoma: Final Results From a Phase I Study. J. Clin. Oncol. 2016, 34, 1104–1111. [Google Scholar] [CrossRef]
- Horton, H.M.; Bernett, M.J.; Pong, E.; Peipp, M.; Karki, S.; Chu, S.Y.; Richards, J.O.; Vostiar, I.; Joyce, P.F.; Repp, R.; et al. Potent In vitro and In vivo Activity of an Fc-Engineered Anti-CD19 Monoclonal Antibody against Lymphoma and Leukemia. Cancer Res. 2008, 68, 8049–8057. [Google Scholar] [CrossRef]
- Blanc, V.; Bousseau, A.; Caron, A.; Carrez, C.; Lutz, R.J.; Lambert, J.M. SAR3419: An anti-CD19-Maytansinoid Immunoconjugate for the treatment of B-cell malignancies. Clin. Cancer Res. 2011, 17, 6448–6458. [Google Scholar] [CrossRef] [PubMed]
- Frampton, J.E. Inebilizumab: First Approval. Drugs 2020, 80, 1259–1264. [Google Scholar] [CrossRef] [PubMed]
- Borate, U.; Fathi, A.T.; Shah, B.D.; DeAngelo, D.J.; Silverman, L.B.; Cooper, T.M.; Albertson, T.M.; O’Meara, M.M.; Sandalic, L.; Stevison, F.; et al. A First-In-Human Phase 1 Study Of The Antibody-Drug Conjugate SGN-CD19A In Relapsed Or Refractory B-Lineage Acute Leukemia and Highly Aggressive Lymphoma. Blood 2013, 122, 1437. [Google Scholar] [CrossRef]
- Moskowitz, C.H.; Fanale, M.A.; Shah, B.D.; Advani, R.H.; Chen, R.; Kim, S.; Kostic, A.; Liu, T.; Peng, J.; Forero-Torres, A. A Phase 1 Study of Denintuzumab Mafodotin (SGN-CD19A) in Relapsed/Refactory B-Lineage Non-Hodgkin Lymphoma. Blood 2015, 126, 182. [Google Scholar] [CrossRef]
- Awan, F.T.; Lapalombella, R.; Trotta, R.; Butchar, J.P.; Yu, B.; Benson, D.M., Jr.; Roda, J.M.; Cheney, C.; Mo, X.; Lehman, A.; et al. CD19 targeting of chronic lymphocytic leukemia with a novel Fc-domain-engineered monoclonal antibody. Blood 2010, 115, 1204–1213. [Google Scholar] [CrossRef] [PubMed]
- Belada, D.; Kopeckova, K.; Bergua, J.M.; André, M.; Persona, E.P.; Pichler, P.; Klöpfer, P.; Brackertz, B.; Lohrmann, E.; Lahiry, A.; et al. First-MIND: A phase Ib, open-label, randomized study to assess safety of tafasitamab (tafa) or tafa + lenalidomide (LEN) in addition to R-CHOP in patients with newly diagnosed DLBCL. J. Clin. Oncol. 2021, 39, 7540. [Google Scholar] [CrossRef]
- Hoy, S.M. Tafasitamab: First Approval. Drugs 2020, 80, 1731–1737. [Google Scholar] [CrossRef]
- Kahl, B.S.; Hamadani, M.; Radford, J.; Carlo-Stella, C.; Caimi, P.; Reid, E.; Feingold, J.M.; Ardeshna, K.M.; Solh, M.; Heffner, L.T.; et al. A Phase I Study of ADCT-402 (Loncastuximab Tesirine), a Novel Pyrrolobenzodiazepine-Based Antibody-Drug Conjugate, in Relapsed/Refractory B-Cell Non-Hodgkin Lymphoma. Clin. Cancer Res. 2019, 25, 6986–6994. [Google Scholar] [CrossRef]
- Dreier, T.; Lorenczewski, G.; Brandl, C.; Hoffmann, P.; Syring, U.; Hanakam, F.; Kufer, P.; Riethmuller, G.; Bargou, R.; Baeuerle, P.A. Extremely potent, rapid and costimulation-independent cytotoxic T-cell response against lymphoma cells catalyzed by a single-chain bispecific antibody. Int. J. Cancer 2002, 100, 690–697. [Google Scholar] [CrossRef]
- Hoffmann, P.; Hofmeister, R.; Brischwein, K.; Brandl, C.; Crommer, S.; Bargou, R.; Itin, C.; Prang, N.; Baeuerle, P.A. Serial killing of tumor cells by cytotoxic T cells redirected with a CD19-/CD3-bispecific single-chain antibody construct. Int. J. Cancer 2005, 115, 98–104. [Google Scholar] [CrossRef]
- Löffler, A.; Gruen, M.; Wuchter, C.; Schriever, F.; Kufer, P.; Dreier, T.; Hanakam, F.; Baeuerle, P.A.; Bommert, K.; Karawajew, L.; et al. Efficient elimination of chronic lymphocytic leukaemia B cells by autologous T cells with a bispecific anti-CD19/anti-CD3 single-chain antibody construct. Leukemia 2003, 17, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Teplyakov, A.; Obmolova, G.; Luo, J.; Gilliland, G.L. Crystal structure of B-cell co-receptor CD19 in complex with antibody B43 reveals an unexpected fold. Proteins 2018, 86, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Vences-Catalán, F.; Kuo, C.-C.; Rajapaksa, R.; Duault, C.; Andor, N.; Czerwinski, D.K.; Levy, R.; Levy, S. CD81 is a novel immunotherapeutic target for B cell lymphoma. J. Exp. Med. 2019, 216, 1497–1508. [Google Scholar] [CrossRef] [PubMed]
- Macauley, M.S.; Crocker, P.R.; Paulson, J.C. Siglec-mediated regulation of immune cell function in disease. Nat. Rev. Immunol. 2014, 14, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.A.; Smith, K.G. CD22: An inhibitory enigma. Immunology 2008, 123, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Tedder, T.F.; Tuscano, J.; Sato, S.; Kehrl, J.H. CD22, a B lymphocyte-specific adhesion molecule that regulates antigen receptor signaling. Annu. Rev. Immunol. 1997, 15, 481–504. [Google Scholar] [CrossRef] [PubMed]
- Clark, E.A.; Giltiay, N.V. CD22: A Regulator of Innate and Adaptive B Cell Responses and Autoimmunity. Front. Immunol. 2018, 9, 2235. [Google Scholar] [CrossRef]
- Nitschke, L. CD22 and Siglec-G regulate inhibition of B-cell signaling by sialic acid ligand binding and control B-cell tolerance. Glycobiology 2014, 24, 807–817. [Google Scholar] [CrossRef]
- Doody, G.M.; Justement, L.B.; Delibrias, C.C.; Matthews, R.J.; Lin, J.; Thomas, M.L.; Fearon, D.T. A role in B cell activation for CD22 and the protein tyrosine phosphatase SHP. Science 1995, 269, 242–244. [Google Scholar] [CrossRef]
- Shah, N.N.; Sokol, L. Targeting CD22 for the Treatment of B-Cell Malignancies. Immunotargets Ther. 2021, 10, 225–236. [Google Scholar] [CrossRef]
- Qu, Z.; Goldenberg, D.M.; Cardillo, T.M.; Shi, V.; Hansen, H.J.; Chang, C.-H. Bispecific anti-CD20/22 antibodies inhibit B-cell lymphoma proliferation by a unique mechanism of action. Blood 2008, 111, 2211–2219. [Google Scholar] [CrossRef] [PubMed]
- Sieger, N.; Fleischer, S.J.; Mei, H.E.; Reiter, K.; Shock, A.; Burmester, G.R.; Daridon, C.; Dörner, T. CD22 ligation inhibits downstream B cell receptor signaling and Ca2+ flux upon activation. Arthritis Rheum. 2013, 65, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Raetz, E.A.; Cairo, M.S.; Borowitz, M.J.; Lu, X.; Devidas, M.; Reid, J.M.; Goldenberg, D.M.; Wegener, W.A.; Zeng, H.; Whitlock, J.A.; et al. Re-induction chemoimmunotherapy with epratuzumab in relapsed acute lymphoblastic leukemia (ALL): Phase II results from Children’s Oncology Group (COG) study ADVL04P2. Pediatr. Blood Cancer 2015, 62, 1171–1175. [Google Scholar] [CrossRef] [PubMed]
- Chevallier, P.; Huguet, F.; Raffoux, E.; Etienne, A.; Leguay, T.; Isnard, F.; Robillard, N.; Guillaume, T.; Delaunay, J.; Charbonnier, A.; et al. Vincristine, dexamethasone and epratuzumab for older relapsed/refractory CD22+ B-acute lymphoblastic leukemia patients: A phase II study. Haematologica 2015, 100, e128–e131. [Google Scholar] [CrossRef]
- Griffiths, G.L.; Govindan, S.V.; Sharkey, R.M.; Fisher, D.R.; Goldenberg, D.M. 90Y-DOTA-hLL2: An agent for radioimmunotherapy of non-Hodgkin’s lymphoma. J. Nucl. Med. 2003, 44, 77–84. [Google Scholar]
- Shor, B.; Gerber, H.P.; Sapra, P. Preclinical and clinical development of inotuzumab-ozogamicin in hematological malignancies. Mol. Immunol. 2015, 67, 107–116. [Google Scholar] [CrossRef]
- Advani, A.; Coiffier, B.; Czuczman, M.S.; Dreyling, M.; Foran, J.; Gine, E.; Gisselbrecht, C.; Ketterer, N.; Nasta, S.; Rohatiner, A.; et al. Safety, pharmacokinetics, and preliminary clinical activity of inotuzumab ozogamicin, a novel immunoconjugate for the treatment of B-cell non-Hodgkin’s lymphoma: Results of a phase I study. J. Clin. Oncol. 2010, 28, 2085–2093. [Google Scholar] [CrossRef]
- Fayad, L.; Offner, F.; Smith, M.R.; Verhoef, G.; Johnson, P.; Kaufman, J.L.; Rohatiner, A.; Advani, A.; Foran, J.; Hess, G.; et al. Safety and clinical activity of a combination therapy comprising two antibody-based targeting agents for the treatment of non-Hodgkin lymphoma: Results of a phase I/II study evaluating the immunoconjugate inotuzumab ozogamicin with rituximab. J. Clin. Oncol. 2013, 31, 573–583. [Google Scholar] [CrossRef]
- Ogura, M.; Tobinai, K.; Hatake, K.; Uchida, T.; Kasai, M.; Oyama, T.; Suzuki, T.; Kobayashi, Y.; Watanabe, T.; Azuma, T.; et al. Phase I study of inotuzumab ozogamicin (CMC-544) in Japanese patients with follicular lymphoma pretreated with rituximab-based therapy. Cancer Sci. 2010, 101, 1840–1845. [Google Scholar] [CrossRef]
- Mansfield, E.; Amlot, P.; Pastan, I.; FitzGerald, D.J. Recombinant RFB4 immunotoxins exhibit potent cytotoxic activity for CD22-bearing cells and tumors. Blood 1997, 90, 2020–2026. [Google Scholar] [CrossRef]
- Dai, H.; Wu, Z.; Jia, H.; Tong, C.; Guo, Y.; Ti, D.; Han, X.; Liu, Y.; Zhang, W.; Wang, C.; et al. Bispecific CAR-T cells targeting both CD19 and CD22 for therapy of adults with relapsed or refractory B cell acute lymphoblastic leukemia. J. Hematol. Oncol. 2020, 13, 30. [Google Scholar] [CrossRef] [PubMed]
- Schneider, D.; Xiong, Y.; Wu, D.; Hu, P.; Alabanza, L.; Steimle, B.; Mahmud, H.; Anthony-Gonda, K.; Krueger, W.; Zhu, Z.; et al. Trispecific CD19-CD20-CD22-targeting duoCAR-T cells eliminate antigen-heterogeneous B cell tumors in preclinical models. Sci. Transl. Med. 2021, 13, eabc6401. [Google Scholar] [CrossRef] [PubMed]



| Monoclonal Antibody | Characteristics | Selected Studies | Disease | Outcomes |
|---|---|---|---|---|
| Anti CD79b | ||||
| Polatuzumab vedotin | CD79b-directed antibody covalently linked to the anti-mitotic cytotoxic agent monomethyl auristatin (MMAE) | Phase Ib/II multicenter trial [51,52] | R/R DLBCL or FL | Pola + BR: ORR was 63% compared to 25% for BR alone. Pola + BR: CR rate was 40% compared to 18% for BR alone. |
| Phase III trial [53]. R-CHOP versus pola-R-CHP | Treatment naïve DLBCL | No difference in OS to date. | ||
| Anti CD19 | ||||
| Coltuximab ravtansine (SAR3419) | Humanized IgG1 anti-CD19 monoclonal antibody conjugated to the maytansinoid tubulin inhibitor, DM4 | Phase I multidose-escalation study [54] | R/R DLBCL, some previously treated with rituximab-based therapy | ORR 23.5% (n = 17) |
| Phase II, single-arm, multicenter study [55] | R/R DLBCL previously treated with rituximab-based therapy | ORR was 44% and CR rate 15% (n = 41) | ||
| Phase II, single-arm, multicenter study combined with rituximab [56] | R/R DLBCL | ORR was 31% and CR rate 8.9% (n = 45) | ||
| Inebilizumab | Humanized, anti-CD19 monoclonal antibody | Phase I dose-escalation study [57] | R/R DLBCL, CLL, FL, and multiple myeloma | ORR was 60% (n = 20) |
| Tafasitamab (XmAb5574) | Humanized anti-CD19 monoclonal antibody with an engineered Fc domain that increases binding to Fc receptors | Phase II single-arm, multi-center, open-label study in combination with lenalidomide [58] | R/R DLBCL | ORR was 57%. |
| Loncastuximab tesirine | Anti-CD19 mAb conjugated to a cytotoxic DNA minor groove inter-strand cross-linking pyrrolobenzodiazepine dimer | Phase II, single-arm study [59] | R/R DLBCL or high-grade B-cell lymphoma | ORR was 48% and CR rate 25% (n = 145) Median duration of response was 12.6 months for responders |
| Blinatumomab | A bispecific T-cell engager (BiTE) that combines anti-CD19 antibody B43 with anti-CD3 | Multicenter, single-arm, open-label phase II study [60]. | Philadelphia-chromosome-negative, primary refractory or relapsed B cell precursor ALL | CR with complete or partial hematologic recovery of 43%, and median overall survival of 6.1 months |
| Multinational, randomized, phase III trial that compared blinatumomab with standard of care chemotherapy [61] | R/R ALL | Median OS was 7.7 months in the blinatumomab arm (95% CI = 5.6, 9.6) and 4 months in the standard of care chemotherapy arm (95% CI = 2.9, 5.3) | ||
| Anti-CD22 | ||||
| Epratuzumab tetraxetan | 90yttrium-labeled humanized IgG anti-CD22 antibody | Multicenter, phase I/II study of epratuzumab tetraxetan alone [62] | R/R NHL | ORR was 62%, CR rate 48%, and median PFS was 9.5 months |
| In combination with the anti-CD20 antibody veltuzumab [63] | R/R NHL | ORR was 53%, CR rate was 18%, and PR rate was 35% | ||
| single-group, phase II trial epratuzumab tetraxetan as consolidation after first-line induction chemoimmunotherapy [64] | Untreated elderly patients with DLBCL. | Estimated 2 year event-free survival was 75% (95% CI 63–84) | ||
| BAY1862864 | (227) thorium-labeled epratuzumab | dose-escalation phase I study [65] | R/R NHL | ORR of 38%, PR of 19% and CR of 5% (n = 21) |
| Inotuzumab ozogamicin | Anti-CD22 antibody attached to calicheamicin | Randomized, phase III INO-VATE study. Inotuzumab versus standard-of-care chemotherapy [66,67] | R/R B cell precursor ALL | Patients treated with inotuzumab ozogamicin had higher CR rates (74% versus 35%), and MRD-negativity (78% versus 28%). The inotuzumab arm had longer PFS (HR, 0.45; 97.5% CI, 0.34–0.61; p < 0.001) and OS (HR, 0.77; 97.5% CI, 0.58–1.03; p = 0.04). |
| Phase II study in combination with lower-intensity chemotherapy and blinatumomab [68] | First relapsed Philadelphia chromosome-negative B-ALL | ORR was 92% and CR 73% (n = 44) | ||
| Single-arm, phase II study, Inotuzumab ozogamicin in combination with low-intensity chemotherapy [69] | Older patients with Philadelphia chromosome-negative ALL | With a median follow-up of 29 months, 2-year PFS was 59%. | ||
| Moxetumomab pasudotox-tdfk | Fv of murine CD22 mAb fused to a truncated portion of Pseudomonas exotoxin A | Multicenter, open-label study [70] | R/R HCL | CR rate was 41% and ORR was 75% |
| CD22 CAR-T therapies | Chimeric antigen receptor T cells targeting CD22 | Phase I, first-in-human, dose escalation trial [71] | R/R CD22 expressing hematopoietic malignancies | CR in 73% (11/15) of patients receiving ≥1 × 106 CD22 CAR T cells/kg |
| Single-center, phase I, 3 1 3 dose-escalation trial [72] | R/R CD22 expressing hematopoietic malignancies | CR was 70% and median OS was 13.4 months (n = 58) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhattacharyya, P.; Christopherson, R.I.; Skarratt, K.K.; Chen, J.Z.; Balle, T.; Fuller, S.J. Combination of High-Resolution Structures for the B Cell Receptor and Co-Receptors Provides an Understanding of Their Interactions with Therapeutic Antibodies. Cancers 2023, 15, 2881. https://doi.org/10.3390/cancers15112881
Bhattacharyya P, Christopherson RI, Skarratt KK, Chen JZ, Balle T, Fuller SJ. Combination of High-Resolution Structures for the B Cell Receptor and Co-Receptors Provides an Understanding of Their Interactions with Therapeutic Antibodies. Cancers. 2023; 15(11):2881. https://doi.org/10.3390/cancers15112881
Chicago/Turabian StyleBhattacharyya, Puja, Richard I. Christopherson, Kristen K. Skarratt, Jake Z. Chen, Thomas Balle, and Stephen J. Fuller. 2023. "Combination of High-Resolution Structures for the B Cell Receptor and Co-Receptors Provides an Understanding of Their Interactions with Therapeutic Antibodies" Cancers 15, no. 11: 2881. https://doi.org/10.3390/cancers15112881





