Pharmacodynamic and Toxicity Studies of 6-Isopropyldithio-2′-guanosine Analogs in Acute T-Lymphoblastic Leukemia
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Study for Exploring the Anticancer Activity of YLS004 and YLS010
2.2.1. Cell Lines and Cultures
2.2.2. In Vitro Toxicity Assays
2.2.3. Apoptosis Detection Assays
2.2.4. Reactive Oxygen Species (ROS) Assays
2.2.5. Mitochondrial Membrane Potential Assay
2.2.6. Lipid Peroxidation Detection
2.2.7. Glutathione Content Assays
2.2.8. Western Blotting
2.2.9. Acute Toxicity Test Study in Mice
2.2.10. Long-Term Toxicity Study in Rats
2.2.11. Animal Model of Acute T-Lymphoblastic Leukemia
2.3. Statistical Analyses
3. Results
3.1. YLS004 Has Better Cytotoxicity against Acute T-Lymphoblastic Leukemia Cells
3.2. The Newly Synthesized Compound YLS010 Is More Cytotoxic Than YLS004
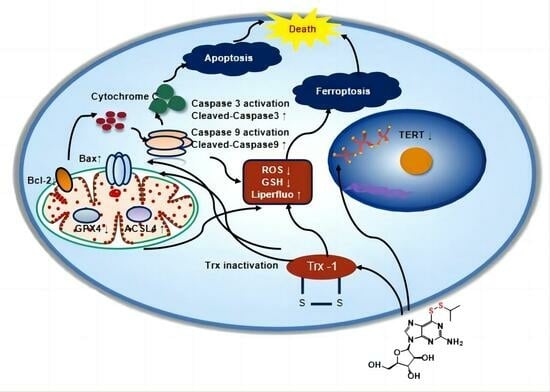
3.3. YLS010 Induces Apoptosis in T-ALL Cells via the Mitochondrial Pathway
3.4. YLS010 Causes Ferroptosis in T-ALL Cells by Inducing Oxidative Stress
3.5. Acute Toxicity Experimental Study of YLS010 on Mice
3.6. Long-Term Toxicity Experimental Study of YLS010 on Rats
3.7. Pharmacodynamic Study of YLS010 in a Mouse Xenograft Model of Acute T-Lymphoblastic Leukemia
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviations | Name |
| WBC | White Blood Cell |
| RBC | Red Blood Cell |
| Hb | Hemoglobin |
| MCV | Mean Corpuscular Volume |
| PLT | Platelets |
| LYMPH | Lymphocytes |
| MXD | Intermediate Cells |
| DLC | Granulocytes |
| P-LCR | Proportion of Large Platelets |
| B-PLT | Number of Large Platelets |
| HCT | Hematocrit |
| MCHC | Mean Corpuscular Hemoglobin Concentration |
| RDW | Coefficient of Variation of Erythrocyte Distribution Width |
| RDW-SD | Standard Deviation of Erythrocyte Distribution Width |
| MCH | Mean corpuscular hemoglobin content |
| MPV | Mean platelet volume |
| PCT | Hematocrit |
| PDW | Platelet distribution width |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| TBIL | Total bilirubin |
| ALB | Albumin |
| CREA | Creatinine |
| BUN | Urea nitrogen |
| TG | Triglycerides |
| TC | Total cholesterol |
| ALP | Basic phosphoric acid |
| GLU | Glucose |
| K | Potassium |
| Cl | Chlorine |
| CK | Creatine muscle enzyme |
References
- Lim, S.S.; Ford, J.B.; Hermiston, M.L. How I treat newly diagnosed and refractory T-cell acute lymphoblastic lymphoma in children and young adults. Blood 2023, 141, 3019–3030. [Google Scholar]
- Abaza, Y.; Kantarjian, H.M.; Faderl, S.; Jabbour, E.; Jain, N.; Thomas, D.; Kadia, T.; Borthakur, G.; Khoury, J.D.; Burger, J.; et al. Hyper-CVAD plus nelarabine in newly diagnosed adult T-cell acute lymphoblastic leukemia and T-lymphoblastic lymphoma. Am. J. Hematol. 2018, 93, 91–99. [Google Scholar] [CrossRef]
- Xiao, H.; Wang, S.; Tang, Y.; Li, S.; Jiang, Y.; Yang, Y.; Zhang, Y.; Han, Y.; Wu, X.; Zheng, L.; et al. Absence of terminal deoxynucleotidyl transferase expression in T-ALL/LBL accumulates chromosomal abnormalities to induce drug resistance. Int. J. Cancer 2023, 152, 2383–2395. [Google Scholar] [CrossRef]
- Teachey, D.T.; Pui, C.H. Comparative features and outcomes between paediatric T-cell and B-cell acute lymphoblastic leukaemia. Lancet Oncol. 2019, 20, e142–e154. [Google Scholar] [CrossRef]
- Raetz, E.A.; Teachey, D.T. T-cell acute lymphoblastic leukemia. Hematol. Am. Soc. Hematol. Educ. Program 2016, 2016, 580–588. [Google Scholar] [CrossRef]
- Jastrząb, A.; Skrzydlewska, E. Thioredoxin-dependent system. Application of inhibitors. J. Enzyme Inhib. Med. Chem. 2021, 36, 362–371. [Google Scholar] [CrossRef]
- Lu, J.; Holmgren, A. The thioredoxin antioxidant system. Free Radic. Biol. Med. 2014, 66, 75–87. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Han, X.; Liu, R.; Fang, J. Targeting the Thioredoxin System for Cancer Therapy. Trends Pharmacol. Sci. 2017, 38, 794–808. [Google Scholar] [CrossRef]
- Baker, A.; Payne, C.M.; Briehl, M.M.; Powis, G. Thioredoxin, a gene found overexpressed in human cancer, inhibits apoptosis in vitro and in vivo. Cancer Res. 1997, 57, 5162–5167. [Google Scholar]
- Xie, W.; Ma, W.; Liu, P.; Zhou, F. Overview of thioredoxin system and targeted therapies for acute leukemia. Mitochondrion 2019, 47, 38–46. [Google Scholar] [CrossRef]
- Shang, W.; Xie, Z.; Lu, F.; Fang, D.; Tang, T.; Bi, R.; Chen, L.; Jiang, L. Increased Thioredoxin-1 Expression Promotes Cancer Progression and Predicts Poor Prognosis in Patients with Gastric Cancer. Oxid. Med. Cell. Longev. 2019, 2019, 9291683. [Google Scholar] [CrossRef]
- Jordan, B.F.; Runquist, M.; Raghunand, N.; Gillies, R.J.; Tate, W.R.; Powis, G.; Baker, A.F. The thioredoxin-1 inhibitor 1-methylpropyl 2-imidazolyl disulfide (PX-12) decreases vascular permeability in tumor xenografts monitored by dynamic contrast enhanced magnetic resonance imaging. Clin. Cancer Res. 2005, 11, 529–536. [Google Scholar] [CrossRef]
- Ramanathan, R.K.; Kirkpatrick, D.L.; Belani, C.P.; Friedland, D.; Green, S.B.; Chow, H.-H.S.; Cordova, C.A.; Stratton, S.P.; Sharlow, E.R.; Baker, A.; et al. A Phase I pharmacokinetic and pharmacodynamic study of PX-12, a novel inhibitor of thioredoxin-1, in patients with advanced solid tumors. Clin. Cancer Res. 2007, 13, 2109–2114. [Google Scholar] [CrossRef]
- Ramanathan, R.K.; Abbruzzese, J.; Dragovich, T.; Kirkpatrick, L.; Guillen, J.M.; Baker, A.F.; Pestano, L.A.; Green, S.; Von Hoff, D.D. Von Hoff. A randomized phase II study of PX-12, an inhibitor of thioredoxin in patients with advanced cancer of the pancreas following progression after a gemcitabine-containing combination. Cancer Chemother. Pharmacol. 2011, 67, 503–509. [Google Scholar] [CrossRef]
- Blackburn, E.H. Switching and signaling at the telomere. Cell 2001, 106, 661–673. [Google Scholar] [CrossRef]
- Lim, C.J.; Cech, T.R. Shaping human telomeres: From shelterin and CST complexes to telomeric chromatin organization. Nat. Rev. Cell Biol. 2021, 22, 283–298. [Google Scholar] [CrossRef]
- Aksenova, A.Y.; Mirkin, S.M. At the Beginning of the End and in the Middle of the Beginning: Structure and Maintenance of Telomeric DNA Repeats and Interstitial Telomeric Sequences. Genes 2019, 10, 118. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, M.; Shi, W.; Yang, Q.; Chen, C.; Wang, Z.; Zhou, X. Arsenic trioxide suppresses transcription of hTERT through down-regulation of multiple transcription factors in HL-60 leukemia cells. Toxicol. Lett. 2015, 232, 481–489. [Google Scholar] [CrossRef]
- Shay, J.W. Role of Telomeres and Telomerase in Aging and Cancer. Cancer Discov. 2016, 6, 584–593. [Google Scholar] [CrossRef]
- Kim, N.W.; Piatyszek, M.A.; Prowse, K.R.; Harley, C.B.; West, M.D.; Ho, P.L.C.; Coviello, G.M.; Wright, W.E.; Weinrich, S.L.; Shay, J.W. Specific association of human telomerase activity with immortal cells and cancer. Science 1994, 266, 2011–2015. [Google Scholar] [CrossRef]
- Mender, I.; Gryaznov, S.; Dikmen, Z.G.; Wright, W.E.; Shay, J.W. Induction of telomere dysfunction mediated by the telomerase substrate precursor 6-thio-2′-deoxyguanosine. Cancer Discov. 2015, 5, 82–95. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, J.; Ye, Q.; Zeng, K.; Pan, J.; Chen, L.; Wang, Y.; Yang, B.; He, Q.; Gao, J.; et al. 6-Dithio-2′-deoxyguanosine analogs induce reactive oxygen species-mediated tumor cell apoptosis via bi-targeting thioredoxin 1 and telomerase. Toxicol. Appl. Phaemacol. 2020, 401, 115079. [Google Scholar] [CrossRef]
- Lambe, C.U.; Averett, D.R.; Paff, M.T.; Reardon, J.E.; Wilson, J.G.; A Krenitsky, T. 2-Amino-6-methoxypurine arabinoside: An agent for T-cell malignancies. Cancer Res. 1995, 55, 3352–3356. [Google Scholar]
- Poprac, P.; Jomova, K.; Simunkova, M.; Kollar, V.; Rhodes, C.J.; Valko, M. Targeting Free Radicals in Oxidative Stress-Related Human Diseases. Trends Pharmacol. Sci. 2017, 38, 592–607. [Google Scholar] [CrossRef]
- Czabotar, P.E.; Lessene, G.; Strasser, A.; Adams, J.M. Control of apoptosis by the BCL-2 protein family: Implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 2014, 15, 49–63. [Google Scholar] [CrossRef]
- Papa, S.; Skulachev, V.P. Reactive oxygen species, mitochondria, apoptosis and aging. Mol. Cell Biochem. 1997, 174, 305–319. [Google Scholar] [CrossRef]
- Gray, D.C.; Mahrus, S.; Wells, J.A. Activation of specific apoptotic caspases with an engineered small-molecule-activated protease. Cell 2010, 142, 637–646. [Google Scholar] [CrossRef]
- Tao, S.; Ren, Y.; Zheng, H.; Zhao, M.; Zhang, X.; Zhu, Y.; Yang, J.; Zheng, S. Salvianolic acid B inhibits intermittent high glucose-induced INS-1 cell apoptosis through regulation of Bcl-2 proteins and mitochondrial membrane potential. Eur. J. Pharmacol. 2017, 814, 56–62. [Google Scholar] [CrossRef]
- Koren, E.; Fuchs, Y. Modes of Regulated Cell Death in Cancer. Cancer Discov. 2021, 11, 245–265. [Google Scholar] [CrossRef]
- Stockwell, B.R. Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell 2022, 185, 2401–2421. [Google Scholar] [CrossRef]
- Hider, R.; Aviles, M.V.; Chen, Y.-L.; Latunde-Dada, G.O. The Role of GSH in Intracellular Iron Trafficking. Int. J. Mol. Sci. 2021, 22, 1278. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Conrad, M. The Metabolic Underpinnings of Ferroptosis. Cell Metab. 2020, 32, 920–937. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, Y. The interaction between ferroptosis and lipid metabolism in cancer. Signal Transduct. Target Ther. 2020, 5, 108. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Kroemer, G. Peroxisome: The new player in ferroptosis. Signal Transduct. Target Ther. 2020, 5, 273. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, L.Q.; Wang, D.G.; Xing, Y.J.; Bai, Y.P.; Zhang, T.; Wang, W.; Zhou, S.M.; Yao, X.M.; Cheng, J.H.; et al. Metformin alleviates glucolipotoxicity-induced pancreatic β cell ferroptosis through regulation of the GPX4/ACSL4 axis. Eur. J. Pharmacol. 2023, 956, 175967. [Google Scholar] [CrossRef]







| Cell Lines | Diseases | IC50 (μM: SEM ± Average, n = 3) |
|---|---|---|
| HUT-78 | Human acute T-lymphoblastic leukemia | 1.84 ± 0.25 |
| MOLT-4 | Human acute T-lymphoblastic leukemia | 0.56 ± 0.02 |
| REH | Human acute non-B non-T lymphoblastic leukemia | 5.34 ± 0.26 |
| Jurkat | Human T-lymphocyte leukemia | 21.8 ± 2.2 |
| Mutz-1 | Human myelodysplastic syndrome | 4.11 ± 0.35 |
| HL-60 | Human promyelocytic leukemia | 53.72 ± 5.61 |
| Kasumi-1 | Human acute promyelocytic leukemia | 7.59 ± 0.74 |
| Skm-1 | Human acute myelogenous leukemia | 3.79 ± 0.26 |
| HEL | Human red leukocyte leukemia | 30.47 ± 3.78 |
| RAJI | Human Burkitt′s lymphoma | 23.21 ± 0.92 |
| U266 | Human multiple myeloma | >100 |
| KM3 | >100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, T.; Yu, Z.; Shen, Q.; Xu, Y.; Hu, H.; Liu, J.; Zeng, K.; Lei, J.; Yu, L. Pharmacodynamic and Toxicity Studies of 6-Isopropyldithio-2′-guanosine Analogs in Acute T-Lymphoblastic Leukemia. Cancers 2024, 16, 1614. https://doi.org/10.3390/cancers16091614
Song T, Yu Z, Shen Q, Xu Y, Hu H, Liu J, Zeng K, Lei J, Yu L. Pharmacodynamic and Toxicity Studies of 6-Isopropyldithio-2′-guanosine Analogs in Acute T-Lymphoblastic Leukemia. Cancers. 2024; 16(9):1614. https://doi.org/10.3390/cancers16091614
Chicago/Turabian StyleSong, Tiantian, Zheming Yu, Qitao Shen, Yu Xu, Haihong Hu, Junqing Liu, Kui Zeng, Jinxiu Lei, and Lushan Yu. 2024. "Pharmacodynamic and Toxicity Studies of 6-Isopropyldithio-2′-guanosine Analogs in Acute T-Lymphoblastic Leukemia" Cancers 16, no. 9: 1614. https://doi.org/10.3390/cancers16091614