Applications of Anti-Cytomegalovirus T Cells for Cancer (Immuno)Therapy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Anti-CMV T Cells—Potent Effector Cells for Cancer Treatment
3. Memory T Cell Inflation
4. Strategies to Exploit Inflationary Anti-CMV T Cells for Elimination of Cancer Cells
4.1. Loading CMV Peptides into Endogenous HLA Complexes on Cancer Cells
4.1.1. TEDbodies
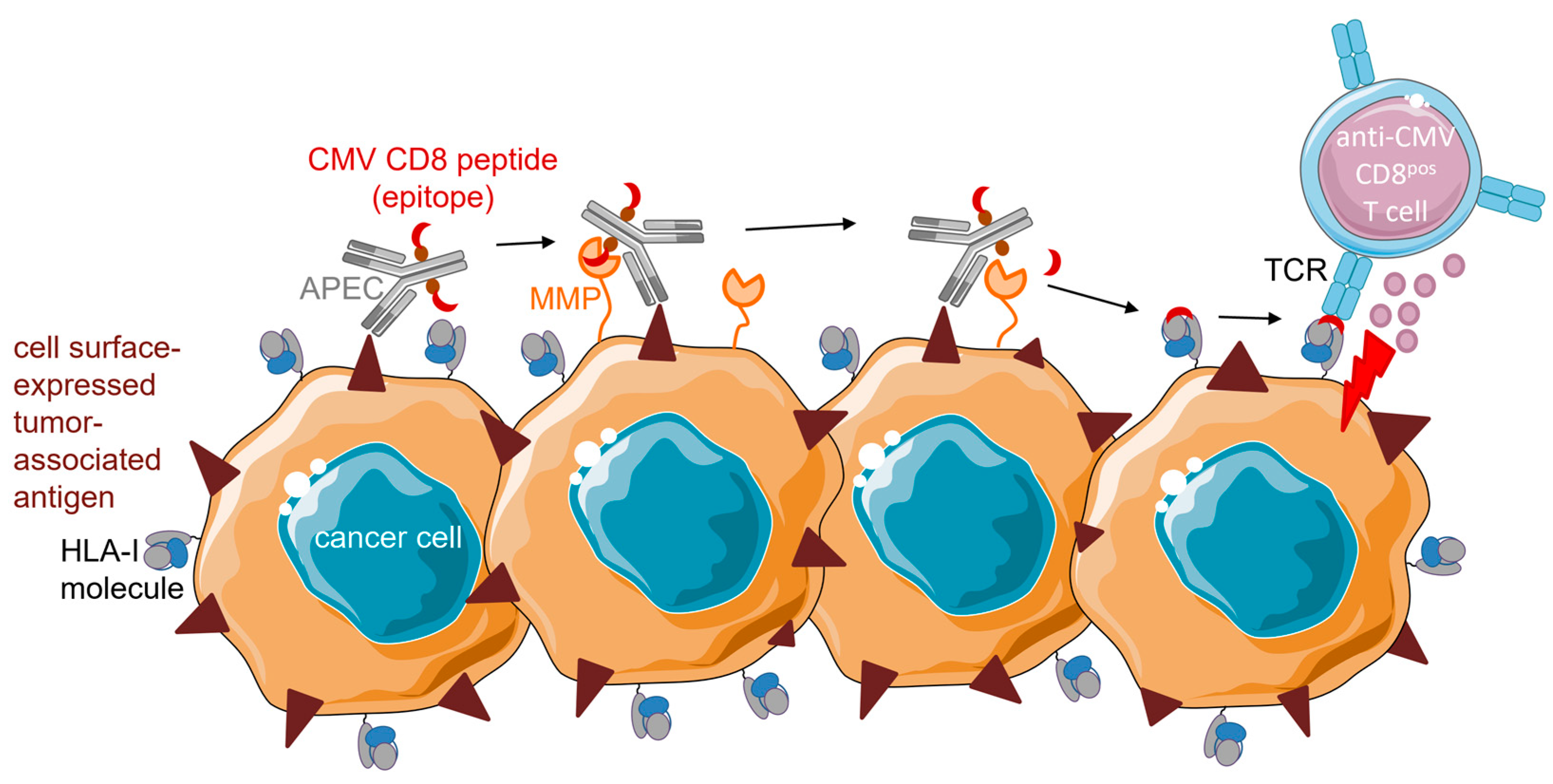
4.1.2. APECs
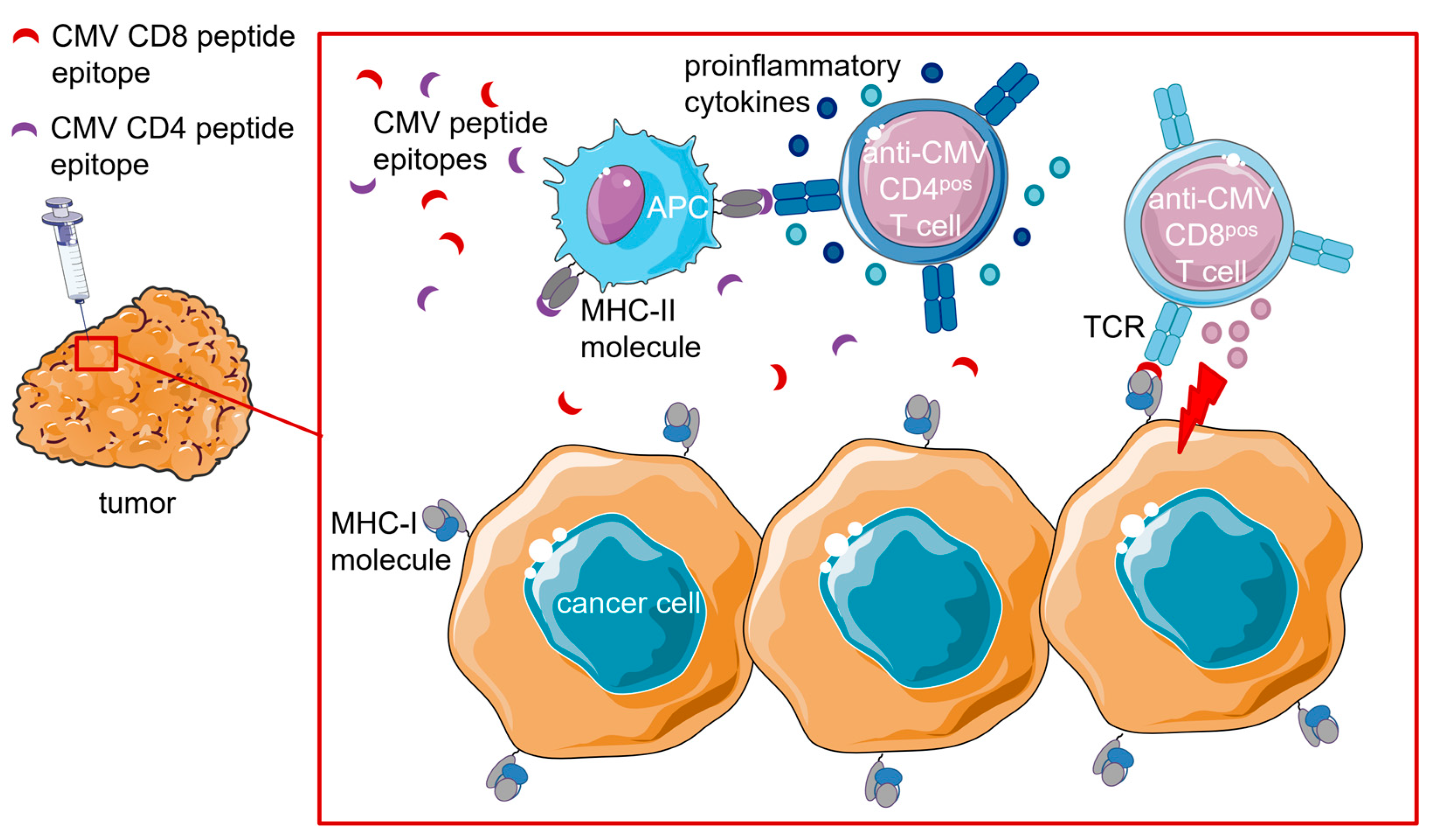
4.1.3. Intratumoral (i.t.) Injection of CMV Peptide Epitopes
4.2. Tumor-Directed Fusion Proteins Comprising CMV Peptide–HLA-I Complexes
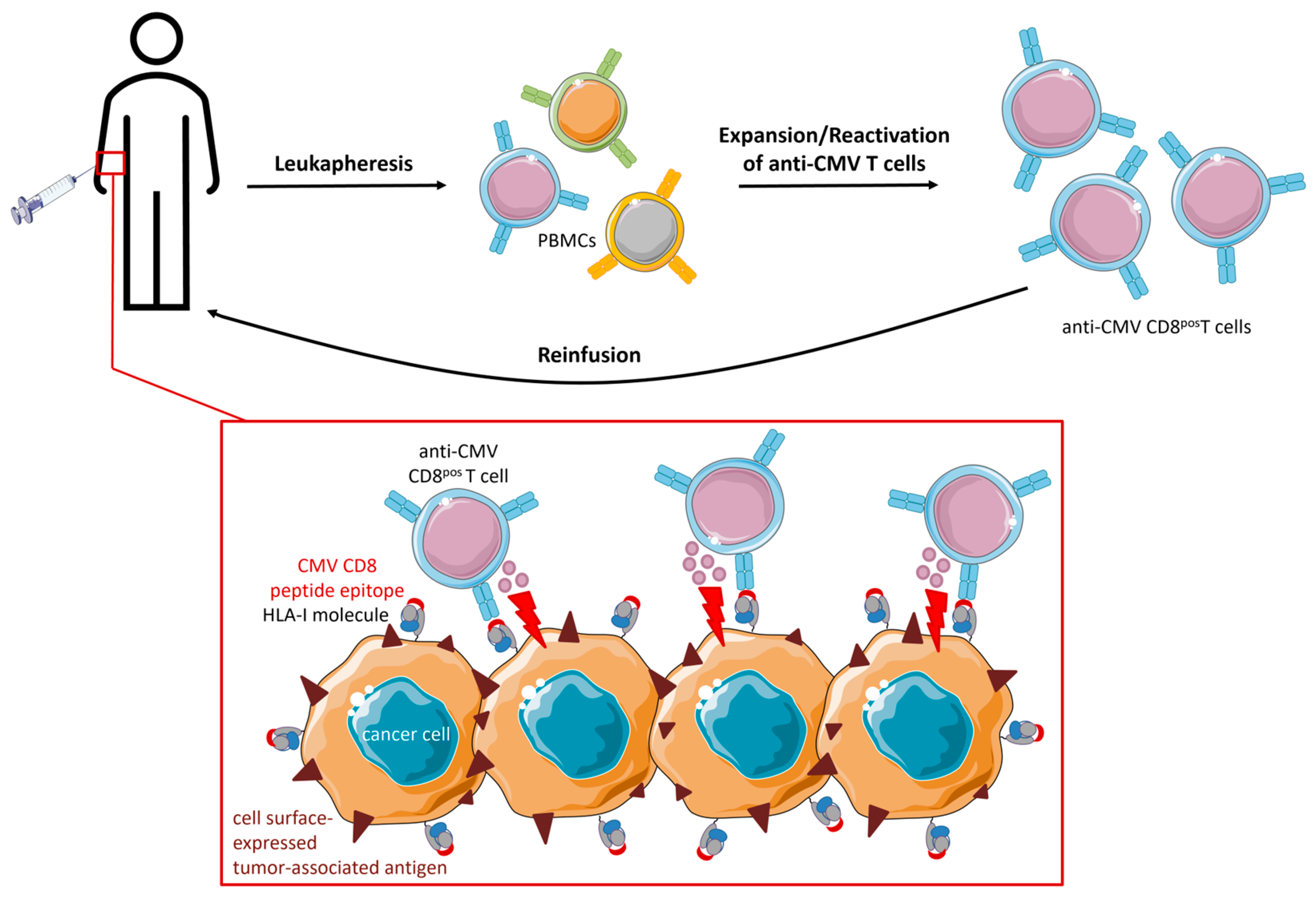
5. Strategies Utilizing (Engineered) CMV T Cells in Adoptive Cell Therapy (ACT)
5.1. CMV-CAR T Cells
5.2. TCR-Engineered CMV T Cells
5.3. Direct Targeting of CMV Peptide Epitopes Expressed in Glioblastoma
6. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zuhair, M.; Smit, G.S.A.; Wallis, G.; Jabbar, F.; Smith, C.; Devleesschauwer, B.; Griffiths, P. Estimation of the worldwide seroprevalence of cytomegalovirus: A systematic review and meta-analysis. Rev. Med. Virol. 2019, 29, e2034. [Google Scholar] [CrossRef] [Green Version]
- Cannon, M.J.; Schmid, D.S.; Hyde, T.B. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev. Med. Virol. 2010, 20, 202–213. [Google Scholar] [CrossRef]
- Dowd, J.B.; Aiello, A.E.; Alley, D.E. Socioeconomic disparities in the seroprevalence of cytomegalovirus infection in the US population: NHANES III. Epidemiology Infect. 2009, 137, 58–65. [Google Scholar] [CrossRef] [Green Version]
- Fowler, K.; Mucha, J.; Neumann, M.; Lewandowski, W.; Kaczanowska, M.; Grys, M.; Schmidt, E.; Natenshon, A.; Talarico, C.; Buck, P.O.; et al. A systematic literature review of the global seroprevalence of cytomegalovirus: Possible implications for treatment, screening, and vaccine development. BMC Public Health 2022, 22, 1659. [Google Scholar] [CrossRef]
- Khan, N.; Shariff, N.; Cobbold, M.; Bruton, R.; Ainsworth, J.A.; Sinclair, A.J.; Nayak, L.; Moss, P.A.H. Cytomegalovirus Seropositivity Drives the CD8 T Cell Repertoire Toward Greater Clonality in Healthy Elderly Individuals. J. Immunol. 2002, 169, 1984–1992. [Google Scholar] [CrossRef] [Green Version]
- Sylwester, A.W.; Mitchell, B.L.; Edgar, J.B.; Taormina, C.; Pelte, C.; Ruchti, F.; Sleath, P.R.; Grabstein, K.H.; Hosken, N.A.; Kern, F.; et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J. Exp. Med. 2005, 202, 673–685. [Google Scholar] [CrossRef] [Green Version]
- Komatsu, H.; Sierro, S.; Cuero, A.V.; Klenerman, P. Population analysis of antiviral T cell responses using MHC class I-peptide tetramers. Clin. Exp. Immunol. 2003, 134, 9–12. [Google Scholar] [CrossRef]
- Pita-Lopez, M.L.; Gayoso, I.; DelaRosa, O.; Casado, J.G.; Alonso, C.; Muñoz-Gomariz, E.; Tarazona, R.; Solana, R. Effect of ageing on CMV-specific CD8 T cells from CMV seropositive healthy donors. Immun. Ageing 2009, 6, 11. [Google Scholar] [CrossRef] [Green Version]
- Klenerman, P.; Oxenius, A. T cell responses to cytomegalovirus. Nat. Rev. Immunol. 2016, 16, 367–377. [Google Scholar] [CrossRef]
- Raa, G.D.T.; Pascutti, M.F.; García-Vallejo, J.J.; Reinen, E.; Remmerswaal, E.B.M.; Berge, I.J.M.T.; van Lier, R.A.W.; Eldering, E.; van Oers, M.H.J.; Tonino, S.H.; et al. CMV-specific CD8+ T-cell function is not impaired in chronic lymphocytic leukemia. Blood 2014, 123, 717–724. [Google Scholar] [CrossRef] [Green Version]
- Simoni, Y.; Becht, E.; Fehlings, M.; Loh, C.Y.; Koo, S.-L.; Teng, K.W.W.; Yeong, J.P.S.; Nahar, R.; Zhang, T.; Kared, H.; et al. Bystander CD8+ T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature 2018, 557, 575–579. [Google Scholar] [CrossRef]
- Leem, G.; Jeon, M.; Kim, K.W.; Jeong, S.; Choi, S.J.; Lee, Y.J.; Kim, E.-S.; Lee, J.-I.; Ha, S.Y.; Park, S.-H.; et al. Tumour-infiltrating bystander CD8+T cells activated by IL-15 contribute to tumour control in non-small cell lung cancer. Thorax 2021, 77, 769–780. [Google Scholar] [CrossRef]
- Von Laer, D.; Meyer-Koenig, U.; Serr, A.; Finke, J.; Kanz, L.; Fauser, A.; Haefelin, D.N.; Brugger, W.; Hufert, F. Detection of cytomegalovirus DNA in CD34+ cells from blood and bone marrow. Blood 1995, 86, 4086–4090. [Google Scholar] [CrossRef]
- Mendelson, M.; Monard, S.; Sissons, P.; Sinclair, J. Detection of endogenous human cytomegalovirus in CD34+ bone marrow progenitors. J. Gen. Virol. 1996, 77, 3099–3102. [Google Scholar] [CrossRef]
- Khaiboullina, S.F.; Maciejewski, J.P.; Crapnell, K.; Spallone, P.A.; Stock, A.D.; Pari, G.S.; Zanjani, E.D.; Jeor, S.S. Human cytomegalovirus persists in myeloid progenitors and is passed to the myeloid progeny in a latent form. Br. J. Haematol. 2004, 126, 410–417. [Google Scholar] [CrossRef]
- Söderberg-Nauclér, C. New mechanistic insights of the pathogenicity of high-risk cytomegalovirus (CMV) strains derived from breast cancer: Hope for new cancer therapy options. Ebiomedicine 2022, 81, 104103. [Google Scholar] [CrossRef]
- Reeves, M.B.; Compton, T. Inhibition of Inflammatory Interleukin-6 Activity via Extracellular Signal-Regulated Kinase–Mitogen-Activated Protein Kinase Signaling Antagonizes Human Cytomegalovirus Reactivation from Dendritic Cells. J. Virol. 2011, 85, 12750–12758. [Google Scholar] [CrossRef] [Green Version]
- Holtappels, R.; Pahl-Seibert, M.-F.; Thomas, D.; Reddehase, M.J. Enrichment of Immediate-Early 1 (m123/pp89) Peptide-Specific CD8 T Cells in a Pulmonary CD62Llo Memory-Effector Cell Pool during Latent Murine Cytomegalovirus Infection of the Lungs. J. Virol. 2000, 74, 11495–11503. [Google Scholar] [CrossRef] [Green Version]
- Karrer, U.; Sierro, S.; Wagner, M.; Oxenius, A.; Hengel, H.; Koszinowski, U.H.; Phillips, R.E.; Klenerman, P. Memory Inflation: Continous Accumulation of Antiviral CD8+ T Cells Over Time. J. Immunol. 2003, 171, 3895. [Google Scholar] [CrossRef] [Green Version]
- Snyder, C.M.; Cho, K.S.; Bonnett, E.L.; van Dommelen, S.; Shellam, G.R.; Hill, A.B. Memory Inflation during Chronic Viral Infection Is Maintained by Continuous Production of Short-Lived, Functional T Cells. Immunity 2008, 29, 650–659. [Google Scholar] [CrossRef] [Green Version]
- Munks, M.W.; Cho, K.S.; Pinto, A.K.; Sierro, S.; Klenerman, P.; Hill, A.B. Four Distinct Patterns of Memory CD8 T Cell Responses to Chronic Murine Cytomegalovirus Infection. J. Immunol. 2006, 177, 450–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turula, H.; Smith, C.J.; Grey, F.; Zurbach, K.A.; Snyder, C.M. Competition between T cells maintains clonal dominance during memory inflation induced by MCMV. Eur. J. Immunol. 2013, 43, 1252–1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almanzar, G.; Schwaiger, S.; Jenewein, B.; Keller, M.; Herndler-Brandstetter, D.; Würzner, R.; Schönitzer, D.; Grubeck-Loebenstein, B. Long-Term Cytomegalovirus Infection Leads to Significant Changes in the Composition of the CD8+T-Cell Repertoire, Which May Be the Basis for an Imbalance in the Cytokine Production Profile in Elderly Persons. J. Virol. 2005, 79, 3675–3683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwanninger, A.; Weinberger, B.; Weiskopf, D.; Herndler-Brandstetter, D.; Reitinger, S.; Gassner, C.; Schennach, H.; Parson, W.; Würzner, R.; Grubeck-Loebenstein, B. Age-related appearance of a CMV-specific high-avidity CD8+ T cell clonotype which does not occur in young adults. Immun. Ageing 2008, 5, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vescovini, R.; Biasini, C.; Fagnoni, F.F.; Telera, A.R.; Zanlari, L.; Pedrazzoni, M.; Bucci, L.; Monti, D.; Medici, M.C.; Chezzi, C.; et al. Massive Load of Functional Effector CD4+ and CD8+ T Cells against Cytomegalovirus in Very Old Subjects. J. Immunol. 2007, 179, 4283–4291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lachmann, R.; Bajwa, M.; Vita, S.; Smith, H.; Cheek, E.; Akbar, A.; Kern, F. Polyfunctional T Cells Accumulate in Large Human Cytomegalovirus-Specific T Cell Responses. J. Virol. 2012, 86, 1001. [Google Scholar] [CrossRef] [Green Version]
- Lang, A.; Brien, J.D.; Nikolich-Zugich, J. Inflation and Long-Term Maintenance of CD8 T Cells Responding to a Latent Herpesvirus Depend upon Establishment of Latency and Presence of Viral Antigens. J. Immunol. 2009, 183, 8077–8087. [Google Scholar] [CrossRef] [Green Version]
- Braga, F.A.V.; Hertoghs, K.M.L.; van Lier, R.A.; van Gisbergen, K.P.J.M. Molecular characterization of HCMV-specific immune responses: Parallels between CD8+T cells, CD4+T cells, and NK cells. Eur. J. Immunol. 2015, 45, 2433–2445. [Google Scholar] [CrossRef]
- Hertoghs, K.M.; Moerland, P.D.; van Stijn, A.; Remmerswaal, E.B.; Yong, S.L.; van de Berg, P.J.; van Ham, S.M.; Baas, F.; Berge, I.J.T.; van Lier, R.A. Molecular profiling of cytomegalovirus-induced human CD8+ T cell differentiation. J. Clin. Investig. 2010, 120, 4077–4090. [Google Scholar] [CrossRef]
- Sierro, S.; Rothkopf, R.; Klenerman, P. Evolution of diverse antiviral CD8+ T cell populations after murine cytomegalovirus infection. Eur. J. Immunol. 2005, 35, 1113–1123. [Google Scholar] [CrossRef]
- Waller, E.C.P.; Day, E.; Sissons, J.G.P.; Wills, M.R. Dynamics of T cell memory in human cytomegalovirus infection. Med. Microbiol. Immunol. 2008, 197, 83–96. [Google Scholar] [CrossRef]
- Derhovanessian, E.; Maier, A.B.; Hähnel, K.; Beck, R.; de Craen, A.J.M.; Slagboom, E.P.; Westendorp, R.G.J.; Pawelec, G. Infection with cytomegalovirus but not herpes simplex virus induces the accumulation of late-differentiated CD4+ and CD8+ T-cells in humans. J. Gen. Virol. 2011, 92, 2746–2756. [Google Scholar] [CrossRef] [PubMed]
- Wills, M.R.; Okecha, G.; Weekes, M.P.; Gandhi, M.K.; Sissons, P.J.G.; Carmichael, A.J. Identification of Naive or Antigen-Experienced Human CD8+ T Cells by Expression of Costimulation and Chemokine Receptors: Analysis of the Human Cytomegalovirus-Specific CD8+ T Cell Response. J. Immunol. 2002, 168, 5455–5464. [Google Scholar] [CrossRef] [PubMed]
- Böttcher, J.P.; Beyer, M.; Meissner, F.; Abdullah, Z.; Sander, J.; Höchst, B.; Eickhoff, S.; Rieckmann, J.C.; Russo, C.; Bauer, T.; et al. Functional classification of memory CD8+ T cells by CX3CR1 expression. Nat. Commun. 2015, 6, 8306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elkington, R.; Walker, S.; Crough, T.; Menzies, M.; Tellam, J.; Bharadwaj, M.; Khanna, R. Ex Vivo Profiling of CD8+-T-Cell Responses to Human Cytomegalovirus Reveals Broad and Multispecific Reactivities in Healthy Virus Carriers. J. Virol. 2003, 77, 5226–5240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, S.E.; Mason, G.M.; Okecha, G.; Sissons, J.G.P.; Wills, M.R. Diverse Specificities, Phenotypes, and Antiviral Activities of Cytomegalovirus-Specific CD8+T Cells. J. Virol. 2014, 88, 10894–10908. [Google Scholar] [CrossRef] [PubMed]
- O’hara, G.A.; Welten, S.P.; Klenerman, P.; Arens, R. Memory T cell inflation: Understanding cause and effect. Trends Immunol. 2012, 33, 84–90. [Google Scholar] [CrossRef]
- Noy, R.; Haus-Cohen, M.; Oved, K.; Voloshin, T.; Reiter, Y. Recruitment of Oligoclonal Viral-Specific T cells to Kill Human Tumor Cells Using Single-Chain Antibody–Peptide–HLA Fusion Molecules. Mol. Cancer Ther. 2015, 14, 1327–1335. [Google Scholar] [CrossRef] [Green Version]
- Jung, K.; Son, M.-J.; Lee, S.-Y.; Kim, J.-A.; Ko, D.-H.; Yoo, S.; Kim, C.-H.; Kim, Y.-S. Antibody-mediated delivery of a viral MHC-I epitope into the cytosol of target tumor cells repurposes virus-specific CD8+ T cells for cancer immunotherapy. Mol. Cancer 2022, 21, 102. [Google Scholar] [CrossRef]
- de Charette, M.; Marabelle, A.; Houot, R. Turning tumour cells into antigen presenting cells: The next step to improve cancer immunotherapy? Eur. J. Cancer 2016, 68, 134–147. [Google Scholar] [CrossRef]
- Millar, D.G.; Ramjiawan, R.R.; Kawaguchi, K.; Gupta, N.; Chen, J.; Zhang, S.; Nojiri, T.; Ho, W.W.; Aoki, S.; Jung, K.; et al. Antibody-mediated delivery of viral epitopes to tumors harnesses CMV-specific T cells for cancer therapy. Nat. Biotechnol. 2020, 38, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yan, C.; Millar, D.G.; Yang, Q.; Heather, J.M.; Langenbucher, A.; Morton, L.T.; Sepulveda, S.; Alpert, E.; Whelton, L.R.; et al. Antibody-Peptide Epitope Conjugates for Personalized Cancer Therapy. Cancer Res. 2022, 82, 773–784. [Google Scholar] [CrossRef]
- Çuburu, N.; Bialkowski, L.; Pontejo, S.M.; Sethi, S.K.; Bell, A.T.; Kim, R.; Thompson, C.D.; Lowy, D.R.; Schiller, J.T. Harnessing anti-cytomegalovirus immunity for local immunotherapy against solid tumors. Proc. Natl. Acad. Sci. USA 2022, 119, e2116738119. [Google Scholar] [CrossRef]
- Mous, R.; Savage, P.; Remmerswaal, E.B.M.; Lier, R.A.W.v.; Eldering, E.; van Oers, M.H.J. Redirection of CMV-specific CTL towards B-CLL via CD20-targeted HLA/CMV complexes. Leukemia 2006, 20, 1096–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmittnaegel, M.; Levitsky, V.; Hoffmann, E.; Georges, G.; Mundigl, O.; Klein, C.; Knoetgen, H. Committing Cytomegalovirus-Specific CD8 T Cells to Eliminate Tumor Cells by Bifunctional Major Histocompatibility Class I Antibody Fusion Molecules. Cancer Immunol. Res. 2015, 3, 764–776. [Google Scholar] [CrossRef] [Green Version]
- Shimabukuro-Vornhagen, A.; Gödel, P.; Subklewe, M.; Stemmler, H.J.; Schlößer, H.A.; Schlaak, M.; Kochanek, M.; Böll, B.; von Bergwelt-Baildon, M.S. Cytokine release syndrome. J. Immunother. Cancer 2018, 6, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmittnaegel, M.; Hoffmann, E.; Imhof-Jung, S.; Fischer, C.; Drabner, G.; Georges, G.; Klein, C.; Knoetgen, H. A New Class of Bifunctional Major Histocompatibility Class I Antibody Fusion Molecules to Redirect CD8 T Cells. Mol. Cancer Ther. 2016, 15, 2130–2142. [Google Scholar] [CrossRef] [Green Version]
- Choudhuri, K.; Wiseman, D.; Brown, M.H.; Gould, K.; van der Merwe, P.A. T-cell receptor triggering is critically dependent on the dimensions of its peptide-MHC ligand. Nature 2005, 436, 578–582. [Google Scholar] [CrossRef]
- Fischer, C.; Munks, M.W.; Hill, A.B.; Kroczek, R.A.; Bissinger, S.; Brand, V.; Schmittnaegel, M.; Imhof-Jung, S.; Hoffmann, E.; Herting, F.; et al. Vaccine-induced CD8 T cells are redirected with peptide-MHC class I-IgG antibody fusion proteins to eliminate tumor cells in vivo. mAbs 2020, 12, 1834818. [Google Scholar] [CrossRef]
- Lacey, S.F.; Villacres, M.C.; La Rosa, C.; Wang, Z.; Longmate, J.; Martinez, J.; Brewer, J.C.; Mekhoubad, S.; Maas, R.; Leedom, J.M.; et al. Relative dominance of HLA-B*07 restricted CD8+ T-Lymphocyte immune responses to human cytomegalovirus pp65 in persons sharing HLA-A*02 and HLA-B*07 alleles. Hum. Immunol. 2003, 64, 440–452. [Google Scholar] [CrossRef]
- Britsch, I.; van Wijngaarden, A.P.; Ke, X.; Hendriks, M.A.; Samplonius, D.F.; Ploeg, E.M.; Helfrich, W. Novel Fab-peptide-HLA-I fusion proteins for redirecting pre-existing anti-CMV T cell immunity to selectively eliminate carcinoma cells. Oncoimmunology 2023, 12, 2207868. [Google Scholar] [CrossRef] [PubMed]
- Seidel, R.D.; Merazga, Z.; Thapa, D.R.; Soriano, J.; Spaulding, E.; Vakkasoglu, A.S.; Ruthardt, P.; Bautista, W.; Quayle, S.N.; Kiener, P.A.; et al. Peptide-HLA-based immunotherapeutics platforms for direct modulation of antigen-specific T cells. Sci. Rep. 2021, 11, 19220. [Google Scholar] [CrossRef] [PubMed]
- Quayle, S.N.; Girgis, N.; Thapa, D.R.; Merazga, Z.; Kemp, M.M.; Histed, A.; Zhao, F.; Moreta, M.; Ruthardt, P.; Hulot, S.; et al. CUE-101, a novel E7-pHLA-IL2-Fc fusion protein, enhances tumor antigen-specific T-cell activation for the treatment of HPV16-driven malignancies. Clin. Cancer Res. 2020, 26, 1953–1964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Garforth, S.J.; O’connor, K.E.; Su, H.; Lee, D.M.; Celikgil, A.; Chaparro, R.J.; Seidel, R.D.; Jones, R.B.; Arav-Boger, R.; et al. T cell receptor–targeted immunotherapeutics drive selective in vivo HIV- and CMV-specific T cell expansion in humanized mice. J. Clin. Investig. 2021, 131, e141051. [Google Scholar] [CrossRef]
- Quayle, S.N.; Girgis, N.; Moniz, R.; Vakkasoglu, A.; Merazga, Z.; Zhang, C.; Saggu, G.; Histed, A.; Diaz, F.; Yeung, K.; et al. Immuno-STAT(Selective Targeting and Alteration of T cells) Platform: Targeting Tumor Heterogeneity and Tumor Escape Mechanisms. In Proceedings of the Frontiers in Cancer Immunotherapy Conference; New York Academy of Sciences: New York, NY, USA, 2021. [Google Scholar]
- Truscott, S.M.; Lybarger, L.; Martinko, J.M.; Mitaksov, V.E.; Kranz, D.M.; Connolly, J.M.; Fremont, D.H.; Hansen, T.H. Disulfide Bond Engineering to Trap Peptides in the MHC Class I Binding Groove. J. Immunol. 2007, 178, 6280–6289. [Google Scholar] [CrossRef] [Green Version]
- Dudley, M.E.; Rosenberg, S.A. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat. Rev. Cancer 2003, 3, 666–675. [Google Scholar] [CrossRef] [Green Version]
- Dudley, M.E.; Wunderlich, J.R.; Yang, J.C.; Sherry, R.M.; Topalian, S.L.; Restifo, N.P.; Royal, R.E.; Kammula, U.; White, D.E.; Mavroukakis, S.A.; et al. Adoptive Cell Transfer Therapy Following Non-Myeloablative but Lymphodepleting Chemotherapy for the Treatment of Patients With Refractory Metastatic Melanoma. J. Clin. Oncol. 2005, 23, 2346–2357. [Google Scholar] [CrossRef] [Green Version]
- Lapteva, N.; Gilbert, M.; Diaconu, I.; Rollins, L.A.; Al-Sabbagh, M.; Naik, S.; Krance, R.A.; Tripic, T.; Hiregange, M.; Raghavan, D.; et al. T-Cell Receptor Stimulation Enhances the Expansion and Function of CD19 Chimeric Antigen Receptor–Expressing T Cells. Clin. Cancer Res. 2019, 25, 7340–7350. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Diamond, D.J.; Forman, S.J.; Nakamura, R. Development of CMV-CD19 bi-specific CAR T cells with post-infusion in vivo boost using an anti-CMV vaccine. Int. J. Hematol. 2021, 114, 544–553. [Google Scholar] [CrossRef]
- Walter, E.A.; Greenberg, P.D.; Gilbert, M.J.; Finch, R.J.; Watanabe, K.S.; Thomas, E.D.; Riddell, S.R. Reconstitution of Cellular Immunity against Cytomegalovirus in Recipients of Allogeneic Bone Marrow by Transfer of T-Cell Clones from the Donor. N. Engl. J. Med. 1995, 333, 1038–1044. [Google Scholar] [CrossRef]
- Bollard, C.M.; Kuehnle, I.; Leen, A.; Rooney, C.M.; Heslop, H. Adoptive immunotherapy for posttransplantation viral infections. Biol. Blood Marrow Transplant. 2004, 10, 143–155. [Google Scholar] [CrossRef] [Green Version]
- Leen, A.M.; Christin, A.; Myers, G.D.; Liu, H.; Cruz, C.R.; Hanley, P.J.; Kennedy-Nasser, A.A.; Leung, K.S.; Gee, A.P.; Krance, R.A.; et al. Cytotoxic T lymphocyte therapy with donor T cells prevents and treats adenovirus and Epstein-Barr virus infections after haploidentical and matched unrelated stem cell transplantation. Blood 2009, 114, 4283–4292. [Google Scholar] [CrossRef] [Green Version]
- Maude, S.L.; Frey, N.; Shaw, P.A.; Aplenc, R.; Barrett, D.M.; Bunin, N.J.; Chew, A.; Gonzalez, V.E.; Zheng, Z.; Lacey, S.F.; et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014, 371, 1507–1517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.W.; Kochenderfer, J.N.; Stetler-Stevenson, M.; Cui, Y.K.; Delbrook, C.; Feldman, S.A.; Fry, T.J.; Orentas, R.; Sabatino, M.; Shah, N.N.; et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: A phase 1 dose-escalation trial. Lancet 2015, 385, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Brawley, V.; Hegde, M.; Bielamowicz, K.; Kalra, M.; Landi, D.; Robertson, C.; Gray, T.L.; Diouf, O.; Wakefield, A.; et al. HER2-Specific Chimeric Antigen Receptor–Modified Virus-Specific T Cells for Progressive Glioblastoma: A Phase 1 Dose-Escalation Trial. JAMA Oncol. 2017, 3, 1094–1101. [Google Scholar] [CrossRef]
- Cruz, C.R.Y.; Micklethwaite, K.P.; Savoldo, B.; Ramos, C.A.; Lam, S.; Ku, S.; Diouf, O.; Liu, E.; Barrett, A.J.; Ito, S.; et al. Infusion of donor-derived CD19-redirected virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: A phase 1 study. Blood 2013, 122, 2965–2973. [Google Scholar] [CrossRef] [PubMed]
- Caruana, I.; Weber, G.; Ballard, B.C.; Wood, M.S.; Savoldo, B.; Dotti, G. K562-Derived Whole-Cell Vaccine Enhances Antitumor Responses of CAR-Redirected Virus-Specific Cytotoxic T Lymphocytes In Vivo. Clin. Cancer Res. 2015, 21, 2952–2962. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wong, C.W.; Urak, R.; Mardiros, A.; Budde, L.E.; Chang, W.C.; Thomas, S.H.; Brown, C.E.; La Rosa, C.; Diamond, D.J.; et al. CMVpp65 vaccine enhances the antitumor efficacy of adoptively transferred CD19-redirected CMV-specific T cells. Clin. Cancer Res. 2015, 21, 2993–3002. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Urak, R.; Walter, M.; Guan, M.; Han, T.; Vyas, V.; Chien, S.-H.; Gittins, B.; Clark, M.C.; Mokhtari, S.; et al. Large-scale manufacturing and characterization of CMV-CD19CAR T cells. J. Immunother. Cancer 2022, 10, e003461. [Google Scholar] [CrossRef]
- La Rosa, C.; Longmate, J.; Martinez, J.; Zhou, Q.; Kaltcheva, T.I.; Tsai, W.; Drake, J.; Carroll, M.; Wussow, F.; Chiuppesi, F.; et al. MVA vaccine encoding CMV antigens safely induces durable expansion of CMV-specific T cells in healthy adults. Blood 2017, 129, 114–125. [Google Scholar] [CrossRef]
- Aldoss, I.; La Rosa, C.; Baden, L.R.; Longmate, J.; Ariza-Heredia, E.J.; Rida, W.N.; Lingaraju, C.R.; Zhou, Q.; Martinez, J.; Kaltcheva, T.; et al. Poxvirus vectored cytomegalovirus vaccine to prevent cytomegalovirus viremia in transplant recipients: A phase 2, randomized clinical trial. Ann. Intern. Med. 2020, 172, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Kalamasz, D.; Long, S.A.; Taniguchi, R.; Buckner, J.H.; Berenson, R.J.; Bonyhadi, M. Optimization of Human T-Cell Expansion Ex Vivo Using Magnetic Beads Conjugated with Anti-CD3 and Anti-CD28 Antibodies. J. Immunother. 2004, 27, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Romero, F.A.; Taur, Y.; Sadelain, M.; Brentjens, R.J.; Hohl, T.M.; Seo, S.K. Cytokine Release Syndrome Grade as a Predictive Marker for Infections in Patients With Relapsed or Refractory B-Cell Acute Lymphoblastic Leukemia Treated With Chimeric Antigen Receptor T Cells. Clin. Infect. Dis. 2018, 67, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.; Li, D.; Hay, K.; Green, M.L.; Cherian, S.; Chen, X.; Riddell, S.R.; Maloney, D.G.; Boeckh, M.; Turtle, C.J. Infectious complications of CD19-targeted chimeric antigen receptor–modified T-cell immunotherapy. Blood 2018, 131, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Tzannou, I.; Papadopoulou, A.; Naik, S.; Leung, K.; Martinez, C.A.; Ramos, C.A.; Carrum, G.; Sasa, G.; Lulla, P.; Watanabe, A.; et al. Off-the-Shelf Virus-Specific T Cells to Treat BK Virus, Human Herpesvirus 6, Cytomegalovirus, Epstein-Barr Virus, and Adenovirus Infections after Allogeneic Hematopoietic Stem-Cell Transplantation. J. Clin. Oncol. 2017, 35, 3547–3557. [Google Scholar] [CrossRef]
- Heemskerk, M.H.M.; Hoogeboom, M.; Hagedoorn, R.; Kester, M.G.D.; Willemze, R.; Falkenburg, J.H.F. Reprogramming of Virus-specific T Cells into Leukemia-reactive T Cells Using T Cell Receptor Gene Transfer. J. Exp. Med. 2004, 199, 885–894. [Google Scholar] [CrossRef]
- van Loenen, M.M.; Hagedoorn, R.S.; Kester, M.G.; Hoogeboom, M.; Willemze, R.; Falkenburg, J.F.; Heemskerk, M.H. Kinetic preservation of dual specificity of coprogrammed minor histocompatibility antigen-reactive virus-specific T cells. Cancer Res. 2009, 69, 2034–2041. [Google Scholar] [CrossRef] [Green Version]
- van Balen, P.; Jedema, I.; van Loenen, M.M.; de Boer, R.; van Egmond, H.M.; Hagedoorn, R.S.; Hoogstaten, C.; Veld, S.A.J.; Hageman, L.; van Liempt, P.A.G.; et al. HA-1H T-Cell Receptor Gene Transfer to Redirect Virus-Specific T Cells for Treatment of Hematological Malignancies After Allogeneic Stem Cell Transplantation: A Phase 1 Clinical Study. Front. Immunol. 2020, 11, 1804. [Google Scholar] [CrossRef] [PubMed]
- Peredo-Harvey, I.; Rahbar, A.; Söderberg-Nauclér, C. Presence of the Human Cytomegalovirus in Glioblastomas—A Systematic Review. Cancers 2021, 13, 5051. [Google Scholar] [CrossRef]
- Ahani, N.; Nikravesh, A.; Shirkoohi, R.; Arzenani, M.K.; Rokouei, M.; Eskandani, M.A. Detection of human cytomegalovirus in glioma tumor tissues. Comp. Clin. Pathol. 2013, 23, 1321–1330. [Google Scholar] [CrossRef]
- Stangherlin, L.M.; Castro, F.L.F.; Medeiros, R.S.S.; Guerra, J.M.; Kimura, L.M.; Shirata, N.K.; Nonogaki, S.; dos Santos, C.J.; Silva, M.C.C. Human Cytomegalovirus DNA Quantification and Gene Expression in Gliomas of Different Grades. PLoS ONE 2016, 11, e0159604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crough, T.; Beagley, L.; Smith, C.; Jones, L.; Walker, D.G.; Khanna, R. Ex vivo functional analysis, expansion and adoptive transfer of cytomegalovirus-specific T-cells in patients with glioblastoma multiforme. Immunol. Cell Biol. 2012, 90, 872–880. [Google Scholar] [CrossRef]
- Fornara, O.; Odeberg, J.; Solberg, N.W.; Tammik, C.; Skarman, P.; Peredo, I.; Stragliotto, G.; Rahbar, A.; Söderberg-Nauclér, C. Poor survival in glioblastoma patients is associated with early signs of immunosenescence in the CD4 T-cell compartment after surgery. Oncoimmunology 2015, 4, e1036211. [Google Scholar] [CrossRef] [Green Version]
- Schuessler, A.; Smith, C.; Beagley, L.; Boyle, G.M.; Rehan, S.; Matthews, K.; Jones, L.; Crough, T.; Dasari, V.; Klein, K.; et al. Autologous T-cell Therapy for Cytomegalovirus as a Consolidative Treatment for Recurrent Glioblastoma. Cancer Res. 2014, 74, 3466–3476. [Google Scholar] [CrossRef] [Green Version]
- Sorkhabi, A.D.; Sarkesh, A.; Saeedi, H.; Marofi, F.; Ghaebi, M.; Silvestris, N.; Baradaran, B.; Brunetti, O. The Basis and Advances in Clinical Application of Cytomegalovirus-Specific Cytotoxic T Cell Immunotherapy for Glioblastoma Multiforme. Front. Oncol. 2022, 12, 818447. [Google Scholar] [CrossRef]
- Smith, C.; Lineburg, K.E.; Martins, J.P.; Ambalathingal, G.R.; Neller, M.A.; Morrison, B.; Matthews, K.K.; Rehan, S.; Crooks, P.; Panikkar, A.; et al. Autologous CMV-specific T cells are a safe adjuvant immunotherapy for primary glioblastoma multiforme. J. Clin. Investig. 2020, 130, 6041–6053. [Google Scholar] [CrossRef] [PubMed]
- Reap, E.A.; Suryadevara, C.M.; Batich, K.A.; Sanchez-Perez, L.; Archer, G.E.; Schmittling, R.J.; Norberg, P.K.; Herndon, J.E.; Healy, P.; Congdon, K.L.; et al. Dendritic Cells Enhance Polyfunctionality of Adoptively Transferred T Cells That Target Cytomegalovirus in Glioblastoma. Cancer Res. 2018, 78, 256–264. [Google Scholar] [CrossRef] [Green Version]
- Gulley, J.L.; Madan, R.A.; Pachynski, R.; Mulders, P.; Sheikh, N.A.; Trager, J.; Drake, C.G. Role of Antigen Spread and Distinctive Characteristics of Immunotherapy in Cancer Treatment. Gynecol. Oncol. 2017, 109, djw261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brossart, P. The Role of Antigen Spreading in the Efficacy of Immunotherapies. Clin. Cancer Res. 2020, 26, 4442–4447. [Google Scholar] [CrossRef]
- Leko, V.; Rosenberg, S.A. Identifying and Targeting Human Tumor Antigens for T Cell-Based Immunotherapy of Solid Tumors. Cancer Cell 2020, 38, 454–472. [Google Scholar] [CrossRef]
- Taher, C.; de Boniface, J.; Mohammad, A.-A.; Religa, P.; Hartman, J.; Yaiw, K.-C.; Frisell, J.; Rahbar, A.; Söderberg-Naucler, C. High Prevalence of Human Cytomegalovirus Proteins and Nucleic Acids in Primary Breast Cancer and Metastatic Sentinel Lymph Nodes. PLoS ONE 2013, 8, e56795. [Google Scholar] [CrossRef] [PubMed]
- Harkins, L.; Volk, A.L.; Samanta, M.; Mikolaenko, I.; Britt, W.J.; Bland, K.; Cobbs, C.S. Specific localisation of human cytomegalovirus nucleic acids and proteins in human colorectal cancer. Lancet 2002, 360, 1557–1563. [Google Scholar] [CrossRef]
- Cooper, L.J.N.; Al-Kadhimi, Z.; Serrano, L.M.; Pfeiffer, T.; Olivares, S.; Castro, A.; Chang, W.-C.; Gonzalez, S.; Smith, D.; Forman, S.J.; et al. Enhanced antilymphoma efficacy of CD19-redirected influenza MP1–specific CTLs by cotransfer of T cells modified to present influenza MP1. Blood 2005, 105, 1622–1631. [Google Scholar] [CrossRef] [PubMed]
- Pule, M.A.; Savoldo, B.; Myers, G.D.; Rossig, C.; Russell, H.V.; Dotti, G.; Huls, M.H.; Liu, E.; Gee, A.P.; Mei, Z.; et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: Persistence and antitumor activity in individuals with neuroblastoma. Nat. Med. 2008, 14, 1264–1270. [Google Scholar] [CrossRef] [PubMed]
- Robert, B.; Guillaume, P.; Luescher, I.; Romero, P.; Mach, J.-P. Antibody-conjugated MHC class I tetramers can target tumor cells for specific lysis by T lymphocytes. Eur. J. Immunol. 2000, 30, 3165–3170. [Google Scholar] [CrossRef] [PubMed]
- Savage, P.; Cowburn, P.; Clayton, A.; Man, S.; McMichael, A.; Lemoine, N.; Epenetos, A.; Ogg, G. Induction of viral and tumour specific CTL responses using antibody targeted HLA class I peptide complexes. Br. J. Cancer 2002, 86, 1336–1342. [Google Scholar] [CrossRef] [Green Version]
- Savage, P.; Cowburn, P.; Clayton, A.; Man, S.; Lawson, T.; Ogg, G.; Lemoine, N.; McMichael, A.; Epenetos, A. Anti-viral cytotoxic T cells inhibit the growth of cancer cells with antibody targeted HLA class I/peptide complexes in SCID mice. Int. J. Cancer 2002, 98, 561–566. [Google Scholar] [CrossRef]
- Lev, A.; Noy, R.; Oved, K.; Novak, H.; Segal, D.; Walden, P.; Zehn, D.; Reiter, Y. Tumor-specific Ab-mediated targeting of MHC-peptide complexes induces regression of human tumor xenografts in vivo. Proc. Natl. Acad. Sci. USA 2004, 101, 9051–9056. [Google Scholar] [CrossRef]
- Oved, K.; Lev, A.; Noy, R.; Segal, D.; Reiter, Y. Antibody-mediated targeting of human single-chain class I MHC with covalently linked peptides induces efficient killing of tumor cells by tumor or viral-specific cytotoxic T lymphocytes. Cancer Immunol. Immunother. 2005, 54, 867–879. [Google Scholar] [CrossRef]
- Novak, H.; Noy, R.; Oved, K.; Segal, D.; Wels, W.S.; Reiter, Y. Selective antibody-mediated targeting of class I MHC to EGFR-expressing tumor cells induces potent antitumor CTL activity in vitro and in vivo. Int. J. Cancer 2006, 120, 329–336. [Google Scholar] [CrossRef]
- Wieland, A.; Kamphorst, A.O.; Adsay, N.V.; Masor, J.J.; Sarmiento, J.; Nasti, T.H.; Darko, S.; Douek, D.C.; Xue, Y.; Curran, W.J.; et al. T cell receptor sequencing of activated CD8 T cells in the blood identifies tumor-infiltrating clones that expand after PD-1 therapy and radiation in a melanoma patient. Cancer Immunol. Immunother. 2018, 67, 1767–1776. [Google Scholar] [CrossRef] [PubMed]
- Fairfax, B.P.; Taylor, C.A.; Watson, R.A.; Nassiri, I.; Danielli, S.; Fang, H.; Mahé, E.A.; Cooper, R.; Woodcock, V.; Traill, Z.; et al. Peripheral CD8+ T cell characteristics associated with durable responses to immune checkpoint blockade in patients with metastatic melanoma. Nat. Med. 2020, 26, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, M.A.; Britsch, I.; Ke, X.; van Wijngarden, A.P.; Samplonius, D.F.; Ploeg, E.M.; Helfrich, W. Cancer cells under immune attack acquire CD47-mediated adaptive immune resistance independent of the myeloid CD47-SIRPα axis. Oncoimmunology 2021, 10, 200534. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Britsch, I.; van Wijngaarden, A.P.; Helfrich, W. Applications of Anti-Cytomegalovirus T Cells for Cancer (Immuno)Therapy. Cancers 2023, 15, 3767. https://doi.org/10.3390/cancers15153767
Britsch I, van Wijngaarden AP, Helfrich W. Applications of Anti-Cytomegalovirus T Cells for Cancer (Immuno)Therapy. Cancers. 2023; 15(15):3767. https://doi.org/10.3390/cancers15153767
Chicago/Turabian StyleBritsch, Isabel, Anne Paulien van Wijngaarden, and Wijnand Helfrich. 2023. "Applications of Anti-Cytomegalovirus T Cells for Cancer (Immuno)Therapy" Cancers 15, no. 15: 3767. https://doi.org/10.3390/cancers15153767




