Phytochemical Modulation of Ion Channels in Oncologic Symptomatology and Treatment
Abstract
:Simple Summary
Abstract
1. Introduction
2. Ion Channels and Cancer
3. Phytochemicals in Cancer Treatment
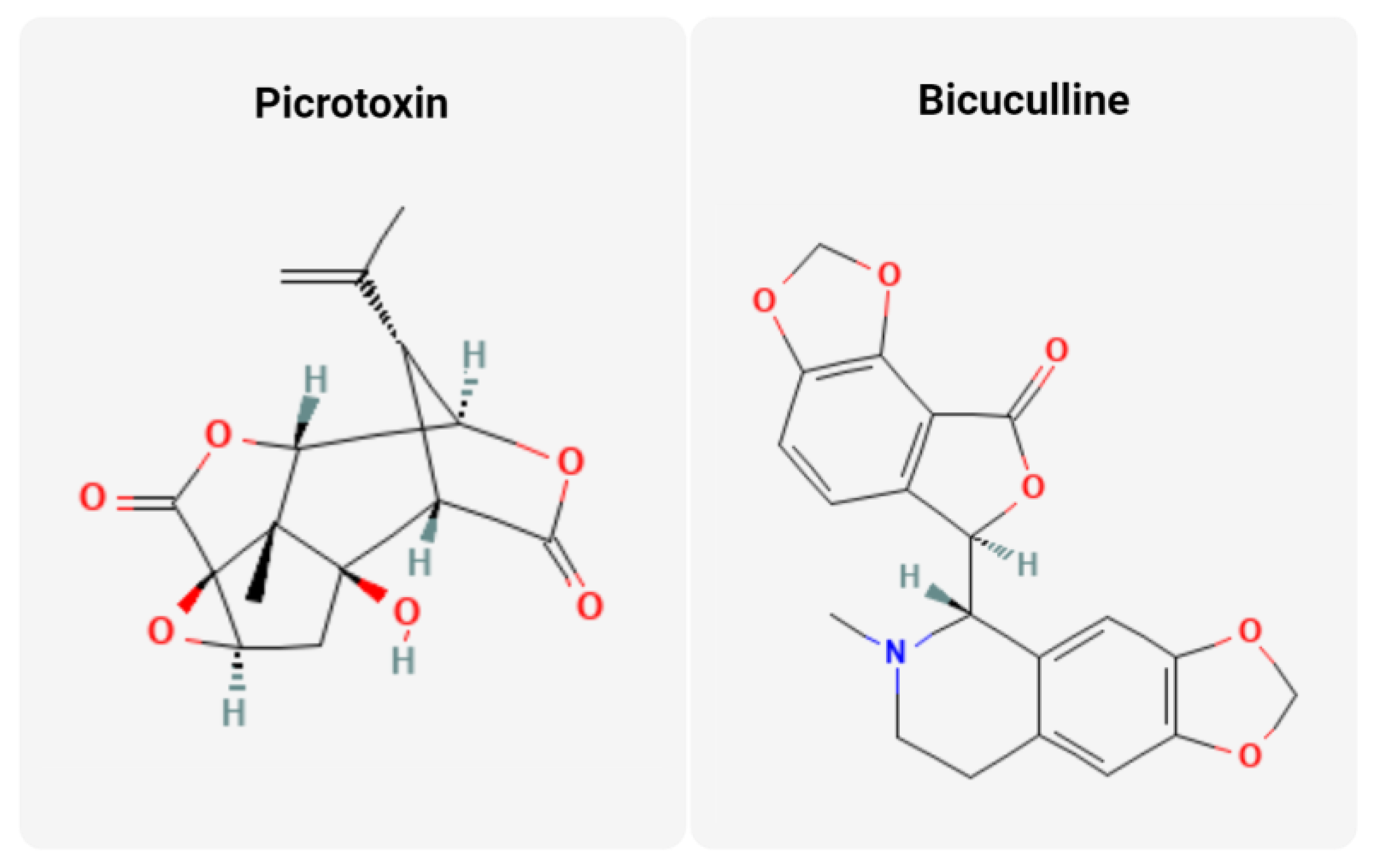
3.1. Anion Channel Modulators
3.2. Cation Channel Modulators
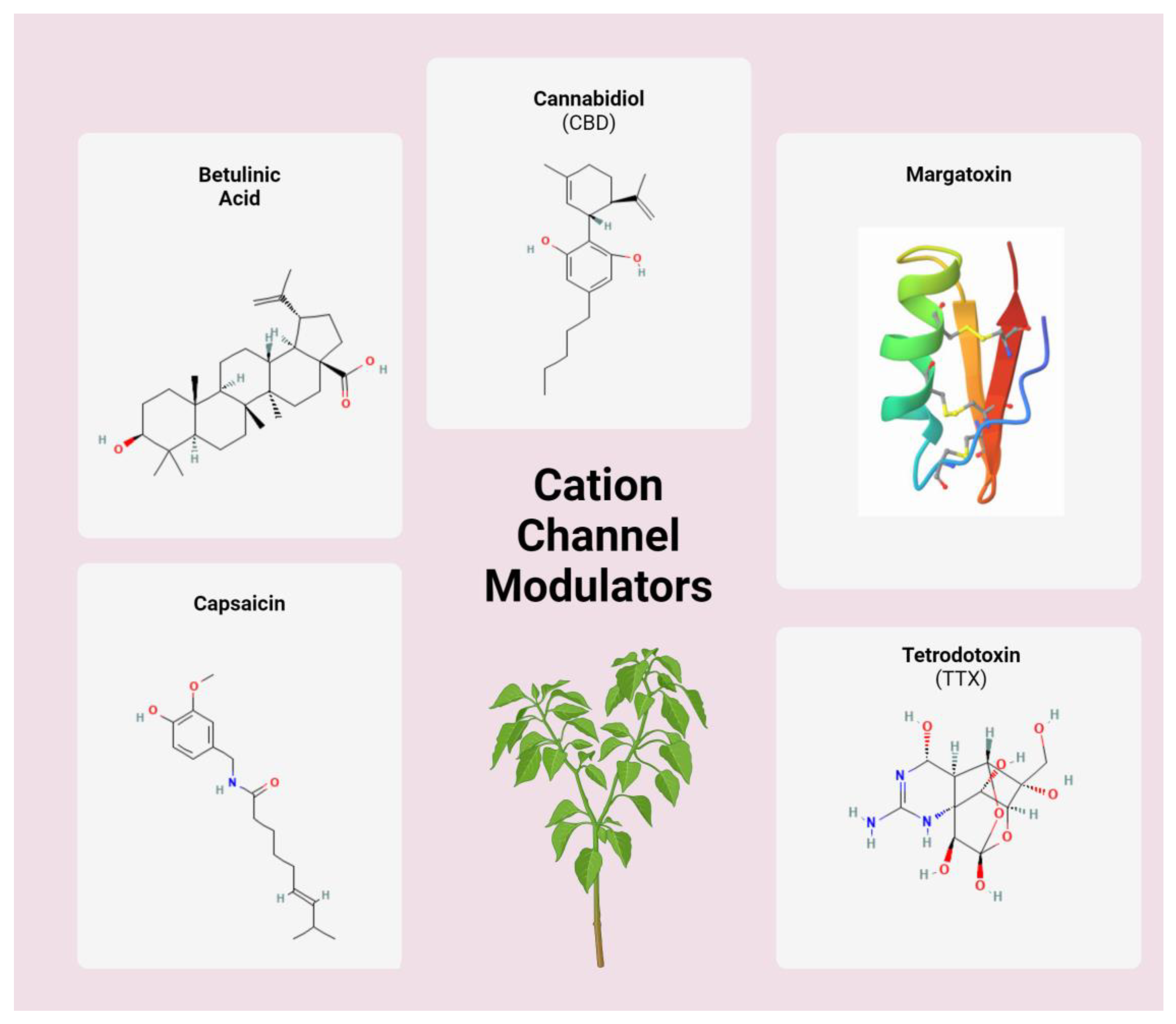
| Chemical | Mechanism | Cancer Subtype(s) | Citations |
|---|---|---|---|
| Picrotoxin | GABA antagonist; Immunomodulator; EGFR-Src pathway inhibition; MAPK/ERK inhibitor | Prostate, colorectal, pancreatic cancers, and melanoma | [42,43,44,49] |
| Bicuculline | GABA antagonist; EGFR-Src pathway inhibition | Pancreatic cancer | [44,51] |
| Betulinic acid | Inhibition of N- and T-type Ca2+ channels; modulation of intracellular mitochondrial apoptotic pathways | Gastrointestinal and pancreatic cancers, myeloid leukemia | [71,72] |
| Cannabidiol | TRPV agonist; intracellular calcium disruption | Chronic myelogenous leukemia, breast, cervical, and lung cancers | [79,80,81,82,106] |
| Margatoxin | Kv1.3 potassium channel inhibitor; cell cycle regulation | Lung adenocarcinoma | [87,89] |
| Tetrodotoxin | NaV inhibitor; modulating cancer metastatic potential | Non-small cell lung, colorectal, and prostate cancers | [95,96,97] |
| Capsaicin | TRPV1 agonist; intracellular calcium disruption | Triple-negative breast, urothelial, prostate, papillary thyroid cancers | [99,100,101,107] |
4. Phytochemicals Modulating Cancer Symptomatology
| Chemical | Mechanism | Symptom(s) | Citation(s) |
|---|---|---|---|
| Resveratrol | P2X receptor inhibitor, sodium channel agonist | Pain management | [42,111,112,114] |
| Puerarin | Decreased P2X receptor expression; NaV inhibitor; EGFR-Src pathway inhibition | Chemotherapy-induced neuropathic pain | [116,117,118] |
| Cannabidiol | TRPV activation; intracellular calcium disruption | Pain management, anxiolytic | [79,80,121,122,123,124] |
| Tetrodotoxin | NaV inhibitor | Pain management | [91,129,130,131,132,133,134,135] |
5. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, F.-S.; Weng, J.-K. Demystifying Traditional Herbal Medicine with Modern Approach. Nat. Plants 2017, 3, 17109. [Google Scholar] [CrossRef]
- Al-Worafi, Y.M. Chapter 14—Herbal Medicines Safety Issues. In Drug Safety in Developing Countries; Al-Worafi, Y., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 163–178. ISBN 978-0-12-819837-7. [Google Scholar]
- Zhang, M.; Moalin, M.; Haenen, G.R.M.M. Connecting West and East. Int. J. Mol. Sci. 2019, 20, 2333. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.A.; Urban, S.; Roessner, U. A Historical Overview of Natural Products in Drug Discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M. Paclitaxel (Taxol): A Success Story with Valuable Lessons for Natural Product Drug Discovery and Development. Med. Res. Rev. 1998, 18, 315–331. [Google Scholar] [CrossRef]
- Dehelean, C.A.; Marcovici, I.; Soica, C.; Mioc, M.; Coricovac, D.; Iurciuc, S.; Cretu, O.M.; Pinzaru, I. Plant-Derived Anticancer Compounds as New Perspectives in Drug Discovery and Alternative Therapy. Molecules 2021, 26, 1109. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Mols, F.; Beijers, T.; Vreugdenhil, G.; van de Poll-Franse, L. Chemotherapy-Induced Peripheral Neuropathy and Its Association with Quality of Life: A Systematic Review. Support. Care Cancer 2014, 22, 2261–2269. [Google Scholar] [CrossRef]
- Nayak, M.G.; George, A.; Vidyasagar, M.; Mathew, S.; Nayak, S.; Nayak, B.S.; Shashidhara, Y.; Kamath, A. Quality of Life among Cancer Patients. Indian. J. Palliat. Care 2017, 23, 445–450. [Google Scholar] [CrossRef]
- Goel, Y.; Fouda, R.; Gupta, K. Endoplasmic Reticulum Stress in Chemotherapy-Induced Peripheral Neuropathy: Emerging Role of Phytochemicals. Antioxidants 2022, 11, 265. [Google Scholar] [CrossRef]
- Singh, J.; Luqman, S.; Meena, A. Emerging Role of Phytochemicals in Targeting Predictive, Prognostic, and Diagnostic Biomarkers of Lung Cancer. Food Chem. Toxicol. 2020, 144, 111592. [Google Scholar] [CrossRef]
- Mao, Q.-Q.; Xu, X.-Y.; Shang, A.; Gan, R.-Y.; Wu, D.-T.; Atanasov, A.G.; Li, H.-B. Phytochemicals for the Prevention and Treatment of Gastric Cancer: Effects and Mechanisms. Int. J. Mol. Sci. 2020, 21, 570. [Google Scholar] [CrossRef] [PubMed]
- Tinoush, B.; Shirdel, I.; Wink, M. Phytochemicals: Potential Lead Molecules for MDR Reversal. Front. Pharmacol. 2020, 11, 832. [Google Scholar] [CrossRef] [PubMed]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Front. Pharmacol. 2019, 10, 1614. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.J.; Huang, X. Ion Channels in Cancer: Orchestrators of Electrical Signaling and Cellular Crosstalk. Rev. Physiol. Biochem. Pharmacol. 2022, 183, 103–133. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.; Shah, S.; Bhattacharya, D.; Toukam, D.K.; Cáceres, R.; Pomeranz Krummel, D.A.; Sengupta, S. Ligand-Gated Ion Channels as Targets for Treatment and Management of Cancers. Front. Physiol. 2022, 13, 839437. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Brackenbury, W.J. Membrane Potential and Cancer Progression. Front. Physiol. 2013, 4, 185. [Google Scholar] [CrossRef] [PubMed]
- Quicke, P.; Sun, Y.; Arias-Garcia, M.; Beykou, M.; Acker, C.D.; Djamgoz, M.B.A.; Bakal, C.; Foust, A.J. Voltage Imaging Reveals the Dynamic Electrical Signatures of Human Breast Cancer Cells. Commun. Biol. 2022, 5, 1178. [Google Scholar] [CrossRef] [PubMed]
- Yu, H. Depolarization or Hyperpolarization: Emerging Role of Altered Bioelectricity in Breast Cancer Metastasis. EBioMedicine 2022, 76, 103853. [Google Scholar] [CrossRef]
- Bonora, M.; Patergnani, S.; Rimessi, A.; De Marchi, E.; Suski, J.M.; Bononi, A.; Giorgi, C.; Marchi, S.; Missiroli, S.; Poletti, F.; et al. ATP Synthesis and Storage. Purinergic Signal 2012, 8, 343–357. [Google Scholar] [CrossRef]
- Schenk, U.; Westendorf, A.M.; Radaelli, E.; Casati, A.; Ferro, M.; Fumagalli, M.; Verderio, C.; Buer, J.; Scanziani, E.; Grassi, F. Purinergic Control of T Cell Activation by ATP Released through Pannexin-1 Hemichannels. Sci. Signal. 2008, 1, ra6. [Google Scholar] [CrossRef]
- Bian, Y.; Tuo, J.; He, L.; Li, W.; Li, S.; Chu, H.; Zhao, Y. Voltage-Gated Sodium Channels in Cancer and Their Specific Inhibitors. Pathol. Res. Pract. 2023, 251, 154909. [Google Scholar] [CrossRef] [PubMed]
- Malcolm, J.R.; Sajjaboontawee, N.; Yerlikaya, S.; Plunkett-Jones, C.; Boxall, P.J.; Brackenbury, W.J. Chapter Four—Voltage-Gated Sodium Channels, Sodium Transport and Progression of Solid Tumours. In Current Topics in Membranes; Gentile, S., Ed.; Ion Channels in Cancer; Academic Press: Cambridge, MA, USA, 2023; Volume 92, pp. 71–98. [Google Scholar]
- Pardo, L.A. Voltage-Gated Potassium Channels Beyond the Action Potential. Bioelectricity 2022, 4, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Pardo, L.A.; del Camino, D.; Sánchez, A.; Alves, F.; Brüggemann, A.; Beckh, S.; Stühmer, W. Oncogenic Potential of EAG K(+) Channels. EMBO J. 1999, 18, 5540–5547. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.; de Queiroz, F.M.; Downie, B.R.; Suckow, A.; Stühmer, W.; Pardo, L.A. Silencing the Activity and Proliferative Properties of the Human EagI Potassium Channel by RNA Interference. J. Biol. Chem. 2006, 281, 13030–13037. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Hoffmann, E.K. Role of Ion Transport in Control of Apoptotic Cell Death. Compr. Physiol. 2012, 2, 2037–2061. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Honisch, S.; Liu, G.; Schmidt, S.; Alkahtani, S.; AlKahtane, A.A.; Stournaras, C.; Lang, F. Up-Regulation of Orai1 Expression and Store Operated Ca2+ Entry Following Activation of Membrane Androgen Receptors in MCF-7 Breast Tumor Cells. BMC Cancer 2015, 15, 995. [Google Scholar] [CrossRef] [PubMed]
- Rhana, P.; Trivelato, R.R.; Beirão, P.S.L.; Cruz, J.S.; Rodrigues, A.L.P. Is There a Role for Voltage-Gated Na+ Channels in the Aggressiveness of Breast Cancer? Braz. J. Med. Biol. Res. 2017, 50, e6011. [Google Scholar] [CrossRef]
- Bhargava, A.; Saha, S. T-Type Voltage Gated Calcium Channels: A Target in Breast Cancer? Breast Cancer Res. Treat. 2019, 173, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Teisseyre, A.; Gąsiorowska, J.; Michalak, K. Voltage-Gated Potassium Channels Kv1.3--Potentially New Molecular Target in Cancer Diagnostics and Therapy. Adv. Clin. Exp. Med. 2015, 24, 517–524. [Google Scholar] [CrossRef]
- Romito, O.; Guéguinou, M.; Raoul, W.; Champion, O.; Robert, A.; Trebak, M.; Goupille, C.; Potier-Cartereau, M. Calcium Signaling: A Therapeutic Target to Overcome Resistance to Therapies in Cancer. Cell Calcium 2022, 108, 102673. [Google Scholar] [CrossRef]
- Kefauver, J.M.; Ward, A.B.; Patapoutian, A. Discoveries in Structure and Physiology of Mechanically Activated Ion Channels. Nature 2020, 587, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Premkumar, L.S. Transient Receptor Potential Channels as Targets for Phytochemicals. ACS Chem. Neurosci. 2014, 5, 1117–1130. [Google Scholar] [CrossRef]
- Levy, R.A.; Anderson, E.G. The Effect of the GABA Antagonists Bicuculline and Picrotoxin on Primary Afferent Terminal Excitability. Brain Res. 1972, 43, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Bause, G.S. From Fish Poison to Merck Picrotoxin. Anesthesiology 2013, 118, 1263. [Google Scholar] [CrossRef] [PubMed]
- Olsen, R.W. Picrotoxin-like Channel Blockers of GABAA Receptors. Proc. Natl. Acad. Sci. USA 2006, 103, 6081–6082. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ren, L.; Li, W.; Zhang, Y.; Zhang, S.; Ge, B.; Yang, H.; Du, G.; Tang, B.; Wang, H.; et al. GABAergic Signaling as a Potential Therapeutic Target in Cancers. Biomed. Pharmacother. 2023, 161, 114410. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Vogelzang, A.; Miyajima, M.; Sugiura, Y.; Wu, Y.; Chamoto, K.; Nakano, R.; Hatae, R.; Menzies, R.J.; Sonomura, K.; et al. B Cell-Derived GABA Elicits IL-10+ Macrophages to Limit Anti-Tumour Immunity. Nature 2021, 599, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Battaglin, F.; Jayachandran, P.; Strelez, C.; Lenz, A.; Algaze, S.; Soni, S.; Lo, J.H.; Yang, Y.; Millstein, J.; Zhang, W.; et al. Neurotransmitter Signaling: A New Frontier in Colorectal Cancer Biology and Treatment. Oncogene 2022, 41, 4769–4778. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Alexander, P.B.; Li, Q.-J.; Wang, X.-F. GABAergic Signaling beyond Synapses: An Emerging Target for Cancer Therapy. Trends Cell Biol. 2023, 33, 403–412. [Google Scholar] [CrossRef]
- Wu, W.; Yang, Q.; Fung, K.-M.; Humphreys, M.R.; Brame, L.S.; Cao, A.; Fang, Y.-T.; Shih, P.-T.; Kropp, B.P.; Lin, H.-K. Linking γ-Aminobutyric Acid A Receptor to Epidermal Growth Factor Receptor Pathways Activation in Human Prostate Cancer. Mol. Cell Endocrinol. 2014, 383, 69–79. [Google Scholar] [CrossRef]
- Xia, D.; Lai, D.V.; Wu, W.; Webb, Z.D.; Yang, Q.; Zhao, L.; Yu, Z.; Thorpe, J.E.; Disch, B.C.; Ihnat, M.A.; et al. Transition from Androgenic to Neurosteroidal Action of 5α-Androstane-3α, 17β-Diol through the Type A γ-Aminobutyric Acid Receptor in Prostate Cancer Progression. J. Steroid Biochem. Mol. Biol. 2018, 178, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Takehara, A.; Hosokawa, M.; Eguchi, H.; Ohigashi, H.; Ishikawa, O.; Nakamura, Y.; Nakagawa, H. Gamma-Aminobutyric Acid (GABA) Stimulates Pancreatic Cancer Growth through Overexpressing GABAA Receptor Pi Subunit. Cancer Res. 2007, 67, 9704–9712. [Google Scholar] [CrossRef] [PubMed]
- Lattanzi, D.; Di Palma, M.; Cuppini, R.; Ambrogini, P. GABAergic Input Affects Intracellular Calcium Levels in Developing Granule Cells of Adult Rat Hippocampus. Int. J. Mol. Sci. 2020, 21, 1715. [Google Scholar] [CrossRef] [PubMed]
- Tagore, M.; Hergenreder, E.; Perlee, S.C.; Cruz, N.M.; Menocal, L.; Suresh, S.; Chan, E.; Baron, M.; Melendez, S.; Dave, A.; et al. GABA Regulates Electrical Activity and Tumor Initiation in Melanoma. Cancer Discov. 2023, 13, 2270–2291. [Google Scholar] [CrossRef] [PubMed]
- PubChem Bicuculline. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/10237 (accessed on 7 March 2024).
- PubChem Picrotoxinin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/442292 (accessed on 7 March 2024).
- Li, T.; Jiang, J.; Tang, Y.; Liang, X. Insights into the Leveraging of GABAergic Signaling in Cancer Therapy. Cancer Med. 2023, 12, 14498–14510. [Google Scholar] [CrossRef] [PubMed]
- Curtis, D.R.; Duggan, A.W.; Felix, D.; Johnston, G.A. GABA, Bicuculline and Central Inhibition. Nature 1970, 226, 1222–1224. [Google Scholar] [CrossRef] [PubMed]
- Mares, P.; Chino, M.; Kubová, H.; Mathern, P.; Veliký, M. Convulsant Action of Systemically Administered Glutamate and Bicuculline Methiodide in Immature Rats. Epilepsy Res. 2000, 42, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Pressly, B.; Vasylieva, N.; Barnych, B.; Singh, V.; Singh, L.; Bruun, D.A.; Hwang, S.H.; Chen, Y.-J.; Fettinger, J.C.; Johnnides, S.; et al. Comparison of the Toxicokinetics of the Convulsants Picrotoxinin and Tetramethylenedisulfotetramine (TETS) in Mice. Arch. Toxicol. 2020, 94, 1995–2007. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, D.; Gawali, V.S.; Kallay, L.; Toukam, D.K.; Koehler, A.; Stambrook, P.; Krummel, D.P.; Sengupta, S. Therapeutically Leveraging GABAA Receptors in Cancer. Exp. Biol. Med. 2021, 246, 2128–2135. [Google Scholar] [CrossRef]
- Sengupta, S.; Weeraratne, S.D.; Sun, H.; Phallen, J.; Rallapalli, S.K.; Teider, N.; Kosaras, B.; Amani, V.; Pierre-Francois, J.; Tang, Y.; et al. A5-GABAA Receptors Negatively Regulate MYC-Amplified Medulloblastoma Growth. Acta Neuropathol. 2014, 127, 593–603. [Google Scholar] [CrossRef]
- Kallay, L.; Keskin, H.; Ross, A.; Rupji, M.; Moody, O.A.; Wang, X.; Li, G.; Ahmed, T.; Rashid, F.; Stephen, M.R.; et al. Modulating Native GABAA Receptors in Medulloblastoma with Positive Allosteric Benzodiazepine-Derivatives Induces Cell Death. J. Neurooncol. 2019, 142, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Jonas, O.; Calligaris, D.; Methuku, K.R.; Poe, M.M.; Francois, J.P.; Tranghese, F.; Changelian, A.; Sieghart, W.; Ernst, M.; Pomeranz Krummel, D.A.; et al. First In Vivo Testing of Compounds Targeting Group 3 Medulloblastomas Using an Implantable Microdevice as a New Paradigm for Drug Development. J. Biomed. Nanotechnol. 2016, 12, 1297–1302. [Google Scholar] [CrossRef] [PubMed]
- Pomeranz Krummel, D.A.; Nasti, T.H.; Kaluzova, M.; Kallay, L.; Bhattacharya, D.; Melms, J.C.; Izar, B.; Xu, M.; Burnham, A.; Ahmed, T.; et al. Melanoma Cell Intrinsic GABAA Receptor Enhancement Potentiates Radiation and Immune Checkpoint Inhibitor Response by Promoting Direct and T Cell-Mediated Antitumor Activity. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 1040–1053. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, D.; Barille, R.; Toukam, D.K.; Gawali, V.S.; Kallay, L.; Ahmed, T.; Brown, H.; Rezvanian, S.; Karve, A.; Desai, P.B.; et al. GABA(A) Receptor Activation Drives GABARAP-Nix Mediated Autophagy to Radiation-Sensitize Primary and Brain-Metastatic Lung Adenocarcinoma Tumors. bioRxiv 2023, preprint. [Google Scholar] [CrossRef] [PubMed]
- PubChem Betulinic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/64971 (accessed on 19 March 2024).
- PubChem Cannabidiol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/644019 (accessed on 19 March 2024).
- PubChem Tetrodotoxin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/11174599 (accessed on 19 March 2024).
- PubChem Capsaicin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/1548943 (accessed on 19 March 2024).
- PubChem Margatoxin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/121596045 (accessed on 19 March 2024).
- Bank, R.P.D. RCSB PDB—1MTX: Determination of the Three-Dimensional Structure of Margatoxin BY 1H, 13C, 15N TRIPLE-Resonance Nuclear Magnetic Resonance Spectroscopy. Available online: https://www.rcsb.org/structure/1mtx (accessed on 26 March 2024).
- Johnson, B.A.; Stevens, S.P.; Williamson, J.M. Determination of the Three-Dimensional Structure of Margatoxin by 1H, 13C, 15N Triple-Resonance Nuclear Magnetic Resonance Spectroscopy. Biochemistry 1994, 33, 15061–15070. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.; Li, H.; Zhang, S.; Lu, H.; Chen, Q. A Review on Preparation of Betulinic Acid and Its Biological Activities. Molecules 2021, 26, 5583. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Li, X.; Dong, S.; Zhou, W. Betulinic Acid in the Treatment of Tumour Diseases: Application and Research Progress. Biomed. Pharmacother. 2021, 142, 111990. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S. Betulinic Acid for Cancer Treatment and Prevention. Int. J. Mol. Sci. 2008, 9, 1096–1107. [Google Scholar] [CrossRef]
- Gao, M.; Lau, P.M.; Kong, S.K. Mitochondrial Toxin Betulinic Acid Induces in Vitro Eryptosis in Human Red Blood Cells through Membrane Permeabilization. Arch. Toxicol. 2014, 88, 755–768. [Google Scholar] [CrossRef]
- Bellampalli, S.S.; Ji, Y.; Moutal, A.; Cai, S.; Kithsiri Wijeratne, E.M.; Gandini, M.A.; Yu, J.; Chefdeville, A.; Dorame, A.; Chew, L.A.; et al. Betulinic Acid, Derived from the Desert Lavender Hyptis Emoryi, Attenuates Paclitaxel-, HIV-, and Nerve Injury-Associated Peripheral Sensory Neuropathy via Block of N- and T-Type Calcium Channels. Pain 2019, 160, 117–135. [Google Scholar] [CrossRef]
- Seca, A.M.L.; Pinto, D.C.G.A. Plant Secondary Metabolites as Anticancer Agents: Successes in Clinical Trials and Therapeutic Application. Int. J. Mol. Sci. 2018, 19, 263. [Google Scholar] [CrossRef] [PubMed]
- Szabo, I.; Zoratti, M.; Biasutto, L. Targeting Mitochondrial Ion Channels for Cancer Therapy. Redox Biol. 2020, 42, 101846. [Google Scholar] [CrossRef] [PubMed]
- Potze, L.; Mullauer, F.B.; Colak, S.; Kessler, J.H.; Medema, J.P. Betulinic Acid-Induced Mitochondria-Dependent Cell Death Is Counterbalanced by an Autophagic Salvage Response. Cell Death Dis. 2014, 5, e1169. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K. Cannabidiol (CBD) in Cancer Management. Cancers 2022, 14, 885. [Google Scholar] [CrossRef] [PubMed]
- Razmovski-Naumovski, V.; Luckett, T.; Amgarth-Duff, I.; Agar, M.R. Efficacy of Medicinal Cannabis for Appetite-Related Symptoms in People with Cancer: A Systematic Review. Palliat. Med. 2022, 36, 912–927. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Kuzontkoski, P.M.; Groopman, J.E.; Prasad, A. Cannabidiol Induces Programmed Cell Death in Breast Cancer Cells by Coordinating the Cross-Talk between Apoptosis and Autophagy. Mol. Cancer Ther. 2011, 10, 1161–1172. [Google Scholar] [CrossRef]
- Howlett, A.C.; Blume, L.C.; Dalton, G.D. CB1 Cannabinoid Receptors and Their Associated Proteins. Curr. Med. Chem. 2010, 17, 1382–1393. [Google Scholar] [CrossRef] [PubMed]
- Koivisto, A.-P.; Belvisi, M.G.; Gaudet, R.; Szallasi, A. Advances in TRP Channel Drug Discovery: From Target Validation to Clinical Studies. Nat. Rev. Drug Discov. 2022, 21, 41–59. [Google Scholar] [CrossRef]
- Seltzer, E.S.; Watters, A.K.; MacKenzie, D.; Granat, L.M.; Zhang, D. Cannabidiol (CBD) as a Promising Anti-Cancer Drug. Cancers 2020, 12, 3203. [Google Scholar] [CrossRef]
- de la Harpe, A.; Beukes, N.; Frost, C.L. CBD Activation of TRPV1 Induces Oxidative Signaling and Subsequent ER Stress in Breast Cancer Cell Lines. Biotechnol. Appl. Biochem. 2022, 69, 420–430. [Google Scholar] [CrossRef]
- Maggi, F.; Morelli, M.B.; Tomassoni, D.; Marinelli, O.; Aguzzi, C.; Zeppa, L.; Nabissi, M.; Santoni, G.; Amantini, C. The Effects of Cannabidiol via TRPV2 Channel in Chronic Myeloid Leukemia Cells and Its Combination with Imatinib. Cancer Sci. 2022, 113, 1235–1249. [Google Scholar] [CrossRef] [PubMed]
- Ramer, R.; Merkord, J.; Rohde, H.; Hinz, B. Cannabidiol Inhibits Cancer Cell Invasion via Upregulation of Tissue Inhibitor of Matrix Metalloproteinases-1. Biochem. Pharmacol. 2010, 79, 955–966. [Google Scholar] [CrossRef] [PubMed]
- Díaz-García, A.; Varela, D. Voltage-Gated K+/Na+ Channels and Scorpion Venom Toxins in Cancer. Front. Pharmacol. 2020, 11, 913. [Google Scholar] [CrossRef] [PubMed]
- Dueñas-Cuellar, R.A.; Santana, C.J.C.; Magalhães, A.C.M.; Pires, O.R.; Fontes, W.; Castro, M.S. Scorpion Toxins and Ion Channels: Potential Applications in Cancer Therapy. Toxins 2020, 12, 326. [Google Scholar] [CrossRef] [PubMed]
- Abdul, M.; Santo, A.; Hoosein, N. Activity of Potassium Channel-Blockers in Breast Cancer. Anticancer. Res. 2003, 23, 3347–3351. [Google Scholar] [PubMed]
- Ghiani, C.A.; Yuan, X.; Eisen, A.M.; Knutson, P.L.; DePinho, R.A.; McBain, C.J.; Gallo, V. Voltage-Activated K+ Channels and Membrane Depolarization Regulate Accumulation of the Cyclin-Dependent Kinase Inhibitors p27Kip1 and p21CIP1 in Glial Progenitor Cells. J. Neurosci. 1999, 19, 5380–5392. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.H.; Choi, S.Y.; Ryu, P.D.; Lee, S.Y. Anti-Proliferative Effect of Kv1.3 Blockers in A549 Human Lung Adenocarcinoma in Vitro and in Vivo. Eur. J. Pharmacol. 2011, 651, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Capitani, C.; Chioccioli Altadonna, G.; Santillo, M.; Lastraioli, E. Ion Channels in Lung Cancer: Biological and Clinical Relevance. Front. Pharmacol. 2023, 14, 1283623. [Google Scholar] [CrossRef] [PubMed]
- Fraser, S.P.; Grimes, J.A.; Djamgoz, M.B.A. Effects of Voltage-Gated Ion Channel Modulators on Rat Prostatic Cancer Cell Proliferation: Comparison of Strongly and Weakly Metastatic Cell Lines. Prostate 2000, 44, 61–76. [Google Scholar] [CrossRef]
- Chen, R.; Chung, S.-H. Mechanism of Tetrodotoxin Block and Resistance in Sodium Channels. Biochem. Biophys. Res. Commun. 2014, 446, 370–374. [Google Scholar] [CrossRef]
- González-Cano, R.; Ruiz-Cantero, M.C.; Santos-Caballero, M.; Gómez-Navas, C.; Tejada, M.Á.; Nieto, F.R. Tetrodotoxin, a Potential Drug for Neuropathic and Cancer Pain Relief? Toxins 2021, 13, 483. [Google Scholar] [CrossRef] [PubMed]
- Bucciarelli, G.M.; Lechner, M.; Fontes, A.; Kats, L.B.; Eisthen, H.L.; Shaffer, H.B. From Poison to Promise: The Evolution of Tetrodotoxin and Its Potential as a Therapeutic. Toxins 2021, 13, 517. [Google Scholar] [CrossRef] [PubMed]
- Diss, J.K.J.; Stewart, D.; Pani, F.; Foster, C.S.; Walker, M.M.; Patel, A.; Djamgoz, M.B.A. A Potential Novel Marker for Human Prostate Cancer: Voltage-Gated Sodium Channel Expression in Vivo. Prostate Cancer Prostatic Dis. 2005, 8, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Lv, Y.; Xu, J.; Mao, X.; Chen, Z.; Lu, W. Over-Expression of Nav1.6 Channels Is Associated with Lymph Node Metastases in Colorectal Cancer. World J. Surg. Oncol. 2019, 17, 175. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Plata, E.; Ortiz, C.S.; Marquina-Castillo, B.; Medina-Martinez, I.; Alfaro, A.; Berumen, J.; Rivera, M.; Gomora, J.C. Overexpression of NaV1.6 Channels Is Associated with the Invasion Capacity of Human Cervical Cancer. Int. J. Cancer 2012, 130, 2013–2023. [Google Scholar] [CrossRef] [PubMed]
- Campbell, T.M.; Main, M.J.; Fitzgerald, E.M. Functional Expression of the Voltage-Gated Na+-Channel Nav1.7 Is Necessary for EGF-Mediated Invasion in Human Non-Small Cell Lung Cancer Cells. J. Cell Sci. 2013, 126, 4939–4949. [Google Scholar] [CrossRef] [PubMed]
- El-Dayem, S.M.A.; Fouda, F.M.; Ali, E.H.A.; Motelp, B.A.A.E. The Antitumor Effects of Tetrodotoxin and/or Doxorubicin on Ehrlich Ascites Carcinoma-Bearing Female Mice. Toxicol. Ind. Health 2013, 29, 404–417. [Google Scholar] [CrossRef]
- Bujak, J.K.; Kosmala, D.; Szopa, I.M.; Majchrzak, K.; Bednarczyk, P. Inflammation, Cancer and Immunity—Implication of TRPV1 Channel. Front. Oncol. 2019, 9, 1087. [Google Scholar] [CrossRef]
- Weber, L.V.; Al-Refae, K.; Wölk, G.; Bonatz, G.; Altmüller, J.; Becker, C.; Gisselmann, G.; Hatt, H. Expression and Functionality of TRPV1 in Breast Cancer Cells. Breast Cancer Targets Ther. 2016, 8, 243–252. [Google Scholar] [CrossRef]
- Caprodossi, S.; Amantini, C.; Nabissi, M.; Morelli, M.B.; Farfariello, V.; Santoni, M.; Gismondi, A.; Santoni, G. Capsaicin Promotes a More Aggressive Gene Expression Phenotype and Invasiveness in Null-TRPV1 Urothelial Cancer Cells. Carcinogenesis 2011, 32, 686–694. [Google Scholar] [CrossRef]
- Pecze, L.; Jósvay, K.; Blum, W.; Petrovics, G.; Vizler, C.; Oláh, Z.; Schwaller, B. Activation of Endogenous TRPV1 Fails to Induce Overstimulation-Based Cytotoxicity in Breast and Prostate Cancer Cells but Not in Pain-Sensing Neurons. Biochim. Biophys. Acta 2016, 1863, 2054–2064. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhang, L.; Cheng, X.; Yu, H.; Bao, J.; Lu, R. Capsaicin Inhibits the Metastasis of Human Papillary Thyroid Carcinoma BCPAP Cells through the Modulation of the TRPV1 Channel. Food Funct. 2018, 9, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, K.C.; Fofaria, N.M.; Gupta, P.; Ranjan, A.; Kim, S.-H.; Srivastava, S.K. Inhibition of β-Catenin Signaling Suppresses Pancreatic Tumor Growth by Disrupting Nuclear β-Catenin/TCF-1 Complex: Critical Role of STAT-3. Oncotarget 2015, 6, 11561–11574. [Google Scholar] [CrossRef] [PubMed]
- Hwang, M.K.; Bode, A.M.; Byun, S.; Song, N.R.; Lee, H.J.; Lee, K.W.; Dong, Z. Cocarcinogenic Effect of Capsaicin Involves Activation of EGFR Signaling but Not TRPV1. Cancer Res. 2010, 70, 6859–6869. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Dai, X.; Wang, P.; Tao, Y.; Chai, D. Capsaicin Induces Cytotoxicity in Human Osteosarcoma MG63 Cells through TRPV1-Dependent and -Independent Pathways. Cell Cycle 2019, 18, 1379–1392. [Google Scholar] [CrossRef] [PubMed]
- Green, R.; Khalil, R.; Mohapatra, S.S.; Mohapatra, S. Role of Cannabidiol for Improvement of the Quality of Life in Cancer Patients: Potential and Challenges. Int. J. Mol. Sci. 2022, 23, 12956. [Google Scholar] [CrossRef] [PubMed]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The Capsaicin Receptor: A Heat-Activated Ion Channel in the Pain Pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Portenoy, R.K.; Ahmed, E. Cancer Pain Syndromes. Hematol. Clin. N. Am. 2018, 32, 371–386. [Google Scholar] [CrossRef]
- Goyal, S.; Goyal, S.; Goins, A.E.; Alles, S.R.A. Plant-Derived Natural Products Targeting Ion Channels for Pain. Neurobiol. Pain. 2023, 13, 100128. [Google Scholar] [CrossRef]
- Wu, B.; Ma, Y.; Yi, Z.; Liu, S.; Rao, S.; Zou, L.; Wang, S.; Xue, Y.; Jia, T.; Zhao, S.; et al. Resveratrol-Decreased Hyperalgesia Mediated by the P2X7 Receptor in Gp120-Treated Rats. Mol. Pain. 2017, 13, 1744806917707667. [Google Scholar] [CrossRef]
- Ko, J.-H.; Sethi, G.; Um, J.-Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The Role of Resveratrol in Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Dong, W.; Zhang, L.; Yang, X. Activating Sirt1 by Resveratrol Suppresses Nav1.7 Expression in DRG through miR-182 and Alleviates Neuropathic Pain in Rats. Channels 2020, 14, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Naiki-Ito, A.; Nakazawa, T.; Hayashi, K.; Naitoh, I.; Miyabe, K.; Shimizu, S.; Kondo, H.; Nishi, Y.; Yoshida, M.; et al. Chemopreventive Effect of Resveratrol and Apocynin on Pancreatic Carcinogenesis via Modulation of Nuclear Phosphorylated GSK3β and ERK1/2. Oncotarget 2015, 6, 42963–42975. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, C.; Ribulla, S.; Magnelli, V.; Patrone, M.; Burlando, B. Resveratrol Induces Intracellular Ca(2+) Rise via T-Type Ca(2+) Channels in a Mesothelioma Cell Line. Life Sci. 2016, 148, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, R.; Takami, A.; Espinoza, J.L. Dietary Phytochemicals and Cancer Chemoprevention: A Review of the Clinical Evidence. Oncotarget 2016, 7, 52517–52529. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Xu, W.; Xu, H.; Xiong, W.; Gao, Y.; Li, G.; Liu, S.; Xie, J.; Tu, G.; Peng, H.; et al. Role of Puerarin in the Signalling of Neuropathic Pain Mediated by P2X3 Receptor of Dorsal Root Ganglion Neurons. Brain Res. Bull. 2012, 87, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-L.; Cao, X.-Y.; Lai, R.-C.; Xie, M.-X.; Zeng, W.-A. Puerarin Relieves Paclitaxel-Induced Neuropathic Pain: The Role of Nav1.8 Β1 Subunit of Sensory Neurons. Front. Pharmacol. 2019, 9, 1510. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Luo, D.; Liang, Z.; Lao, L.; Rong, J. Plant Natural Product Puerarin Ameliorates Depressive Behaviors and Chronic Pain in Mice with Spared Nerve Injury (SNI). Mol. Neurobiol. 2017, 54, 2801–2812. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Song, B.; Hu, Y.; Chen, J.; Zhang, S.; Chen, D.; Wang, J. Puerarin Ameliorates 5-Fluorouracil-Induced Intestinal Mucositis in Mice by Inhibiting JAKs. J. Pharmacol. Exp. Ther. 2021, 379, 147–155. [Google Scholar] [CrossRef]
- Brasky, T.M.; Newton, A.M.; Conroy, S.; Adib, A.; Adley, N.C.; Strassels, S.A.; Hays, J.L.; Cooper, Z.D.; Wagener, T.L.; Stevens, E.; et al. Marijuana and Cannabidiol Use Prevalence and Symptom Management Among Patients with Cancer. Cancer Res. Commun. 2023, 3, 1917–1926. [Google Scholar] [CrossRef]
- Etemad, L.; Karimi, G.; Alavi, M.S.; Roohbakhsh, A. Pharmacological Effects of Cannabidiol by Transient Receptor Potential Channels. Life Sci. 2022, 300, 120582. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.C.; Guimarães, F.S. Evidence for a Potential Role for TRPV1 Receptors in the Dorsolateral Periaqueductal Gray in the Attenuation of the Anxiolytic Effects of Cannabinoids. Progress. Neuro-Psychopharmacol. Biol. Psychiatry 2009, 33, 1517–1521. [Google Scholar] [CrossRef]
- Heider, C.G.; Itenberg, S.A.; Rao, J.; Ma, H.; Wu, X. Mechanisms of Cannabidiol (CBD) in Cancer Treatment: A Review. Biology 2022, 11, 817. [Google Scholar] [CrossRef]
- Costa, B.; Giagnoni, G.; Franke, C.; Trovato, A.E.; Colleoni, M. Vanilloid TRPV1 Receptor Mediates the Antihyperalgesic Effect of the Nonpsychoactive Cannabinoid, Cannabidiol, in a Rat Model of Acute Inflammation. Br. J. Pharmacol. 2004, 143, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Black, J.A.; Nikolajsen, L.; Kroner, K.; Jensen, T.S.; Waxman, S.G. Multiple Sodium Channel Isoforms and Mitogen-Activated Protein Kinases Are Present in Painful Human Neuromas. Ann. Neurol. 2008, 64, 644–653. [Google Scholar] [CrossRef]
- Coward, K.; Aitken, A.; Powell, A.; Plumpton, C.; Birch, R.; Tate, S.; Bountra, C.; Anand, P. Plasticity of TTX-Sensitive Sodium Channels PN1 and Brain III in Injured Human Nerves. Neuroreport 2001, 12, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Berta, T.; Kim, Y.H.; Lee, S.; Lee, S.-Y.; Ji, R.-R. Expression and Role of Voltage-Gated Sodium Channels in Human Dorsal Root Ganglion Neurons with Special Focus on Nav1.7, Species Differences, and Regulation by Paclitaxel. Neurosci. Bull. 2018, 34, 4–12. [Google Scholar] [CrossRef]
- Nozaki-Taguchi, N.; Chaplan, S.R.; Higuera, E.S.; Ajakwe, R.C.; Yaksh, T.L. Vincristine-Induced Allodynia in the Rat. Pain 2001, 93, 69–76. [Google Scholar] [CrossRef]
- Alvarez, P.; Levine, J.D. Antihyperalgesic Effect of Tetrodotoxin in Rat Models of Persistent Muscle Pain. Neuroscience 2015, 311, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Nieto, F.R.; Entrena, J.M.; Cendán, C.M.; Del Pozo, E.; Vela, J.M.; Baeyens, J.M. Tetrodotoxin Inhibits the Development and Expression of Neuropathic Pain Induced by Paclitaxel in Mice. Pain 2008, 137, 520–531. [Google Scholar] [CrossRef]
- Hagen, N.; Lapointe, B.; Ong-Lam, M.; Dubuc, B.; Walde, D.; Gagnon, B.; Love, R.; Goel, R.; Hawley, P.; Ngoc, A.H.; et al. A Multicentre Open-Label Safety and Efficacy Study of Tetrodotoxin for Cancer Pain. Curr. Oncol. 2011, 18, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Hagen, N.A.; Fisher, K.M.; Lapointe, B.; du Souich, P.; Chary, S.; Moulin, D.; Sellers, E.; Ngoc, A.H. An Open-Label, Multi-Dose Efficacy and Safety Study of Intramuscular Tetrodotoxin in Patients with Severe Cancer-Related Pain. J. Pain. Symptom Manag. 2007, 34, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Hagen, N.A.; Cantin, L.; Constant, J.; Haller, T.; Blaise, G.; Ong-Lam, M.; du Souich, P.; Korz, W.; Lapointe, B. Tetrodotoxin for Moderate to Severe Cancer-Related Pain: A Multicentre, Randomized, Double-Blind, Placebo-Controlled, Parallel-Design Trial. Pain. Res. Manag. 2017, 2017, 7212713. [Google Scholar] [CrossRef] [PubMed]
- Goldlust, S.A.; Kavoosi, M.; Nezzer, J.; Kavoosi, M.; Korz, W.; Deck, K. Tetrodotoxin for Chemotherapy-Induced Neuropathic Pain: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Dose Finding Trial. Toxins 2021, 13, 235. [Google Scholar] [CrossRef] [PubMed]
- Huerta, M.Á.; de la Nava, J.; Artacho-Cordón, A.; Nieto, F.R. Efficacy and Security of Tetrodotoxin in the Treatment of Cancer-Related Pain: Systematic Review and Meta-Analysis. Mar. Drugs 2023, 21, 316. [Google Scholar] [CrossRef] [PubMed]
- Schwartzberg, L.; Kim, E.S.; Liu, D.; Schrag, D. Precision Oncology: Who, How, What, When, and When Not? Am. Soc. Clin. Oncol. Educ. Book. 2017, 37, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Qazi, A.K.; Siddiqui, J.A.; Jahan, R.; Chaudhary, S.; Walker, L.A.; Sayed, Z.; Jones, D.T.; Batra, S.K.; Macha, M.A. Emerging Therapeutic Potential of Graviola and Its Constituents in Cancers. Carcinogenesis 2018, 39, 522–533. [Google Scholar] [CrossRef]
- Moertel, C.G.; Fleming, T.R.; Rubin, J.; Kvols, L.K.; Sarna, G.; Koch, R.; Currie, V.E.; Young, C.W.; Jones, S.E.; Davignon, J.P. A Clinical Trial of Amygdalin (Laetrile) in the Treatment of Human Cancer. N. Engl. J. Med. 1982, 306, 201–206. [Google Scholar] [CrossRef]
- Crespo-Ortiz, M.P.; Wei, M.Q. Antitumor Activity of Artemisinin and Its Derivatives: From a Well-Known Antimalarial Agent to a Potential Anticancer Drug. J. Biomed. Biotechnol. 2012, 2012, 247597. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural Products in Drug Discovery: Advances and Opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, R.; Mohammed, C.; Alschuler, L.; Pomeranz Krummel, D.A.; Sengupta, S. Phytochemical Modulation of Ion Channels in Oncologic Symptomatology and Treatment. Cancers 2024, 16, 1786. https://doi.org/10.3390/cancers16091786
Rao R, Mohammed C, Alschuler L, Pomeranz Krummel DA, Sengupta S. Phytochemical Modulation of Ion Channels in Oncologic Symptomatology and Treatment. Cancers. 2024; 16(9):1786. https://doi.org/10.3390/cancers16091786
Chicago/Turabian StyleRao, Rohan, Caroline Mohammed, Lise Alschuler, Daniel A. Pomeranz Krummel, and Soma Sengupta. 2024. "Phytochemical Modulation of Ion Channels in Oncologic Symptomatology and Treatment" Cancers 16, no. 9: 1786. https://doi.org/10.3390/cancers16091786







