Unlocking New Horizons in Small-Cell Lung Cancer Treatment: The Onset of Antibody–Drug Conjugates
Abstract
:Simple Summary
Abstract
1. Introduction
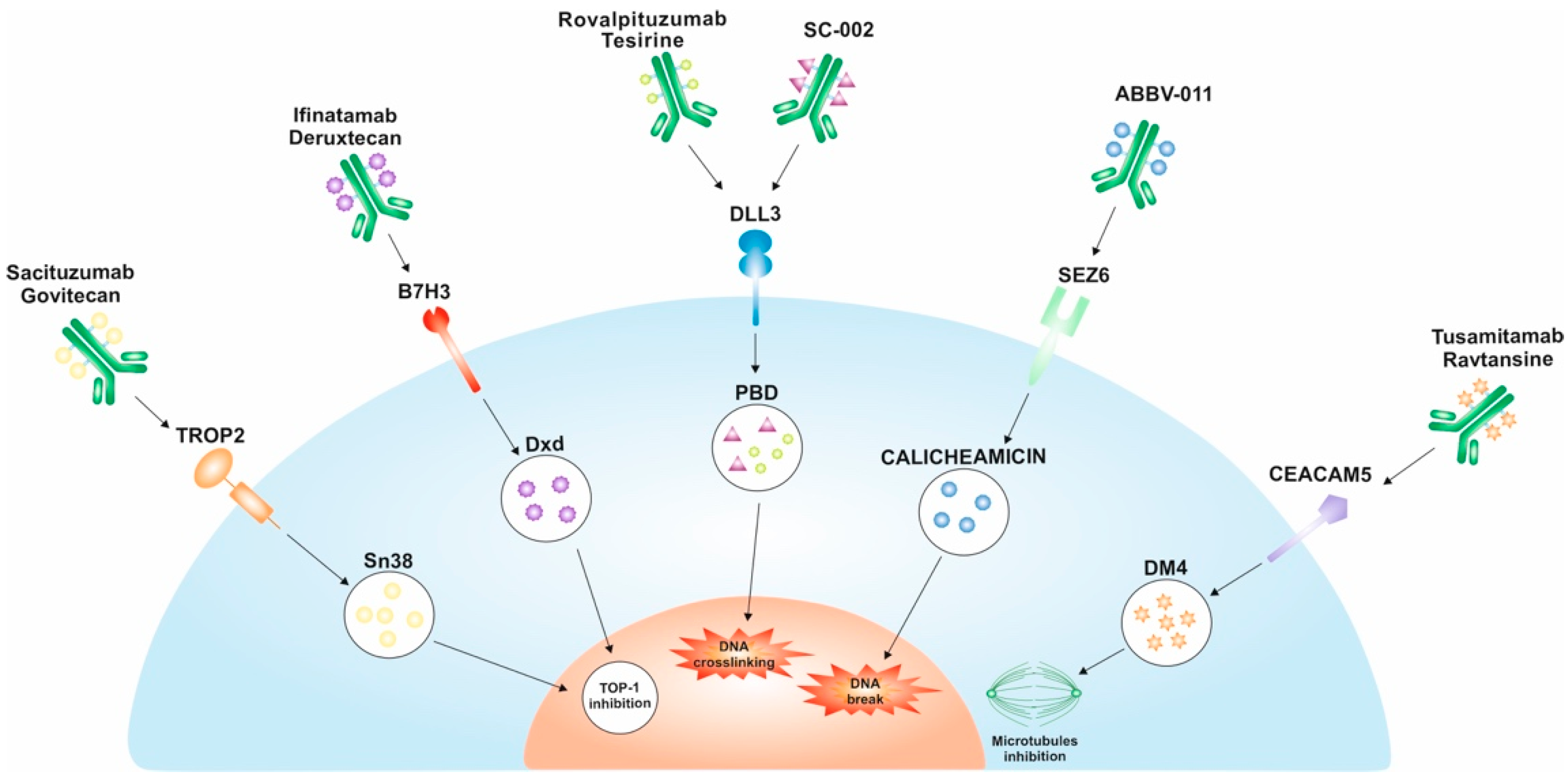
2. Trophoblast Cell Surface Antigen (TROP2)
Anti-TROP2 ADCs
3. Delta-like Ligand 3 (DLL3)
Anti-DLL3 ADCs
4. B7-H3 (CD276)
Anti-B7-H3 ADCs
5. Seizure-Related Homolog 6 (SEZ6)
Anti-SEZ6 ADCs
6. Carcinoembryonic Antigen-Related Cell Adhesion Molecule 5 (CEACAM5 or CD66e)
Anti-CEACAM5 ADCs
7. Future Perspectives
8. Discussion
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Q.; Gümüş, Z.H.; Colarossi, C.; Memeo, L.; Wang, X.; Kong, C.Y.; Boffetta, P. SCLC: Epidemiology, Risk Factors, Genetic Susceptibility, Molecular Pathology, Screening, and Early Detection. J. Thorac. Oncol. 2023, 18, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Bogart, J.A.; Waqar, S.N.; Mix, M.D. Radiation and Systemic Therapy for Limited-Stage Small-Cell Lung Cancer. J. Clin. Oncol. 2022, 40, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.V.; Reck, M.; Mansfield, A.S.; Mok, T.; Scherpereel, A.; Reinmuth, N.; Garassino, M.C.; De Castro Carpeno, J.; Califano, R.; Nishio, M.; et al. Updated Overall Survival and PD-L1 Subgroup Analysis of Patients With Extensive-Stage Small-Cell Lung Cancer Treated With Atezolizumab, Carboplatin, and Etoposide (IMpower133). J. Clin. Oncol. 2021, 39, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Özgüroğlu, M.; Ji, J.H.; Garassino, M.C.; et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer: 3-year overall survival update from CASPIAN. ESMO Open 2022, 7, 100408. [Google Scholar] [CrossRef] [PubMed]
- Abdollahpour-Alitappeh, M.; Lotfinia, M.; Gharibi, T.; Mardaneh, J.; Farhadihosseinabadi, B.; Larki, P.; Faghfourian, B.; Sepehr, K.S.; Abbaszadeh-Goudarzi, K.; Abbaszadeh-Goudarzi, G.; et al. Antibody-drug conjugates (ADCs) for cancer therapy: Strategies, challenges, and successes. J. Cell. Physiol. 2019, 234, 5628–5642. [Google Scholar] [CrossRef] [PubMed]
- Natsume, A.; Niwa, R.; Satoh, M. Improving effector functions of antibodies for cancer treatment: Enhancing ADCC and CDC. Drug Des. Dev. Ther. 2009, 3, 7–16. [Google Scholar] [CrossRef]
- Damelin, M.; Zhong, W.; Myers, J.; Sapra, P. Evolving Strategies for Target Selection for Antibody-Drug Conjugates. Pharm. Res. 2015, 32, 3494–3507. [Google Scholar] [CrossRef]
- Birrer, M.J.; Moore, K.N.; Betella, I.; Bates, R.C. Antibody-Drug Conjugate-Based Therapeutics: State of the Science. J. Natl. Cancer Inst. 2019, 111, 538–549. [Google Scholar] [CrossRef]
- Diamantis, N.; Banerji, U. Antibody-drug conjugates—An emerging class of cancer treatment. Br. J. Cancer 2016, 114, 362–367. [Google Scholar] [CrossRef]
- Rosner, S.; Valdivia, A.; Hoe, H.J.; Murray, J.C.; Levy, B.; Felip, E.; Solomon, B.J. Antibody-Drug Conjugates for Lung Cancer: Payloads and Progress. Am. Soc. Clin. Oncol. Educ. Book 2023, 43, e389968. [Google Scholar] [CrossRef]
- Belluomini, L.; Avancini, A.; Sposito, M.; Milella, M.; Rossi, A.; Pilotto, S. Antibody-drug conjugates (ADCs) targeting TROP-2 in lung cancer. Expert Opin. Biol. Ther. 2023, 1–11. [Google Scholar] [CrossRef]
- Lenárt, S.; Lenárt, P.; Šmarda, J.; Remšík, J.; Souček, K.; Beneš, P. Trop2: Jack of All Trades, Master of None. Cancers 2020, 12, 3328. [Google Scholar] [CrossRef]
- Stein, R.; Basu, A.; Chen, S.; Shih, L.B.; Goldenberg, D.M. Specificity and properties of MAb RS7-3G11 and the antigen defined by this pancarcinoma monoclonal antibody. Int. J. Cancer 1993, 55, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, D.M.; Stein, R.; Sharkey, R.M. The emergence of trophoblast cell-surface antigen 2 (TROP-2) as a novel cancer target. Oncotarget 2018, 9, 28989–29006. [Google Scholar] [CrossRef]
- Zhao, W.; Jia, L.; Kuai, X.; Tang, Q.; Huang, X.; Yang, T.; Qiu, Z.; Zhu, J.; Huang, J.; Huang, W.; et al. The role and molecular mechanism of Trop2 induced epithelial-mesenchymal transition through mediated β-catenin in gastric cancer. Cancer Med. 2019, 8, 1135–1147. [Google Scholar] [CrossRef] [PubMed]
- Trerotola, M.; Li, J.; Alberti, S.; Languino, L.R. Trop-2 inhibits prostate cancer cell adhesion to fibronectin through the β1 integrin-RACK1 axis. J. Cell. Physiol. 2012, 227, 3670–3677. [Google Scholar] [CrossRef]
- Guerra, E.; Trerotola, M.; Aloisi, A.L.; Tripaldi, R.; Vacca, G.; La Sorda, R.; Lattanzio, R.; Piantelli, M.; Alberti, S. The Trop-2 signalling network in cancer growth. Oncogene 2013, 32, 1594–1600. [Google Scholar] [CrossRef] [PubMed]
- Inamura, K.; Yokouchi, Y.; Kobayashi, M.; Ninomiya, H.; Sakakibara, R.; Subat, S.; Nagano, H.; Nomura, K.; Okumura, S.; Shibutani, T.; et al. Association of tumor TROP2 expression with prognosis varies among lung cancer subtypes. Oncotarget 2017, 8, 28725–28735. [Google Scholar] [CrossRef]
- Cardillo, T.M.; Rossi, D.L.; Zalath, M.B.; Liu, D.; Arrojo, R.; Sharkey, R.M.; Chang, C.H.; Goldenberg, D.M. Predictive biomarkers for sacituzumab govitecan efficacy in Trop-2-expressing triple-negative breast cancer. Oncotarget 2020, 11, 3849–3862. [Google Scholar] [CrossRef]
- Heist, R.S.; Guarino, M.J.; Masters, G.; Purcell, W.T.; Starodub, A.N.; Horn, L.; Scheff, R.J.; Bardia, A.; Messersmith, W.A.; Berlin, J.; et al. Therapy of Advanced Non-Small-Cell Lung Cancer With an SN-38-Anti-Trop-2 Drug Conjugate, Sacituzumab Govitecan. J. Clin. Oncol. 2017, 35, 2790–2797. [Google Scholar] [CrossRef]
- Bardia, A.; Messersmith, W.A.; Kio, E.A.; Berlin, J.D.; Vahdat, L.; Masters, G.A.; Moroose, R.; Santin, A.D.; Kalinsky, K.; Picozzi, V.; et al. Sacituzumab govitecan, a Trop-2-directed antibody-drug conjugate, for patients with epithelial cancer: Final safety and efficacy results from the phase I/II IMMU-132-01 basket trial. Ann. Oncol. 2021, 32, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.E.; Heist, R.S.; Starodub, A.N.; Camidge, D.R.; Kio, E.A.; Masters, G.A.; Purcell, W.T.; Guarino, M.J.; Misleh, J.; Schneider, C.J.; et al. Therapy of Small Cell Lung Cancer (SCLC) with a Topoisomerase-I-inhibiting Antibody-Drug Conjugate (ADC) Targeting Trop-2, Sacituzumab Govitecan. Clin. Cancer Res. 2017, 23, 5711–5719. [Google Scholar] [CrossRef] [PubMed]
- Morgensztern, D.; Besse, B.; Greillier, L.; Santana-Davila, R.; Ready, N.; Hann, C.L.; Glisson, B.S.; Farago, A.F.; Dowlati, A.; Rudin, C.M.; et al. Efficacy and Safety of Rovalpituzumab Tesirine in Third-Line and Beyond Patients with DLL3-Expressing, Relapsed/Refractory Small-Cell Lung Cancer: Results From the Phase II TRINITY Study. Clin. Cancer Res. 2019, 25, 6958–6966. [Google Scholar] [CrossRef]
- Blackhall, F.; Jao, K.; Greillier, L.; Cho, B.C.; Penkov, K.; Reguart, N.; Majem, M.; Nackaerts, K.; Syrigos, K.; Hansen, K.; et al. Efficacy and Safety of Rovalpituzumab Tesirine Compared With Topotecan as Second-Line Therapy in DLL3-High SCLC: Results From the Phase 3 TAHOE Study. J. Thorac. Oncol. 2021, 16, 1547–1558. [Google Scholar] [CrossRef]
- Johnson, M.L.; Zvirbule, Z.; Laktionov, K.; Helland, A.; Cho, B.C.; Gutierrez, V.; Colinet, B.; Lena, H.; Wolf, M.; Gottfried, M.; et al. Rovalpituzumab Tesirine as a Maintenance Therapy After First-Line Platinum-Based Chemotherapy in Patients With Extensive-Stage-SCLC: Results From the Phase 3 MERU Study. J. Thorac. Oncol. 2021, 16, 1570–1581. [Google Scholar] [CrossRef]
- Morgensztern, D.; Johnson, M.; Rudin, C.M.; Rossi, M.; Lazarov, M.; Brickman, D.; Fong, A. SC-002 in patients with relapsed or refractory small cell lung cancer and large cell neuroendocrine carcinoma: Phase 1 study. Lung Cancer 2020, 145, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; Awad, M.; Koyama, T.; Gutierrez, M.; Falchook, G.S.; Piha-Paul, S.A.; Doi, T.; Satoh, T.; Okamoto, N.; Singh, J.; et al. OA05.05 Ifinatamab Deruxtecan (I-DXd; DS-7300) in Patients with Refractory SCLC: A Subgroup Analysis of a Phase 1/2 Study. J. Thorac. Oncol. 2023, 18 (Suppl. 54–55). [Google Scholar] [CrossRef]
- Morgensztern, D.; Ready, N.E.; Johnson, M.L.; Dowlati, A.; Choudhury, N.J.; Carbone, D.P.; Schaefer, E.S.; Arnold, S.M.; Puri, S.; Piotrowska, Z.; et al. First-in-human study of ABBV-011, a seizure-related homolog protein 6 (SEZ6)–targeting antibody-drug conjugate, in patients with small cell lung cancer. J. Clin. Oncol. 2023, 41 (Suppl. 16), 3002. [Google Scholar] [CrossRef]
- Tabernero, J.; Bedard, P.L.; Bang, Y.J.; Vieito, M.; Ryu, M.H.; Fagniez, N.; Chadjaa, M.; Soufflet, C.; Masson, N.; Gazzah, A. Tusamitamab Ravtansine in Patients with Advanced Solid Tumors: Phase I Study of Safety, Pharmacokinetics, and Antitumor Activity Using Alternative Dosing Regimens. Cancer Res. Commun. 2023, 3, 1662–1671. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Li, X.; Yuan, X.; Chu, Q. Targeting the Notch signaling pathway and the Notch ligand, DLL3, in small cell lung cancer. Biomed. Pharmacother. 2023, 159, 114248. [Google Scholar] [CrossRef]
- Huang, J.; Cao, D.; Sha, J.; Zhu, X.; Han, S. DLL3 is regulated by LIN28B and miR-518d-5p and regulates cell proliferation, migration and chemotherapy response in advanced small cell lung cancer. Biochem. Biophys. Res. Commun. 2019, 514, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Geffers, I.; Serth, K.; Chapman, G.; Jaekel, R.; Schuster-Gossler, K.; Cordes, R.; Sparrow, D.B.; Kremmer, E.; Dunwoodie, S.L.; Klein, T.; et al. Divergent functions and distinct localization of the Notch ligands DLL1 and DLL3 in vivo. J. Cell Biol. 2007, 178, 465–476. [Google Scholar] [CrossRef]
- Rojo, F.; Corassa, M.; Mavroudis, D.; Öz, A.B.; Biesma, B.; Brcic, L.; Pauwels, P.; Sailer, V.; Gosney, J.; Miljkovic, D.; et al. International real-world study of DLL3 expression in patients with small cell lung cancer. Lung Cancer 2020, 147, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Kuempers, C.; Jagomast, T.; Krupar, R.; Paulsen, F.O.; Heidel, C.; Ribbat-Idel, J.; Idel, C.; Märkl, B.; Anlauf, M.; Berezowska, S.; et al. Delta-Like Protein 3 Expression in Paired Chemonaive and Chemorelapsed Small Cell Lung Cancer Samples. Front. Med. 2021, 8, 734901. [Google Scholar] [CrossRef]
- Rudin, C.M.; Pietanza, M.C.; Bauer, T.M.; Ready, N.; Morgensztern, D.; Glisson, B.S.; Byers, L.A.; Johnson, M.L.; Burris, H.A., 3rd; Robert, F.; et al. Rovalpituzumab tesirine, a DLL3-targeted antibody-drug conjugate, in recurrent small-cell lung cancer: A first-in-human, first-in-class, open-label, phase 1 study. Lancet Oncol. 2017, 18, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Schwartzberg, L.; Korytowsky, B.; Penrod, J.; Yuan, Y.; Gu, T.; Le, T.; Abraham, P.; Selvaggi, G. P1.12-21 Developing a Real-World 3L Comparator to CheckMate 032: Overall Survival (OS) in Patients with Small Cell Lung Cancer (SCLC). J. Thorac. Oncol. 2018, 13, S581–S582. [Google Scholar] [CrossRef]
- Staudacher, A.H.; Brown, M.P. Antibody drug conjugates and bystander killing: Is antigen-dependent internalisation required? Br. J. Cancer 2017, 117, 1736–1742. [Google Scholar] [CrossRef]
- Steinberger, P.; Majdic, O.; Derdak, S.V.; Pfistershammer, K.; Kirchberger, S.; Klauser, C.; Zlabinger, G.; Pickl, W.F.; Stöckl, J.; Knapp, W. Molecular characterization of human 4Ig-B7-H3, a member of the B7 family with four Ig-like domains. J. Immunol. 2004, 172, 2352–2359. [Google Scholar] [CrossRef]
- Arigami, T.; Narita, N.; Mizuno, R.; Nguyen, L.; Ye, X.; Chung, A.; Giuliano, A.E.; Hoon, D.S. B7-h3 ligand expression by primary breast cancer and associated with regional nodal metastasis. Ann. Surg. 2010, 252, 1044–1051. [Google Scholar] [CrossRef]
- Crispen, P.L.; Sheinin, Y.; Roth, T.J.; Lohse, C.M.; Kuntz, S.M.; Frigola, X.; Thompson, R.H.; Boorjian, S.A.; Dong, H.; Leibovich, B.C.; et al. Tumor cell and tumor vasculature expression of B7-H3 predict survival in clear cell renal cell carcinoma. Clin. Cancer Res. 2008, 14, 5150–5157. [Google Scholar] [CrossRef]
- Wang, J.; Chong, K.K.; Nakamura, Y.; Nguyen, L.; Huang, S.K.; Kuo, C.; Zhang, W.; Yu, H.; Morton, D.L.; Hoon, D.S. B7-H3 associated with tumor progression and epigenetic regulatory activity in cutaneous melanoma. J. Investig. Dermatol. 2013, 133, 2050–2058. [Google Scholar] [CrossRef]
- Loos, M.; Hedderich, D.M.; Ottenhausen, M.; Giese, N.A.; Laschinger, M.; Esposito, I.; Kleeff, J.; Friess, H. Expression of the costimulatory molecule B7-H3 is associated with prolonged survival in human pancreatic cancer. BMC Cancer 2009, 9, 463. [Google Scholar] [CrossRef]
- Wu, C.P.; Jiang, J.T.; Tan, M.; Zhu, Y.B.; Ji, M.; Xu, K.F.; Zhao, J.M.; Zhang, G.B.; Zhang, X.G. Relationship between co-stimulatory molecule B7-H3 expression and gastric carcinoma histology and prognosis. World J. Gastroenterol. 2006, 12, 457–459. [Google Scholar] [CrossRef]
- Bendell, J.C.; Doi, T.; Patel, M.R.; Piha-Paul, S.A.; Sen, S.; Shimizu, T.; Cheng, B.; Mekan, S.F.; Myobatake, Y.; Okuda, Y.; et al. A phase I/II, two-part, multicenter, first-in-human study of DS-7300a in patients with advanced solid malignant tumors. J. Clin. Oncol. 2020, 38 (Suppl. 15), TPS3646. [Google Scholar] [CrossRef]
- Qiu, W.Q.; Luo, S.; Ma, S.A.; Saminathan, P.; Li, H.; Gunnersen, J.M.; Gelbard, H.A.; Hammond, J.W. The Sez6 Family Inhibits Complement by Facilitating Factor I Cleavage of C3b and Accelerating the Decay of C3 Convertases. Front. Immunol. 2021, 12, 607641. [Google Scholar] [CrossRef] [PubMed]
- Wiedemeyer, W.R.; Gavrilyuk, J.; Schammel, A.; Zhao, X.; Sarvaiya, H.; Pysz, M.; Gu, C.; You, M.; Isse, K.; Sullivan, T.; et al. ABBV-011, A Novel, Calicheamicin-Based Antibody-Drug Conjugate, Targets SEZ6 to Eradicate Small Cell Lung Cancer Tumors. Mol. Cancer Ther. 2022, 21, 986–998. [Google Scholar] [CrossRef]
- Vollmar, B.S.; Frantz, C.; Schutten, M.M.; Zhong, F.; Del Rosario, G.; Go, M.A.T.; Yu, S.F.; Leipold, D.D.; Kamath, A.V.; Ng, C.; et al. Calicheamicin Antibody-Drug Conjugates with Improved Properties. Mol. Cancer Ther. 2021, 20, 1112–1120. [Google Scholar] [CrossRef]
- Beauchemin, N.; Arabzadeh, A. Carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) in cancer progression and metastasis. Cancer Metastasis Rev. 2013, 32, 643–671. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, R.D.; Hansen, H.J.; Goldenberg, D.M. Inhibition of adhesion, invasion, and metastasis by antibodies targeting CEACAM6 (NCA-90) and CEACAM5 (Carcinoembryonic Antigen). Cancer Res. 2005, 65, 8809–8817. [Google Scholar] [CrossRef]
- Decary, S.; Berne, P.F.; Nicolazzi, C.; Lefebvre, A.M.; Dabdoubi, T.; Cameron, B.; Rival, P.; Devaud, C.; Prades, C.; Bouchard, H.; et al. Preclinical Activity of SAR408701: A Novel Anti-CEACAM5-maytansinoid Antibody-drug Conjugate for the Treatment of CEACAM5-positive Epithelial Tumors. Clin. Cancer Res. 2020, 26, 6589–6599. [Google Scholar] [CrossRef]
- Kuespert, K.; Pils, S.; Hauck, C.R. CEACAMs: Their role in physiology and pathophysiology. Curr. Opin. Cell Biol. 2006, 18, 565–571. [Google Scholar] [CrossRef]
- Gazzah, A.; Bedard, P.L.; Hierro, C.; Kang, Y.K.; Abdul Razak, A.; Ryu, M.H.; Demers, B.; Fagniez, N.; Henry, C.; Hospitel, M.; et al. Safety, pharmacokinetics, and antitumor activity of the anti-CEACAM5-DM4 antibody-drug conjugate tusamitamab ravtansine (SAR408701) in patients with advanced solid tumors: First-in-human dose-escalation study. Ann. Oncol. 2022, 33, 416–425. [Google Scholar] [CrossRef]
- Cao, Y.; Shen, Q.; Ding, M.; Chen, Y.; Lu, W.; Zhu, S. Development of HSP90 inhibitors-SN38 conjugates for cancer treatment. Bioorg. Chem. 2023, 137, 106582. [Google Scholar] [CrossRef]
- Falchook, G.S.; Bendell, J.C.; Ulahannan, S.V.; Sen, S.; Vilimas, R.; Kriksciukaite, K.; Mei, L.; Jerkovic, G.; Sarapa, N.; Bilodeau, M.; et al. Pen-866, a miniature drug conjugate of a heat shock protein 90 (HSP90) ligand linked to SN38 for patients with advanced solid malignancies: Phase I and expansion cohort results. J. Clin. Oncol. 2020, 38 (Suppl. 15), 3515. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Hirai, S.; Idogawa, M.; Sumi, T.; Uchida, H.; Fujitani, N.; Takahashi, M.; Sakuma, Y. Junctional adhesion molecule 3 is a potential therapeutic target for small cell lung carcinoma. Exp. Cell Res. 2023, 426, 113570. [Google Scholar] [CrossRef] [PubMed]
- Yotsumoto, T.; Maemura, K.; Watanabe, K.; Amano, Y.; Matsumoto, Y.; Zokumasu, K.; Ando, T.; Kawakami, M.; Kage, H.; Nakajima, J.; et al. NRXN1 as a novel potential target of antibody-drug conjugates for small cell lung cancer. Oncotarget 2020, 11, 3590–3600. [Google Scholar] [CrossRef]
- Kim, K.H.; Kim, J.O.; Park, J.Y.; Seo, M.D.; Park, S.G. Antibody-Drug Conjugate Targeting c-Kit for the Treatment of Small Cell Lung Cancer. Int. J. Mol. Sci. 2022, 23, 2264. [Google Scholar] [CrossRef]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef]
- Hurvitz, S.A.; Hegg, R.; Chung, W.P.; Im, S.A.; Jacot, W.; Ganju, V.; Chiu, J.W.Y.; Xu, B.; Hamilton, E.; Madhusudan, S.; et al. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: Updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet 2023, 401, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, J.E.; Powles, T.; Sonpavde, G.P.; Loriot, Y.; Duran, I.; Lee, J.L.; Matsubara, N.; Vulsteke, C.; Castellano, D.; Mamtani, R.; et al. EV-301 long-term outcomes: 24-month findings from the phase III trial of enfortumab vedotin vs chemotherapy in patients with previously treated advanced urothelial carcinoma. Ann. Oncol. 2023, 386, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Li, B.T.; Smit, E.F.; Goto, Y.; Nakagawa, K.; Udagawa, H.; Mazières, J.; Nagasaka, M.; Bazhenova, L.; Saltos, A.N.; Felip, E.; et al. Trastuzumab Deruxtecan in HER2-Mutant Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2022, 386, 241–251. [Google Scholar] [CrossRef]
- Eaton, J.S.; Miller, P.E.; Mannis, M.J.; Murphy, C.J. Ocular Adverse Events Associated with Antibody-Drug Conjugates in Human Clinical Trials. J. Ocul. Pharmacol. Ther. 2015, 31, 589–604. [Google Scholar] [CrossRef]
- Fu, Z.; Li, S.; Han, S.; Shi, C.; Zhang, Y. Antibody drug conjugate: The “biological missile” for targeted cancer therapy. Signal Transduct. Target. Ther. 2022, 7, 93. [Google Scholar] [CrossRef]
- Vitorino, P.; Chuang, C.H.; Iannello, A.; Zhao, X.; Anderson, W.; Ferrando, R.; Zhang, Z.; Madhavan, S.; Karsunky, H.; Saunders, L.R. Rova-T enhances the anti-tumor activity of anti-PD1 in a murine model of small cell lung cancer with endogenous Dll3 expression. Transl. Oncol. 2021, 14, 100883. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, J.; Nikolinakos, P.; Leal, T.; Lehman, J.; Morgensztern, D.; Patel, J.D.; Wrangle, J.M.; Curigliano, G.; Greillier, L.; Johnson, M.L.; et al. A Phase 1-2 Study of Rovalpituzumab Tesirine in Combination With Nivolumab Plus or Minus Ipilimumab in Patients With Previously Treated Extensive-Stage SCLC. J. Thorac. Oncol. 2021, 16, 1559–1569. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.J.; Svoboda, J.; Chong, E.A.; Nasta, S.D.; Mato, A.R.; Anak, Ö.; Brogdon, J.L.; Pruteanu-Malinici, I.; Bhoj, V.; Landsburg, D.; et al. Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas. N. Engl. J. Med. 2017, 377, 2545–2554. [Google Scholar] [CrossRef] [PubMed]
- Flemming, A. Cytotoxic CD4(+) CAR T cells implicated in long-term leukaemia remission. Nat. Rev. Immunol. 2022, 22, 146. [Google Scholar] [CrossRef]
- Jaspers, J.E.; Khan, J.F.; Godfrey, W.D.; Lopez, A.V.; Ciampricotti, M.; Rudin, C.M.; Brentjens, R.J. IL-18-secreting CAR T cells targeting DLL3 are highly effective in small cell lung cancer models. J. Clin. Investig. 2023, 133, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tacheva-Grigorova, S.K.; Sutton, J.; Melton, Z.; Mak, Y.S.L.; Lay, C.; Smith, B.A.; Sai, T.; Van Blarcom, T.; Sasu, B.J.; et al. Allogeneic CAR T Cells Targeting DLL3 Are Efficacious and Safe in Preclinical Models of Small Cell Lung Cancer. Clin. Cancer Res. 2023, 29, 971–985. [Google Scholar] [CrossRef]
- Zhao, W.; Jia, L.; Zhang, M.; Huang, X.; Qian, P.; Tang, Q.; Zhu, J.; Feng, Z. The killing effect of novel bi-specific Trop2/PD-L1 CAR-T cell targeted gastric cancer. Am. J. Cancer Res. 2019, 9, 1846–1856. [Google Scholar]
- Paz-Ares, L.; Champiat, S.; Lai, W.V.; Izumi, H.; Govindan, R.; Boyer, M.; Hummel, H.D.; Borghaei, H.; Johnson, M.L.; Steeghs, N.; et al. Tarlatamab, a First-in-Class DLL3-Targeted Bispecific T-Cell Engager, in Recurrent Small-Cell Lung Cancer: An Open-Label, Phase I Study. J. Clin. Oncol. 2023, 41, 2893–2903. [Google Scholar] [CrossRef] [PubMed]
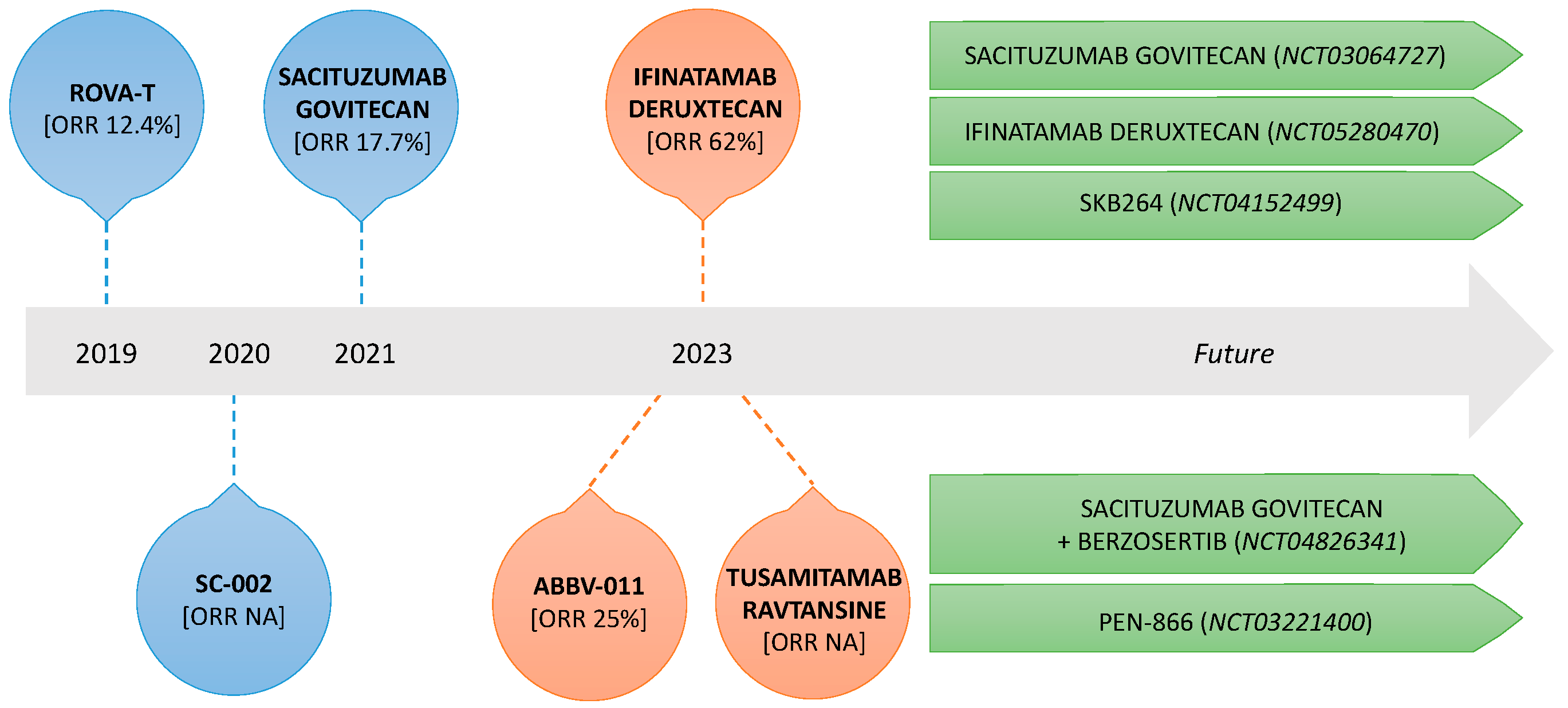
| Trial | ADC | Target | Payload | SCLC (N) | Activity | Toxicity (≥G3) |
|---|---|---|---|---|---|---|
| Bardia et al. [21] IMMU-132-01 | Sacituzumab govitecan | TROP2 | SN–38 | 495 | ORR 17.7% (CI 9.2–29.5) mDOR 5.7 mo (CI 3.6–19.9) mPFS 3.7 mo (CI 2.1–4.8) mOS 7.1 mo (CI 5.6–8.1) | Total 59.6%:
|
| Morgensztern et al. [23] TRINITY | Rovalpituzumab tesirine | DLL3 | Pyrrolobenzodiazepine | 261 | ORR 12.4% (CI 9.1–16.4) mOS 5.6 mo (CI 4.9–6.1) mPFS 3.5 mo (CI 3.0–3.9) | Total 40%:
|
| Blackhall et al. [24] TAHOE | 296 | mOS 6.3 mo (CI 5.6–7.3) mPFS 3 mo (CI 2.9–3.6) ORR 15% | Total 64%:
| |||
| Johnson et al. [25] MERU | 372 | mOS 8.5 mo (CI 7.3–10.2) mPFS 3.7 mo (CI 2.9–4) ORR 9% | Total 59%:
| |||
| Morgensztern et al. [26] | SC-002 | DLL3 | Pyrrolobenzodiazepine | 35 § | PR 14% CR 0% SD 40% PD 31% | Total 69% |
| Johnson et al. [27] | Ifinatamab deruxtecan | B7-H3 | Deruxtecan | 21 | ORR 52% (CI 29.8–74.3%) mDOR 5.9 mo (CI 2.8–7.5) mPFS 5.8 mo (CI 3.9–8.1) mOS 9.9 mo (CI 5.8–NR) | Total 23% *:
|
| Morgensztern et al. [28] | ABBV-011 | SEZ6 | Calicheamicin | 99 | ORR 25% mDOR 4.2 mo (CI 2.6–6.7) mPFS 3.5 mo CBR 65% | Total 45%:
|
| Tabernero et al. [29] | Tusamitamab ravtansine | CECAM5 | Maytansinoid DM4 | 1 | NA | Total 22.6% |
| ClinicalTrial.gov ID | Phase | Setting | N | Treatment | Target | Primary Endpoint | Selected Secondary Endpoints | Status |
|---|---|---|---|---|---|---|---|---|
| NCT03964727 | II | Pretreated solid tumors, including SCLC | 165 | Sacituzumab govitecan | Trop2 | ORR | DOR, PFS, CBR, OS | Active, not recruiting |
| NCT05280470 | II | Pretreated SCLC | 91 | Ifinatamab deruxtecan | B7-H3 | ORR | TEAEs, PFS, OS, DOR | Active, not recruiting |
| NCT04152499 | I–II | Pretreated solid tumors, including SCLC | 430 | SKB264 | Trop2 | MTD, RDE, ORR | DLTs, DOR, PFS, OS | Recruiting |
| NCT04826341 | I–II | Pretreated solid tumors, including SCLC, small-cell neuroendocrine cancers, and HRD solid cancer | 85 | Sacituzumab govitecan + berzosertib | Trop2 + ATR inhibitor | MTD, ORR | PFS, OS, DOR | Recruiting |
| NCT03221400 | I–IIa | Solid tumors | 340 | PEN-866 | HSP90 | DLT, ORR | DCR, PFS, OS, DOR | Recruiting |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belluomini, L.; Sposito, M.; Avancini, A.; Insolda, J.; Milella, M.; Rossi, A.; Pilotto, S. Unlocking New Horizons in Small-Cell Lung Cancer Treatment: The Onset of Antibody–Drug Conjugates. Cancers 2023, 15, 5368. https://doi.org/10.3390/cancers15225368
Belluomini L, Sposito M, Avancini A, Insolda J, Milella M, Rossi A, Pilotto S. Unlocking New Horizons in Small-Cell Lung Cancer Treatment: The Onset of Antibody–Drug Conjugates. Cancers. 2023; 15(22):5368. https://doi.org/10.3390/cancers15225368
Chicago/Turabian StyleBelluomini, Lorenzo, Marco Sposito, Alice Avancini, Jessica Insolda, Michele Milella, Antonio Rossi, and Sara Pilotto. 2023. "Unlocking New Horizons in Small-Cell Lung Cancer Treatment: The Onset of Antibody–Drug Conjugates" Cancers 15, no. 22: 5368. https://doi.org/10.3390/cancers15225368





