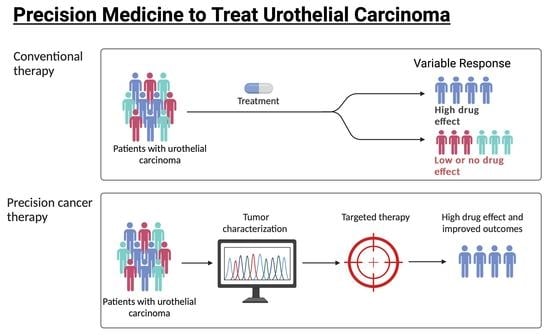
Precision Medicine to Treat Urothelial Carcinoma—The Way Forward
Abstract
:Simple Summary
Abstract
1. Introduction
2. Better Understanding of Tumor Biology and Emergence of Liquid Biopsies
3. Immune Checkpoint Inhibitors and Prediction of Therapeutic Response
4. FGFR and Other Receptor Tyrosine Kinase Inhibitors
5. Antibody Drug Conjugates
6. Combination Therapy—Current Developments
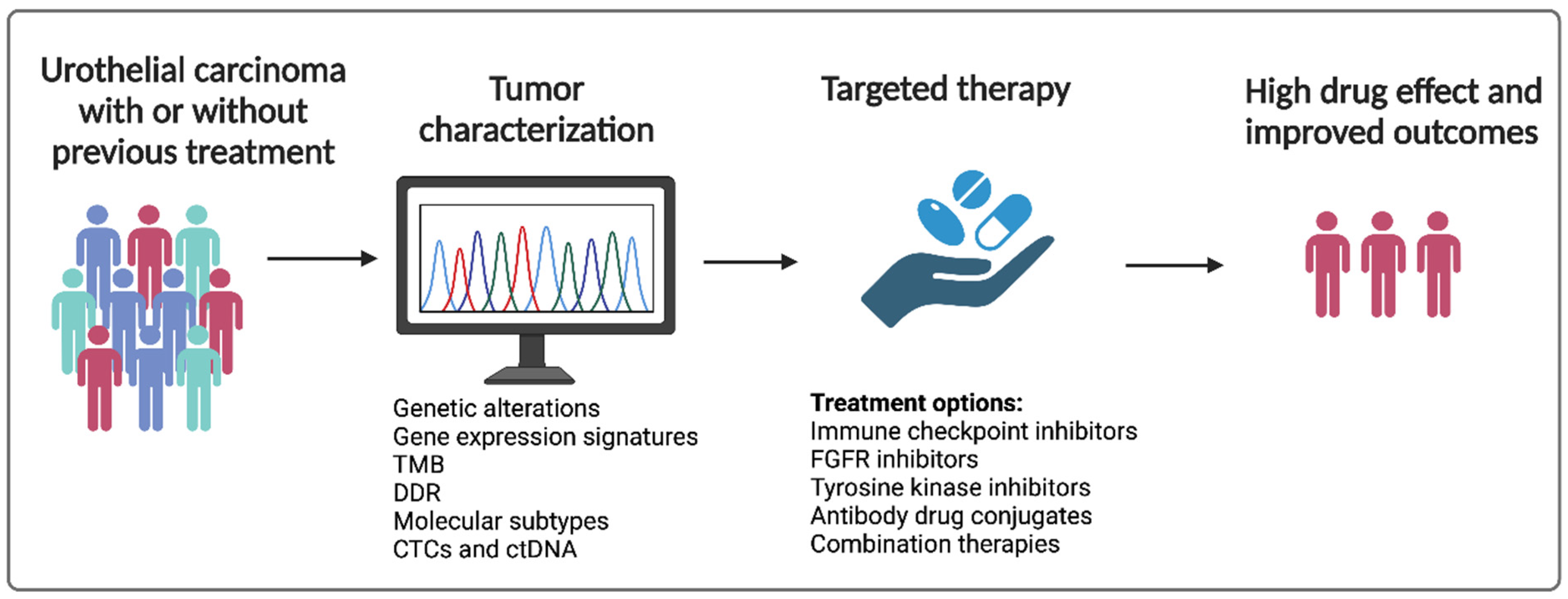
7. The Way Forward—Where Do We Go from Here?
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Disclosures
References
- Maheswaran, S.; Haber, D.A. Circulating tumor cells: A window into cancer biology and metastasis. Curr. Opin. Genet. Dev. 2010, 20, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Zhu, H.; Zhang, C.; Sun, X.; Gao, X.; Chen, G. “Liquid biopsy”-ctDNA detection with great potential and challenges. Ann. Transl. Med. 2015, 3, 235. [Google Scholar] [CrossRef]
- Vandekerkhove, G.; Lavoie, J.M.; Annala, M.; Murtha, A.J.; Sundahl, N.; Walz, S.; Sano, T.; Taavitsainen, S.; Ritch, E.; Fazli, L.; et al. Plasma ctDNA is a tumor tissue surrogate and enables clinical-genomic stratification of metastatic bladder cancer. Nat. Commun. 2021, 12, 184. [Google Scholar] [CrossRef]
- Soave, A.; Riethdorf, S.; Dahlem, R.; von Amsberg, G.; Minner, S.; Weisbach, L.; Engel, O.; Fisch, M.; Pantel, K.; Rink, M. A nonrandomized, prospective, clinical study on the impact of circulating tumor cells on outcomes of urothelial carcinoma of the bladder patients treated with radical cystectomy with or without adjuvant chemotherapy. Int. J. Cancer 2017, 140, 381–389. [Google Scholar] [CrossRef]
- Alva, A.; Friedlander, T.; Clark, M.; Huebner, T.; Daignault, S.; Hussain, M.; Lee, C.; Hafez, K.; Hollenbeck, B.; Weizer, A.; et al. Circulating Tumor Cells as Potential Biomarkers in Bladder Cancer. J. Urol. 2015, 194, 790–798. [Google Scholar] [CrossRef]
- Christensen, E.; Birkenkamp-Demtroder, K.; Sethi, H.; Shchegrova, S.; Salari, R.; Nordentoft, I.; Wu, H.T.; Knudsen, M.; Lamy, P.; Lindskrog, S.V.; et al. Early Detection of Metastatic Relapse and Monitoring of Therapeutic Efficacy by Ultra-Deep Sequencing of Plasma Cell-Free DNA in Patients With Urothelial Bladder Carcinoma. J. Clin. Oncol. 2019, 37, 1547–1557. [Google Scholar] [CrossRef]
- Bellmunt, J.; Hussain, M.; Gschwend, J.E.; Albers, P.; Oudard, S.; Castellano, D.; Daneshmand, S.; Nishiyama, H.; Majchrowicz, M.; Degaonkar, V.; et al. Adjuvant atezolizumab versus observation in muscle-invasive urothelial carcinoma (IMvigor010): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Assaf, Z.J.; Davarpanah, N.; Banchereau, R.; Szabados, B.E.; Yuen, K.C.; Grivas, P.; Hussain, M.; Oudard, S.; Gschwend, J.E.; et al. ctDNA guiding adjuvant immunotherapy in urothelial carcinoma. Nature 2021, 595, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.B.; Assaf, Z.J.; Davarpanah, N.; Hussain, M.; Oudard, S.; Gschwend, J.E.; Albers, P.; Castellano, D.; Nishiyama, H.; Daneshmand, S.; et al. 1O Clinical outcomes in post-operative ctDNA-positive muscle-invasive urothelial carcinoma (MIUC) patients after atezolizumab adjuvant therapy. Ann. Oncol. 2020, 31, S1417. [Google Scholar] [CrossRef]
- Nuzzo, P.V.; Berchuck, J.E.; Korthauer, K.; Spisak, S.; Nassar, A.H.; Abou Alaiwi, S.; Chakravarthy, A.; Shen, S.Y.; Bakouny, Z.; Boccardo, F.; et al. Detection of renal cell carcinoma using plasma and urine cell-free DNA methylomes. Nat. Med. 2020, 26, 1041–1043. [Google Scholar] [CrossRef]
- Rose, K.M.; Murthy, P.; Gould, B.; Huelster, H.L.; Davaro, F.; Du, P.; Jia, S.; Li, R. Cell-free urinary tumor DNA to detect minimal residual disease prior to repeat-transurethral resection of bladder tumor in non–muscle-invasive bladder cancer: A prospective study. J. Clin. Oncol. 2023, 41, LBA445. [Google Scholar] [CrossRef]
- Christensen, E.; Nordentoft, I.; Birkenkamp-Demtroder, K.; Elbaek, S.K.; Lindskrog, S.V.; Taber, A.; Andreasen, T.G.; Strandgaard, T.; Knudsen, M.; Lamy, P.; et al. Cell-free urine- and plasma DNA mutational analysis predicts neoadjuvant chemotherapy response and outcome in patients with muscle invasive bladder cancer. Clin. Cancer Res. 2023, 29, 1582–1591. [Google Scholar] [CrossRef] [PubMed]
- Stewart, T.F.; Shenoy, A.; Stuart, S.M.; McClintock, K.; Bagaria, S.; So, T.; Bagrodia, A.; Salmasi, A.; Kader, A.K.; Monga, M.; et al. Comparison of urine cell-free DNA with blood-based screening for detection of bladder cancer. J. Clin. Oncol. 2023, 41, 457. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Chen, K.; Babbra, R.K.; Feng, W.; Pejovic, N.; Nallicheri, A.; Harris, P.K.; Dienstbach, K.; Atkocius, A.; Maguire, L.; et al. Urine tumor DNA detection of minimal residual disease in muscle-invasive bladder cancer treated with curative-intent radical cystectomy: A cohort study. PLoS Med. 2021, 18, e1003732. [Google Scholar] [CrossRef]
- Fradet, Y.; Bellmunt, J.; Vaughn, D.J.; Lee, J.L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; Necchi, A.; et al. Randomized phase III KEYNOTE-045 trial of pembrolizumab versus paclitaxel, docetaxel, or vinflunine in recurrent advanced urothelial cancer: Results of >2 years of follow-up. Ann. Oncol. 2019, 30, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Retz, M.; Siefker-Radtke, A.; Baron, A.; Necchi, A.; Bedke, J.; Plimack, E.R.; Vaena, D.; Grimm, M.O.; Bracarda, S.; et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017, 18, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.R.; Ellerton, J.; Infante, J.R.; Agrawal, M.; Gordon, M.; Aljumaily, R.; Britten, C.D.; Dirix, L.; Lee, K.W.; Taylor, M.; et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): Pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol. 2018, 19, 51–64. [Google Scholar] [CrossRef]
- Powles, T.; Park, S.H.; Voog, E.; Caserta, C.; Valderrama, B.P.; Gurney, H.; Kalofonos, H.; Radulović, S.; Demey, W.; Ullén, A.; et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2020, 383, 1218–1230. [Google Scholar] [CrossRef]
- Sonpavde, G.; Manitz, J.; Gao, C.; Tayama, D.; Kaiser, C.; Hennessy, D.; Makari, D.; Gupta, A.; Abdullah, S.E.; Niegisch, G.; et al. Five-Factor Prognostic Model for Survival of Post-Platinum Patients with Metastatic Urothelial Carcinoma Receiving PD-L1 Inhibitors. J. Urol. 2020, 204, 1173–1179. [Google Scholar] [CrossRef]
- Sha, D.; Jin, Z.; Budczies, J.; Kluck, K.; Stenzinger, A.; Sinicrope, F.A. Tumor Mutational Burden as a Predictive Biomarker in Solid Tumors. Cancer Discov. 2020, 10, 1808–1825. [Google Scholar] [CrossRef]
- Powles, T.; Durán, I.; van der Heijden, M.S.; Loriot, Y.; Vogelzang, N.J.; De Giorgi, U.; Oudard, S.; Retz, M.M.; Castellano, D.; Bamias, A.; et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): A multicentre, open-label, phase 3 randomised controlled trial. Lancet 2018, 391, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Galsky, M.D.; Saci, A.; Szabo, P.M.; Han, G.C.; Grossfeld, G.; Collette, S.; Siefker-Radtke, A.; Necchi, A.; Sharma, P. Nivolumab in Patients with Advanced Platinum-resistant Urothelial Carcinoma: Efficacy, Safety, and Biomarker Analyses with Extended Follow-up from CheckMate 275. Clin. Cancer Res. 2020, 26, 5120–5128. [Google Scholar] [CrossRef]
- Powles, T.; Sridhar, S.S.; Loriot, Y.; Bellmunt, J.; Mu, X.J.; Ching, K.A.; Pu, J.; Sternberg, C.N.; Petrylak, D.P.; Tambaro, R.; et al. Avelumab maintenance in advanced urothelial carcinoma: Biomarker analysis of the phase 3 JAVELIN Bladder 100 trial. Nat. Med. 2021, 27, 2200–2211. [Google Scholar] [CrossRef]
- Nassar, A.H.; Mouw, K.W.; Jegede, O.; Shinagare, A.B.; Kim, J.; Liu, C.-J.; Pomerantz, M.; Harshman, L.C.; Van Allen, E.M.; Wei, X.X.; et al. A model combining clinical and genomic factors to predict response to PD-1/PD-L1 blockade in advanced urothelial carcinoma. Br. J. Cancer 2020, 122, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Loriot, Y.; Necchi, A.; Park, S.H.; Garcia-Donas, J.; Huddart, R.; Burgess, E.; Fleming, M.; Rezazadeh, A.; Mellado, B.; Varlamov, S.; et al. Erdafitinib in Locally Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2019, 381, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Marcus, L.; Fashoyin-Aje, L.A.; Donoghue, M.; Yuan, M.; Rodriguez, L.; Gallagher, P.S.; Philip, R.; Ghosh, S.; Theoret, M.R.; Beaver, J.A.; et al. FDA Approval Summary: Pembrolizumab for the Treatment of Tumor Mutational Burden-High Solid Tumors. Clin. Cancer Res. 2021, 27, 4685–4689. [Google Scholar] [CrossRef]
- Goswami, S.; Chen, Y.; Anandhan, S.; Szabo, P.M.; Basu, S.; Blando, J.M.; Liu, W.; Zhang, J.; Natarajan, S.M.; Xiong, L.; et al. ARID1A mutation plus CXCL13 expression act as combinatorial biomarkers to predict responses to immune checkpoint therapy in mUCC. Sci. Transl. Med. 2020, 12, eabc4220. [Google Scholar] [CrossRef]
- Teo, M.Y.; Seier, K.; Ostrovnaya, I.; Regazzi, A.M.; Kania, B.E.; Moran, M.M.; Cipolla, C.K.; Bluth, M.J.; Chaim, J.; Al-Ahmadie, H.; et al. Alterations in DNA Damage Response and Repair Genes as Potential Marker of Clinical Benefit From PD-1/PD-L1 Blockade in Advanced Urothelial Cancers. J. Clin. Oncol. 2018, 36, 1685–1694. [Google Scholar] [CrossRef]
- Seiler, R.; Ashab, H.A.D.; Erho, N.; van Rhijn, B.W.G.; Winters, B.; Douglas, J.; Van Kessel, K.E.; Fransen van de Putte, E.E.; Sommerlad, M.; Wang, N.Q.; et al. Impact of Molecular Subtypes in Muscle-invasive Bladder Cancer on Predicting Response and Survival after Neoadjuvant Chemotherapy. Eur. Urol. 2017, 72, 544–554. [Google Scholar] [CrossRef]
- Sjodahl, G.; Abrahamsson, J.; Holmsten, K.; Bernardo, C.; Chebil, G.; Eriksson, P.; Johansson, I.; Kollberg, P.; Lindh, C.; Lovgren, K.; et al. Different Responses to Neoadjuvant Chemotherapy in Urothelial Carcinoma Molecular Subtypes. Eur. Urol. 2022, 81, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Kockx, M.; Rodriguez-Vida, A.; Duran, I.; Crabb, S.J.; Van Der Heijden, M.S.; Szabados, B.; Pous, A.F.; Gravis, G.; Herranz, U.A.; et al. Clinical efficacy and biomarker analysis of neoadjuvant atezolizumab in operable urothelial carcinoma in the ABACUS trial. Nat. Med. 2019, 25, 1706–1714. [Google Scholar] [CrossRef]
- Necchi, A.; Raggi, D.; Gallina, A.; Madison, R.; Colecchia, M.; Lucianò, R.; Montironi, R.; Giannatempo, P.; Farè, E.; Pederzoli, F.; et al. Updated Results of PURE-01 with Preliminary Activity of Neoadjuvant Pembrolizumab in Patients with Muscle-invasive Bladder Carcinoma with Variant Histologies. Eur. Urol. 2020, 77, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Robertson, A.G.; Meghani, K.; Cooley, L.F.; McLaughlin, K.A.; Fall, L.A.; Yu, Y.; Castro, M.A.A.; Groeneveld, C.S.; de Reynies, A.; Nazarov, V.I.; et al. Expression-based subtypes define pathologic response to neoadjuvant immune-checkpoint inhibitors in muscle-invasive bladder cancer. Nat. Commun. 2023, 14, 2126. [Google Scholar] [CrossRef]
- van Dijk, N.; Gil-Jimenez, A.; Silina, K.; Hendricksen, K.; Smit, L.A.; de Feijter, J.M.; van Montfoort, M.L.; van Rooijen, C.; Peters, D.; Broeks, A.; et al. Preoperative ipilimumab plus nivolumab in locoregionally advanced urothelial cancer: The NABUCCO trial. Nat. Med. 2020, 26, 1839–1844. [Google Scholar] [CrossRef]
- Grande, E.; Guerrero, F.; Puente, J.; Galante, I.; Duran, I.; Dominguez, M.; Alonso Gordoa, T.; Burgos, J.; Font, A.; Pinto, A.; et al. DUTRENEO Trial: A randomized phase II trial of DUrvalumab and TREmelimumab versus chemotherapy as a NEOadjuvant approach to muscle-invasive urothelial bladder cancer (MIBC) patients (pts) prospectively selected by an interferon (INF)-gamma immune signature. J. Clin. Oncol. 2020, 38, 5012. [Google Scholar] [CrossRef]
- Pottier, C.; Fresnais, M.; Gilon, M.; Jérusalem, G.; Longuespée, R.; Sounni, N.E. Tyrosine Kinase Inhibitors in Cancer: Breakthrough and Challenges of Targeted Therapy. Cancers 2020, 12, 731. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.S.; Wang, K.; Khaira, D.; Ali, S.M.; Fisher, H.A.; Mian, B.; Nazeer, T.; Elvin, J.A.; Palma, N.; Yelensky, R.; et al. Comprehensive genomic profiling of 295 cases of clinically advanced urothelial carcinoma of the urinary bladder reveals a high frequency of clinically relevant genomic alterations. Cancer 2016, 122, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, C.N.; Petrylak, D.P.; Bellmunt, J.; Nishiyama, H.; Necchi, A.; Gurney, H.; Lee, J.-L.; van der Heijden, M.S.; Rosenbaum, E.; Penel, N.; et al. FORT-1: Phase II/III Study of Rogaratinib Versus Chemotherapy in Patients with Locally Advanced or Metastatic Urothelial Carcinoma Selected Based on FGFR1/3 mRNA Expression. J. Clin. Oncol. 2022, 41, 629–639. [Google Scholar] [CrossRef]
- Pal, S.K.; Daneshmand, S.; Matin, S.F.; Loriot, Y.; Sridhar, S.S.; Grivas, P.; Gupta, S.; Sonpavde, G.; Fleming, M.T.; Lerner, S.P.; et al. PROOF 302: A randomized, double-blind, placebo-controlled, phase III trial of infigratinib as adjuvant therapy in patients with invasive urothelial carcinoma harboring FGFR3 alterations. J. Clin. Oncol. 2020, 38, TPS600. [Google Scholar] [CrossRef]
- Kommalapati, A.; Tella, S.H.; Borad, M.; Javle, M.; Mahipal, A. FGFR Inhibitors in Oncology: Insight on the Management of Toxicities in Clinical Practice. Cancers 2021, 13, 2968. [Google Scholar] [CrossRef] [PubMed]
- Chau, C.H.; Steeg, P.S.; Figg, W.D. Antibody–drug conjugates for cancer. Lancet 2019, 394, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Rosenberg, J.E.; Sonpavde, G.P.; Loriot, Y.; Durán, I.; Lee, J.L.; Matsubara, N.; Vulsteke, C.; Castellano, D.; Wu, C.; et al. Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma. N. Engl. J. Med. 2021, 384, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Tagawa, S.T.; Balar, A.V.; Petrylak, D.P.; Kalebasty, A.R.; Loriot, Y.; Fléchon, A.; Jain, R.K.; Agarwal, N.; Bupathi, M.; Barthelemy, P.; et al. TROPHY-U-01: A Phase II Open-Label Study of Sacituzumab Govitecan in Patients With Metastatic Urothelial Carcinoma Progressing After Platinum-Based Chemotherapy and Checkpoint Inhibitors. J. Clin. Oncol. 2021, 39, 2474–2485. [Google Scholar] [CrossRef]
- Rosenberg, J.E.; O’Donnell, P.H.; Balar, A.V.; McGregor, B.A.; Heath, E.I.; Yu, E.Y.; Galsky, M.D.; Hahn, N.M.; Gartner, E.M.; Pinelli, J.M.; et al. Pivotal Trial of Enfortumab Vedotin in Urothelial Carcinoma After Platinum and Anti-Programmed Death 1/Programmed Death Ligand 1 Therapy. J. Clin. Oncol. 2019, 37, 2592–2600. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Liu, Y.; Zhou, X.; Shen, P.; Xue, R.; Zhang, M. Disitamab vedotin: A novel antibody-drug conjugates for cancer therapy. Drug. Deliv. 2022, 29, 1335–1344. [Google Scholar] [CrossRef]
- Sheng, X.; Yan, X.; Wang, L.; Shi, Y.; Yao, X.; Luo, H.; Shi, B.; Liu, J.; He, Z.; Yu, G.; et al. Open-label, Multicenter, Phase II Study of RC48-ADC, a HER2-Targeting Antibody–Drug Conjugate, in Patients with Locally Advanced or Metastatic Urothelial Carcinoma. Clin. Cancer Res. 2021, 27, 43–51. [Google Scholar] [CrossRef]
- Chu, C.E.; Sjöström, M.; Egusa, E.A.; Gibb, E.A.; Badura, M.L.; Zhu, J.; Koshkin, V.S.; Stohr, B.A.; Meng, M.V.; Pruthi, R.S.; et al. Heterogeneity in NECTIN4 Expression Across Molecular Subtypes of Urothelial Cancer Mediates Sensitivity to Enfortumab Vedotin. Clin. Cancer Res. 2021, 27, 5123–5130. [Google Scholar] [CrossRef]
- Jindal, T.; Zhang, L.; Chou, J.; Shui, D.; Porten, S.P.; Wong, A.C.; Chan, E.; Stohr, B.A.; de Kouchkovsky, I.; Borno, H.; et al. Biomarkers predictive of response to enfortumab vedotin (EV) treatment in advanced urothelial cancer (aUC). J. Clin. Oncol. 2022, 40, 531. [Google Scholar] [CrossRef]
- Jindal, T.; Kilari, D.; Alhalabi, O.; Nizam, A.; Khaki, A.R.; Basu, A.; Barata, P.C.; Bilen, M.A.; Shah, S.; Zakharia, Y.; et al. Biomarkers of response to enfortumab vedotin (EV) in patients (pts) with advanced urothelial carcinoma (aUC): Analysis of the UNITE study. J. Clin. Oncol. 2023, 41, 450. [Google Scholar] [CrossRef]
- Aggen, D.H.; Chu, C.E.; Rosenberg, J.E. Scratching the Surface: NECTIN-4 as a Surrogate for Enfortumab Vedotin Resistance. Clin. Cancer Res. 2023, 29, 1377–1380. [Google Scholar] [CrossRef]
- Chou, J.; Trepka, K.; Sjöström, M.; Egusa, E.A.; Chu, C.E.; Zhu, J.; Chan, E.; Gibb, E.A.; Badura, M.L.; Contreras-Sanz, A.; et al. TROP2 Expression Across Molecular Subtypes of Urothelial Carcinoma and Enfortumab Vedotin-resistant Cells. Eur. Urol. Oncol. 2022, 5, 714–718. [Google Scholar] [CrossRef]
- Fan, Y.; Li, Q.; Shen, Q.; Liu, Z.; Zhang, Z.; Hu, S.; Yu, W.; He, Z.; He, Q.; Zhang, Q. Head-to-Head Comparison of the Expression Differences of NECTIN-4, TROP-2, and HER2 in Urothelial Carcinoma and Its Histologic Variants. Front. Oncol. 2022, 12, 858865. [Google Scholar] [CrossRef]
- Galsky, M.D.; Arija JÁ, A.; Bamias, A.; Davis, I.D.; De Santis, M.; Kikuchi, E.; Garcia-Del-Muro, X.; De Giorgi, U.; Mencinger, M.; Izumi, K.; et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): A multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2020, 395, 1547–1557. [Google Scholar] [CrossRef] [PubMed]
- Bamias, A.; Davis, I.D.; Galsky, M.D.; Arranz Arija, J.A.; Kikuchi, E.; Grande, E.; Garcia del Muro, X.; Park, S.H.; De Giorgi, U.; Alekseev, B.; et al. Final overall survival (OS) analysis of atezolizumab (atezo) monotherapy vs chemotherapy (chemo) in untreated locally advanced or metastatic urothelial carcinoma (mUC) from the Phase 3 IMvigor130 study. J. Clin. Oncol. 2023, 41, LBA441. [Google Scholar] [CrossRef]
- Powles, T.; Csőszi, T.; Özgüroğlu, M.; Matsubara, N.; Géczi, L.; Cheng, S.Y.S.; Fradet, Y.; Oudard, S.; Vulsteke, C.; Morales Barrera, R.; et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 931–945. [Google Scholar] [CrossRef]
- Rosenberg, J.E.; Gajate, P.; Morales-Barrera, R.; Lee, J.-L.; Necchi, A.; Penel, N.; Zagonel, V.; Sierecki, M.R.; Piciu, A.-M.; Ellinghaus, P.; et al. Safety and preliminary efficacy of rogaratinib in combination with atezolizumab in a phase Ib/II study (FORT-2) of first-line treatment in cisplatin-ineligible patients (pts) with locally advanced or metastatic urothelial cancer (UC) and FGFR mRNA overexpression. J. Clin. Oncol. 2020, 38, 5014. [Google Scholar] [CrossRef]
- Siefker-Radtke, A.O.; Loriot, Y.; Siena, S.; Beato, C.; Duran, M.A.C.; Varlamov, S.; Duran, I.; Tagawa, S.T.; Geoffrois, L.; Mellado, B.; et al. 752P Updated data from the NORSE trial of erdafitinib (ERDA) plus cetrelimab (CET) in patients (pts) with metastatic or locally advanced urothelial carcinoma (mUC) and specific fibroblast growth factor receptor (FGFR) alterations. Ann. Oncol. 2020, 31, S584–S585. [Google Scholar] [CrossRef]
- Loriot, Y.; Grivas, P.; De Wit, R.; Balar, A.V.; Siefker-Radtke, A.O.; Zolnierek, J.; Csoszi, T.; Shin, S.J.; Park, S.H.; Atduev, V.; et al. First-line pembrolizumab (pembro) with or without lenvatinib (lenva) in patients with advanced urothelial carcinoma (LEAP-011): A phase 3, randomized, double-blind study. J. Clin. Oncol. 2022, 40, 432. [Google Scholar] [CrossRef]
- Sharma, P.; Siefker-Radtke, A.; de Braud, F.; Basso, U.; Calvo, E.; Bono, P.; Morse, M.A.; Ascierto, P.A.; Lopez-Martin, J.; Brossart, P.; et al. Nivolumab Alone and With Ipilimumab in Previously Treated Metastatic Urothelial Carcinoma: CheckMate 032 Nivolumab 1 mg/kg Plus Ipilimumab 3 mg/kg Expansion Cohort Results. J. Clin. Oncol. 2019, 37, 1608–1616. [Google Scholar] [CrossRef] [PubMed]
- Hoimes, C.J.; Flaig, T.W.; Milowsky, M.I.; Friedlander, T.W.; Bilen, M.A.; Gupta, S.; Srinivas, S.; Merchan, J.R.; McKay, R.R.; Petrylak, D.P.; et al. Enfortumab Vedotin Plus Pembrolizumab in Previously Untreated Advanced Urothelial Cancer. J. Clin. Oncol. 2022, 41, 22–31. [Google Scholar] [CrossRef]
- Rosenberg, J. LBA73—Study EV-103 Cohort K: Antitumor Activity of Enfortumab Vedotin (EV) Monotherapy or in Combination with Pembrolizumab (P) in Previously Untreated Cisplatin-Ineligible Patients (pts) with Locally Advanced or Metastatic Urothelial Cancer (la/mUC); European Society for Medical Oncology: Lugano, Switzerland, 2022. [Google Scholar]
- Sheng, X.; Zhou, L.; He, Z.; Guo, H.; Yan, X.; Li, S.; Xu, H.; Li, J.; Chi, Z.; Si, L.; et al. Preliminary results of a phase Ib/II combination study of RC48-ADC, a novel humanized anti-HER2 antibody-drug conjugate (ADC) with toripalimab, a humanized IgG4 mAb against programmed death-1 (PD-1) in patients with locally advanced or metastatic urothelial carcinoma. J. Clin. Oncol. 2022, 40, 4518. [Google Scholar] [CrossRef]
- Balar, A.V.; Moreno, V.; Angevin, E.; Gan, H.K.; Vieito, M.; Italiano, A.; Danielli, R.; Massarelli, E.; Opdam, F.; Chisamore, M.J.; et al. Inducible T-cell co-stimulatory (ICOS) receptor agonist, feladilimab (fela), alone and in combination (combo) with pembrolizumab (P): Results from INDUCE-1 urothelial carcinoma (UC) expansion cohorts (ECs). J. Clin. Oncol. 2021, 39, 4519. [Google Scholar] [CrossRef]
- Sadeghi, S.; Quinn, D.; Dorff, T.; Pal, S.; Groshen, S.; Tsao-Wei, D.; Parikh, R.; Devitt, M.; Parikh, M.; Jackovich, A.; et al. EphrinB2 Inhibition and Pembrolizumab in Metastatic Urothelial Carcinoma. J. Clin. Oncol. 2022, 41, 640–650. [Google Scholar] [CrossRef]
- Iyer, G.; Hanrahan, A.J.; Milowsky, M.I.; Al-Ahmadie, H.; Scott, S.N.; Janakiraman, M.; Pirun, M.; Sander, C.; Socci, N.D.; Ostrovnaya, I.; et al. Genome sequencing identifies a basis for everolimus sensitivity. Science 2012, 338, 221. [Google Scholar] [CrossRef] [PubMed]
- Bellmunt, J.; Lalani, A.A.; Jacobus, S.; Wankowicz, S.A.; Polacek, L.; Takeda, D.Y.; Harshman, L.C.; Wagle, N.; Moreno, I.; Lundgren, K.; et al. Everolimus and pazopanib (E/P) benefit genomically selected patients with metastatic urothelial carcinoma. Br. J. Cancer 2018, 119, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Adib, E.; Klonowska, K.; Giannikou, K.; Do, K.T.; Pruitt-Thompson, S.; Bhushan, K.; Milstein, M.I.; Hedglin, J.; Kargus, K.E.; Sholl, L.M.; et al. Phase II Clinical Trial of Everolimus in a Pan-Cancer Cohort of Patients with mTOR Pathway Alterations. Clin. Cancer Res. 2021, 27, 3845–3853. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, J.E.; Park, S.H.; Kozlov, V.; Dao, T.V.; Castellano, D.; Li, J.R.; Mukherjee, S.D.; Howells, K.; Dry, H.; Lanasa, M.C.; et al. Durvalumab Plus Olaparib in Previously Untreated, Platinum-Ineligible Patients With Metastatic Urothelial Carcinoma: A Multicenter, Randomized, Phase II Trial (BAYOU). J. Clin. Oncol. 2023, 41, 43–53. [Google Scholar] [CrossRef]
- Pantuck, M.; Palaskas, N.; Drakaki, A. Next generation T-cell therapy for genitourinary malignancies, part A: Introduction and current state of the art. Cancer Treat. Res. Commun. 2018, 17, 8–12. [Google Scholar] [CrossRef]
- Vuky, J.; Balar, A.V.; Castellano, D.; O'Donnell, P.H.; Grivas, P.; Bellmunt, J.; Powles, T.; Bajorin, D.; Hahn, N.M.; Savage, M.J.; et al. Long-Term Outcomes in KEYNOTE-052: Phase II Study Investigating First-Line Pembrolizumab in Cisplatin-Ineligible Patients with Locally Advanced or Metastatic Urothelial Cancer. J. Clin. Oncol. 2020, 38, 2658–2666. [Google Scholar] [CrossRef]
- Plimack, E.R.; Bellmunt, J.; Gupta, S.; Berger, R.; Chow, L.Q.M.; Juco, J.; Lunceford, J.; Saraf, S.; Perini, R.F.; O’Donnell, P.H. Safety and activity of pembrolizumab in patients with locally advanced or metastatic urothelial cancer (KEYNOTE-012): A non-randomised, open-label, phase 1b study. Lancet Oncol. 2017, 18, 212–220. [Google Scholar] [CrossRef]
- Galsky, M.D.; Mortazavi, A.; Milowsky, M.I.; George, S.; Gupta, S.; Fleming, M.T.; Dang, L.H.; Geynisman, D.M.; Walling, R.; Alter, R.S.; et al. Randomized Double-Blind Phase II Study of Maintenance Pembrolizumab Versus Placebo After First-Line Chemotherapy in Patients with Metastatic Urothelial Cancer. J. Clin. Oncol. 2020, 38, 1797–1806. [Google Scholar] [CrossRef]
- Balar, A.V.; Galsky, M.D.; Rosenberg, J.E.; Powles, T.; Petrylak, D.P.; Bellmunt, J.; Loriot, Y.; Necchi, A.; Hoffman-Censits, J.; Perez-Gracia, J.L.; et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: A single-arm, multicentre, phase 2 trial. Lancet 2017, 389, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Galsky, M.D.; Retz, M.; Siefker-Radtke, A.O.; Baron, A.; Necchi, A.; Bedke, J.; Plimack, E.R.; Vaena, D.; Grimm, M.O.; Bracarda, S.; et al. Efficacy and safety of nivolumab monotherapy in patients with metastatic urothelial cancer (mUC) who have received prior treatment: Results from the phase II CheckMate 275 study. Ann. Oncol. 2016, 27, vi567. [Google Scholar] [CrossRef]
- Bajorin, D.F.; Witjes, J.A.; Gschwend, J.E.; Schenker, M.; Valderrama, B.P.; Tomita, Y.; Bamias, A.; Lebret, T.; Shariat, S.F.; Park, S.H.; et al. Adjuvant Nivolumab versus Placebo in Muscle-Invasive Urothelial Carcinoma. N. Engl. J. Med. 2021, 384, 2102–2114. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; O’Donnell, P.H.; Massard, C.; Arkenau, H.T.; Friedlander, T.W.; Hoimes, C.J.; Lee, J.L.; Ong, M.; Sridhar, S.S.; Vogelzang, N.J.; et al. Efficacy and Safety of Durvalumab in Locally Advanced or Metastatic Urothelial Carcinoma: Updated Results From a Phase 1/2 Open-label Study. JAMA Oncol. 2017, 3, e172411. [Google Scholar] [CrossRef]
- Powles, T.; van der Heijden, M.S.; Castellano, D.; Galsky, M.D.; Loriot, Y.; Petrylak, D.P.; Ogawa, O.; Park, S.H.; Lee, J.-L.; De Giorgi, U.; et al. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): A randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2020, 21, 1574–1588. [Google Scholar] [CrossRef]
- Powles, T.B.; Chistyakov, V.; Beliakouski, V.; Semenov, A.; Everaert, E.; Baranau, Y.; Moreno, V.; Perez Valderrama, B.; Vano, Y.; Del Conte, G.; et al. LBA27 Erdafitinib (ERDA) or ERDA plus cetrelimab (CET) for patients with metastatic or locally advanced urothelial carcinoma (mUC) and Fibroblast Growth Factor Receptor alterations (FGFRa): First phase (Ph) II results from the NORSE study. Ann. Oncol. 2021, 32, S1303. [Google Scholar] [CrossRef]
- Yu, E.Y.; Petrylak, D.P.; O’Donnell, P.H.; Lee, J.-L.; van der Heijden, M.S.; Loriot, Y.; Stein, M.N.; Necchi, A.; Kojima, T.; Harrison, M.R.; et al. Enfortumab vedotin after PD-1 or PD-L1 inhibitors in cisplatin-ineligible patients with advanced urothelial carcinoma (EV-201): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2021, 22, 872–882. [Google Scholar] [CrossRef]
- Rosenberg, J.E.; Sridhar, S.S.; Zhang, J.; Smith, D.C.; Ruether, J.D.; Flaig, T.W.; Baranda, J.C.; Lang, J.M.; Plimack, E.R.; Sangha, R.S.; et al. Mature results from EV-101: A phase I study of enfortumab vedotin in patients with metastatic urothelial cancer (mUC). J. Clin. Oncol. 2019, 37, 377. [Google Scholar] [CrossRef]
- Hong, D.S.; Van Tine, B.A.; Biswas, S.; McAlpine, C.; Johnson, M.L.; Olszanski, A.J.; Clarke, J.M.; Araujo, D.; Blumenschein, G.R.; Kebriaei, P.; et al. Autologous T cell therapy for MAGE-A4+ solid cancers in HLA-A*02+ patients: A phase 1 trial. Nat. Med. 2023, 29, 104–114. [Google Scholar] [CrossRef] [PubMed]

| Study | Study Population | Treatment Arms | Biomarker | Improved Outcomes |
|---|---|---|---|---|
| Phase III IMvigor211 [21] | locally advanced or mUC after progression with platinum-based chemotherapy | atezolizumab vs. chemotherapy | TMB | median OS was longer with atezolizumab (HR 0.68, CI 95% 0.51–0.90) in the TMB-high subgroup |
| Phase II Checkmate 275 [16] | metastatic or surgically unresectable locally advanced UC | nivolumab | TMB | TMB alone showed a positive association with ORR with nivolumab [OR (95% CI): 2.13 (1.26–3.60), p < 0.05] |
| ARID1A + CXCL13 | ARID1A mutation and high CXCL13 was associated with median PFS of 3.7 months (85% CI, 1.8 to NA) and OS of 19.1 months (95% CI, 6.1 to NA) compared to patients with neither alteration who had a median PFS of 1.9 months (95% CI, 1.7–2.0) and median OS of 5.3 months (95% CI, 3.6 to 11.4) | |||
| molecular subtypes (luminal 1, luminal 2, basal 1, basal 2) | Basal 1 (cluster 1) and luminal 2 (cluster 2) were found to have higher CR rates to nivolumab compared to the other subtypes (Basal 2 CR, 0%; luminal 1 CR, 1.5%; luminal 2 CR, 1.8%). | |||
| High interferon gamma signature | more likely to respond to nivolumab than those with low expression (p = 0.0003) | |||
| Nassar et al.—A model combining clinical and genomic factors to predict response to PD-1/PD-L1 blockade in advanced urothelial carcinoma [24] | mUC | Retrospective study of ICIs | TMB/SNV count, visceral metastases and NLR highly | Biomarkers highly correlated with tumor regression [AUC (95% CI) = 0.90 (0.80, 0.99)] [24] |
| Phase II IMvigor210 [28] | Locally advanced or mUC | Atezolizumab | ARID1A + CXCL13 | expression of both ARID1A mutation plus baseline CXCL13 expression had a median OS of 17.8 months (95% CI, 10.4 to NA) compared to 7.1 months (95% CI, 5.5 to 9.9) in patients with neither |
| Study | Cancer Type | Treatment Arms | Biomarker | Outcomes |
|---|---|---|---|---|
| ABACUS Study [32] | MIBC | neoadjuvant atezolizumab | preexisting activated T-cells | Patients with a high presence of intraepithelial CD8+ cells had a pCR rate of 40% (95% CI: 26–57%) compared to a rate of 20% (95% CI: 67–94%) for patients with an absence of CD |
| PURE-01 [33,34] | MIBC | neoadjuvant pembrolizumab | histone demethylase KDM5B | inhibition of KDM5B enhances immunogenicity in FGFR3-mutated UC cells |
| NABUCCO trial [35] | MIBC | neoadjuvant ipilimumab plus nivolumab | Tertiary lymphoid structures | induction of TLS was observed in responding patients |
| DUTRENEO trial [36] | MIBC | Neoadjuvant durvalumab and tremelimumab vs. chemotherapy | Patients were classified as having a “hot” or “cold” tumor using a TIS score based on an 18-gene interferon-y signaling related expression | This stratification failed to select patients more likely to benefit from immunotherapy vs. chemotherapy. |
| Study | Cancer Type | Study Design | Biomarker | Conclusions/Outcomes |
|---|---|---|---|---|
| Chu et al.—Heterogeneity in NECTIN4 Expression Across Molecular Subtypes of UC Mediates Sensitivity to EV [48] | MIBC | Retrospective study with molecular subtyping and NECTIN4 expression data from seven MIBC clinical cohorts | Molecular subtyping and NECTIN4 expression | Sensitivity to EV is mediated by expression of NECTIN4, which is enriched in luminal subtypes of bladder cancer |
| Jindal et al.—Biomarkers predictive of response to EV treatment in advanced UC [49] | Advanced UC | Retrospective study assessing molecular and clinical characteristics | TP53, CDKN2A, CDKN2B | The presence of TP53 and absence of CDKN2A and CDKN2B alterations were associated with favorable responses and improved clinical outcomes. |
| UNITE study [50] | Advanced UC | Retrospective study assessing molecular and clinical characteristics | ERBB2, TSC1 | Observed responses were higher in patients with ERBB2 (67% vs. 44%; p = 0.05) and TSC1 (68% vs. 25%; p = 0.04) alterations vs. wild-type. |
| High TMB | patients with high TMB were found to have longer median OS (13.5 vs. 8.3 months, p = 0.02) | |||
| CDKN2A, CDKN2B, MTAP alterations | Shorter median PFS was found in patients with CDKN2A (4.4 vs. 6.0 months, p = 0.02), CDKN2B (4.4 vs. 6.0 months, p ≤ 0.01), and MTAP alterations (4.6 vs. 6.0 months, p = 0.05). |
| Name(s) | Target/MOA | Trials | Phase | n | Study Arms | Primary Endpoint(s) |
|---|---|---|---|---|---|---|
| Atezolizumab + CRT | PD-L1 + CRT | NCT03775265 | III, active, recruiting | 475 | RT + chemotherapy vs. CRT + atezolizumab in localized MIBC | Bladder intact event-free survival |
| Pembrolizumab + CRT | PD-1 + CRT | NCT04241185 (KEYNOTE-992) | III, active, recruiting | 636 | Pembrolizumab + CRT vs. placebo + CRT in MIBC | Bladder intact event-free survival |
| Enfortumab vedotin + pembrolizumab + platinum-based chemotherapy | Nectin-4 and monomethyl auristatin E (MMAE) + PD-1 | NCT03288545 (EV-103) | I/II, active, not recruiting | 457 | UC | ORR, pCR, AEs |
| Saciuzumab-govitecan + pembrolizumab | Anti-Trop-2 humanized monoclonal antibody + PD-1/PD-L1 | NCT05535218 (SURE-02) | II, active, recruiting | 48 | MIBC | pCR |
| Sacituzumab-govitecan + Atezolizumab | NCT03869190 (MORPHEUS-UC) | Ib/II, active, recruiting | 645 | MIBC or locally advanced or mUC who progressed with platinum therapy | ORR, pCR | |
| Sacituzumab-govitecan + Avelumab | NCT05327530 (JAVELIN Bladder Medley) | II, active, recruiting | 252 | MIBC or locally advanced or mUC who progressed with platinum therapy | PFS, AEs | |
| Disitamab Vedotin + Pembrolizumab | HER2 (hertuzumab and MMAE) + PD-1/PD-L1 | NCT04879329 | II, active | 270 | Disitamab Vedotin monotherapy (only cohort C) for HER2+ locally advanced unresectable or mUC | Confirmed ORR |
| Disitamab Vedotin + Toripalimab | NCT05302284 | III, active, recruiting | 456 | untreated unresectable locally advanced or metastatic HER2-positive UC | PFS, OS | |
| RC48-ADC (Disitamab Vedotin) + Toripalimab | NCT04264936 | Ib/II, active, unknown recruitment status | 36 | RC48-ACD and JS001 for locally advanced or mUC | AEs and maximal tolerated dose | |
| EphB4-human serum albumin + pembrolizumab | EphB4-human serum albumin + PD-1 | NCT02717156 | II, active, recruiting | 170 | EphB4-HAS + pembrolizumab in solid tumors | Toxicities and AEs |
| Cabozantinib + avelumab (VEGF TKI + PD-L1 inhibitor) | VEGF TKI + PD-L1 inhibitor | NCT05092958 (MAIN-CAV) | III, active, recruiting | 654 | Avelumab vs. avelumab + cabozantinib in mUC | OS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luceno, C.F.; Jeon, W.J.; Samaeekia, R.; Shin, J.; Sonpavde, G.P. Precision Medicine to Treat Urothelial Carcinoma—The Way Forward. Cancers 2023, 15, 3024. https://doi.org/10.3390/cancers15113024
Luceno CF, Jeon WJ, Samaeekia R, Shin J, Sonpavde GP. Precision Medicine to Treat Urothelial Carcinoma—The Way Forward. Cancers. 2023; 15(11):3024. https://doi.org/10.3390/cancers15113024
Chicago/Turabian StyleLuceno, Carvy Floyd, Won Jin Jeon, Ravand Samaeekia, John Shin, and Guru P. Sonpavde. 2023. "Precision Medicine to Treat Urothelial Carcinoma—The Way Forward" Cancers 15, no. 11: 3024. https://doi.org/10.3390/cancers15113024