Galectins as Cancer Biomarkers
Abstract
:1. Introduction
2. Discussion
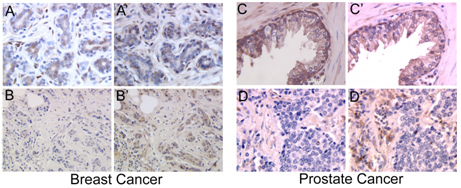
2.1. Galectins in Breast and Ovarian Cancer
2.2. Galectins in Bladder Cancer
2.3. Galectins in Gastric and Colon Cancer
2.4. Galectins in Melanomas
2.5. Galectins in Prostate Cancer
2.6. Galectins in Lung Cancer
2.7. Galectins in Lymphoma
2.8. Galectins in Gliomas and Meningiomas
2.9. Galectins in Pancreatic Cancer
2.10. Galectins in Squamous Cancer
2.11. Galectins in Thyroid Cancer
3. Concluding Remarks

Acknowledgements
References
- Dumic, J.; Dabelic, S.; Flögel, M. Galectin-3: an open-ended story. Biochim. Biophys. Acta 2006, 1760, 616–635. [Google Scholar] [CrossRef]
- Vasta, G.R. Roles of galectins in infection. Nat. Rev. Microbiol. 2009, 7, 424–438. [Google Scholar] [CrossRef]
- Barondes, S.H.; Cooper, D.N.; Gitt, M.A.; Leffler, H. Galectins. Structure and function of a large family of animal lectins. J. Biol. Chem. 1994, 269, 20807–20810. [Google Scholar]
- Califice, S.; Castronovo, V.; Van Den Brule, F. Galectin-3 and cancer. Int. J. Oncol. 2004, 25, 983–992. [Google Scholar]
- Ochieng, J.; Fridman, R.; Nangia-Makker, P.; Kleiner, D.E.; Liotta, L.A.; Stetler-Stevenson, W.G.; Raz, A. Galectin-3 is a novel substrate for human matrix metalloproteinases-2 and -9. Biochemistry (Moscow) 1994, 33, 14109–14114. [Google Scholar] [CrossRef]
- Nagae, M.; Nishi, N.; Murata, T.; Usui, T.; Nakamura, T.; Wakatsuki, S.; Kato, R. Crystal structure of the galectin-9 N-terminal carbohydrate recognition domain from Mus musculus reveals the basic mechanism of carbohydrate recognition. J. Biol. Chem. 2006, 281, 35884–35893. [Google Scholar] [CrossRef]
- Rabinovich, G.A.; Liu, F.T.; Hirashima, M.; Anderson, A. An emerging role for galectins in tuning the immune response: lessons from experimental models of inflammatory disease, autoimmunity and cancer. Scand. J. Immunol. 2007, 66, 143–158. [Google Scholar] [CrossRef]
- Castronovo, V.; Van Den Brule, F.A.; Jackers, P.; Clausse, N.; Liu, F.T.; Gillet, C.; Sobel, M.E. Decreased expression of galectin-3 is associated with progression of human breast cancer. J. Pathol. 1996, 179, 43–48. [Google Scholar] [CrossRef]
- Idikio, H. Galectin-3 expression in human breast carcinoma: correlation with cancer histologic grade. Int. J. Oncol. 1998, 12, 1287–1290. [Google Scholar]
- Nangia-Makker, P.; Thompson, E.; Hogan, V.; Ochieng, J.; Raz, A. Induction of tumorigenicity by galectin-3 in a non-tumorigenic human breast carcinoma cell line. Int. J. Oncol. 1995, 7, 1079–1087. [Google Scholar]
- Honjo, Y.; Nangia-Makker, P.; Inohara, H.; Raz, A. Down-regulation of galectin-3 suppresses tumorigenicity of human breast carcinoma cells. Clin. Cancer Res. 2001, 7, 661–668. [Google Scholar]
- Shekhar, M.P.; Nangia-Makker, P.; Tait, L.; Miller, F.; Raz, A. Alterations in galectin-3 expression and distribution correlate with breast cancer progression: functional analysis of galectin-3 in breast epithelial-endothelial interactions. Am. J. Pathol. 2004, 165, 1931–1941. [Google Scholar] [CrossRef]
- Elston, C.W.; Ellis, I.O. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 2002, 41, 154–161. [Google Scholar] [CrossRef]
- Fernandez-Aguilar, S.; Noel, J.C. Expression of cathepsin D and galectin 3 in tubular carcinomas of the breast. APMIS 2008, 116, 33–40. [Google Scholar] [CrossRef]
- Nangia-Makker, P.; Raz, T.; Tait, L.; Hogan, V.; Fridman, R.; Raz, A. Galectin-3 cleavage: a novel surrogate marker for matrix metalloproteinase activity in growing breast cancers. Cancer Res. 2007, 67, 11760–11768. [Google Scholar] [CrossRef]
- Irie, A.; Yamauchi, A.; Kontani, K.; Kihara, M.; Liu, D.; Shirato, Y.; Seki, M.; Nishi, N.; Nakamura, T.; Yokomise, H.; Hirashima, M. Galectin-9 as a prognostic factor with antimetastatic potential in breast cancer. Clin. Cancer Res. 2005, 11, 2962–2968. [Google Scholar] [CrossRef]
- Huflejt, M.E.; Leffler, H. Galectin-4 in normal tissues and cancer. Glycoconj. J. 2004, 20, 247–255. [Google Scholar] [CrossRef]
- Balasubramanian, K.; Vasudevamurthy, R.; Venkateshaiah, S.U.; Thomas, A.; Vishweshwara, A.; Dharmesh, S.M. Galectin-3 in urine of cancer patients: stage and tissue specificity. J. Cancer Res. Clin. Oncol. 2009, 135, 355–363. [Google Scholar] [CrossRef]
- Heinzelmann-Schwarz, V.A.; Gardiner-Garden, M.; Henshall, S.M.; Scurry, J.P.; Scolyer, R.A.; Smith, A.N.; Bali, A.; Vanden Bergh, P.; Baron-Hay, S.; Scott, C.; Fink, D.; Hacker, N.F.; Sutherland, R.L.; O'Brien, P.M. A distinct molecular profile associated with mucinous epithelial ovarian cancer. Br. J. Cancer 2006, 94, 904–913. [Google Scholar] [CrossRef]
- Brustmann, H. Epidermal growth factor receptor expression in serous ovarian carcinoma: an immunohistochemical study with galectin-3 and cyclin D1 and outcome. Int. J. Gynecol. Pathol. 2008, 27, 380–389. [Google Scholar] [CrossRef]
- Cindolo, L.; Benvenuto, G.; Salvatore, P.; Pero, R.; Salvatore, G.; Mirone, V.; Prezioso, D.; Altieri, V.; Bruni, C.B.; Chiariotti, L. galectin-1 and galectin-3 expression in human bladder transitional-cell carcinomas. Int. J. Cancer 1999, 84, 39–43. [Google Scholar] [CrossRef]
- Sakaki, M.; Oka, N.; Nakanishi, R.; Yamaguchi, K.; Fukumori, T.; Kanayama, H.O. Serum level of galectin-3 in human bladder cancer. J. Med. Invest. 2008, 55, 127–132. [Google Scholar] [CrossRef]
- Lotan, R.; Ito, H.; Yasui, W.; Yokozaki, H.; Lotan, D.; Tahara, E. Expression of a 31-kDa lactoside-binding lectin in normal human gastric mucosa and in primary and metastatic gastric carcinomas. Int. J. Cancer 1994, 56, 474–480. [Google Scholar] [CrossRef]
- Baldus, S.E.; Zirbes, T.K.; Weingarten, M.; Fromm, S.; Glossmann, J.; Hanisch, F.G.; Monig, S.P.; Schroder, W.; Flucke, U.; Thiele, J.; Holscher, A.H.; Dienes, H.P. Increased galectin-3 expression in gastric cancer: correlations with histopathological subtypes, galactosylated antigens and tumor cell proliferation. Tumour Biol. 2000, 21, 258–266. [Google Scholar] [CrossRef]
- Miyazaki, J.; Hokari, R.; Kato, S.; Tsuzuki, Y.; Kawaguchi, A.; Nagao, S.; Itoh, K.; Miura, S. Increased expression of galectin-3 in primary gastric cancer and the metastatic lymph nodes. Oncol. Rep. 2002, 9, 1307–1312. [Google Scholar]
- Kim, S.J.; Choi, I.J.; Cheong, T.C.; Lee, S.J.; Lotan, R.; Park, S.H.; Chun, K.H. Galectin-3 Increases Gastric Cancer Cell Motility by Up-Regulating Fascin-1 Expression. Gastroenterology 2009, 138, 1035–1045. [Google Scholar]
- Hippo, Y.; Yashiro, M.; Ishii, M.; Taniguchi, H.; Tsutsumi, S.; Hirakawa, K.; Kodama, T.; Aburatani, H. Differential gene expression profiles of scirrhous gastric cancer cells with high metastatic potential to peritoneum or lymph nodes. Cancer Res. 2001, 61, 889–895. [Google Scholar]
- Endo, K.; Kohnoe, S.; Tsujita, E.; Watanabe, A.; Nakashima, H.; Baba, H.; Maehara, Y. Galectin-3 expression is a potent prognostic marker in colorectal cancer. Anticancer Res. 2005, 25, 3117–3121. [Google Scholar]
- Nagy, N.; Legendre, H.; Engels, O.; André, S.; Kaltner, H.; Wasano, K.; Zick, Y.; Pector, J.-C.; Decaestecker, C.; Gabius, H.-J.; Salmon, I.; Kiss, R. Refined prognostic evaluation in colon carcinoma using immunohistochemical galectin fingerprinting. Cancer 2003, 97, 1849–1858. [Google Scholar] [CrossRef]
- Hittelet, A.; Legendre, H.; Nagy, N.; Bronckart, Y.; Pector, J.-C.; Salmon, I.; Yeaton, P.; Gabius, H.-J.; Kiss, R.; Camby, I. Upregulation of galectins-1 and -3 in human colon cancer and their role in regulating cell migration. Int. J. Cancer 2003, 103, 370–379. [Google Scholar] [CrossRef]
- Greco, C.; Vona, R.; Cosimelli, M.; Matarrese, P.; Straface, E.; Scordati, P.; Giannarelli, D.; Casale, V.; Assisi, D.; Mottolese, M.; Moles, A.; Malorni, W. Cell surface overexpression of galectin-3 and the presence of its ligand 90k in the blood plasma as determinants in colon neoplastic lesions. Glycobiology 2004, 14, 783–792. [Google Scholar] [CrossRef]
- Schoeppner, H.L.; Raz, A.; Ho, S.B.; Bresalier, R.S. Expression of an endogenous galactose-binding lectin correlates with neoplastic progression in the colon. Cancer 1995, 75, 2818–2826. [Google Scholar] [CrossRef]
- Nakamura, M.; Inufusa, H.; Adachi, T.; Aga, M.; Kurimoto, M.; Nakatani, Y.; Wakano, T.; Nakajima, A.; Hida, J.I.; Miyake, M.; Shindo, K.; Yasutomi, M. Involvement of galectin-3 expression in colorectal cancer progression and metastasis. Int. J. Oncol. 1999, 15, 143–148. [Google Scholar]
- van den Brule, F.A.; Buicu, C.; Berchuck, A.; Bast, R.C.; Deprez, M.; Liu, F.T.; Cooper, D.N.; Pieters, C.; Sobel, M.E.; Castronovo, V. Expression of the 67-kD laminin receptor, galectin-1, and galectin-3 in advanced human uterine adenocarcinom. Hum. Pathol. 1996, 27, 1185–1191. [Google Scholar] [CrossRef]
- Lotz, M.M.; Andrews, C.W., Jr.; Korzelius, C.A.; Lee, E.C.; Steele, G.D., Jr.; Clarke, A.; Mercurio, A.M. Decreased expression of Mac-2 (carbohydrate binding protein 35) and loss of its nuclear localization are associated with the neoplastic progression of colon carcinoma. Proc. Natl. Acad. Sci. USA 1993, 90, 3466–3470. [Google Scholar] [CrossRef]
- Bidon-Wagner, N.; Le Pennec, J.P. Human galectin-8 isoforms and cancer. Glycoconj. J. 2004, 19, 557–563. [Google Scholar] [CrossRef]
- Vereecken, P.; Debray, C.; Petein, M.; Awada, A.; Lalmand, M.C.; Laporte, M.; Van Den Heule, B.; Verhest, A.; Pochet, R.; Heenen, M. Expression of galectin-3 in primary and metastatic melanoma: immunohistochemical studies on human lesions and nude mice xenograft tumors. Arch. Dermatol. Res. 2005, 296, 353–358. [Google Scholar] [CrossRef]
- Vereecken, P.; Zouaoui Boudjeltia, K.; Debray, C.; Awada, A.; Legssyer, I.; Sales, F.; Petein, M.; Vanhaeverbeek, M.; Ghanem, G.; Heenen, M. High serum galectin-3 in advanced melanoma: preliminary results. Clin. Exp. Dermatol. 2006, 31, 105–109. [Google Scholar] [CrossRef]
- Vereecken, P.; Awada, A.; Suciu, S.; Castro, G.; Morandini, R.; Litynska, A.; Lienard, D.; Ezzedine, K.; Ghanem, G.; Heenen, M. Evaluation of the prognostic significance of serum galectin-3 in American Joint Committee on Cancer stage III and stage IV melanoma patients. Melanoma Res. 2009, 19, 316–320. [Google Scholar] [CrossRef]
- Prieto, V.G.; Mourad-Zeidan, A.A.; Melnikova, V.; Johnson, M.M.; Lopez, A.; Diwan, A.H.; Lazar, A.J.; Shen, S.S.; Zhang, P.S.; Reed, J.A.; Gershenwald, J.E.; Raz, A.; Bar-Eli, M. Galectin-3 expression is associated with tumor progression and pattern of sun exposure in melanoma. Clin. Cancer Res. 2006, 12, 6709–6715. [Google Scholar] [CrossRef]
- Ellerhorst, J.; Troncoso, P.; Xu, X.C.; Lee, J.; Lotan, R. Galectin-1 and galectin-3 expression in human prostate tissue and prostate cancer. Urol. Res. 1999, 27, 362–367. [Google Scholar] [CrossRef]
- Pacis, R.A.; Pilat, M.J.; Pienta, K.J.; Wojno, K.; Raz, A.; Hogan, V.; Cooper, C.R. Decreased galectin-3 expression in prostate cancer. Prostate 2000, 44, 118–123. [Google Scholar] [CrossRef]
- van den Brule, F.A.; Waltregny, D.; Castronovo, V. Increased expression of galectin-1 in carcinoma-associated stroma predicts poor outcome in prostate carcinoma patients. J. Pathol. 2001, 193, 80–87. [Google Scholar] [CrossRef]
- van den Brule, F.A.; Waltregny, D.; Liu, F.T.; Castronovo, V. Alteration of the cytoplasmic/nuclear expression pattern of galectin-3 correlates with prostate carcinoma progression. Int. J. Cancer 2000, 89, 361–367. [Google Scholar] [CrossRef]
- Ahmed, H.; Banerjee, P.P.; Vasta, G.R. Differential expression of galectins in normal, benign and malignant prostate epithelial cells: silencing of galectin-3 expression in prostate cancer by its promoter methylation. Biochem. Biophys. Res. Commun. 2007, 358, 241–246. [Google Scholar] [CrossRef]
- Wang, Y.; Nangia-Makker, P.; Tait, L.; Balan, V.; Hogan, V.; Pienta, K.J.; Raz, A. Regulation of prostate cancer progression by galectin-3. Am. J. Pathol. 2009, 174, 1515–1523. [Google Scholar] [CrossRef]
- Yoshimura, A.; Gemma, A.; Hosoya, Y.; Komaki, E.; Hosomi, Y.; Okano, T.; Takenaka, K.; Matuda, K.; Seike, M.; Uematsu, K.; Hibino, S.; Shibuya, M.; Yamada, T.; Hirohashi, S.; Kudoh, S. Increased expression of the LGALS3 (galectin 3) gene in human non-small-cell lung cancer. Gen. Chrom. Cancer 2003, 37, 159–164. [Google Scholar] [CrossRef]
- Buttery, R.; Monaghan, H.; Salter, D.M.; Sethi, T. Galectin-3: differential expression between small-cell and non-small-cell lung cancer. Histopathology 2004, 44, 339–344. [Google Scholar] [CrossRef]
- Jeong, S.H.; Lee, H.W.; Han, J.H.; Kang, S.Y.; Choi, J.H.; Jung, Y.M.; Choi, H.; Oh, Y.T.; Park, K.J.; Hwang, S.C.; Sheen, S.S.; Oh, Y.J.; Kim, J.H.; Lim, H.Y. Low expression of Bax predicts poor prognosis in resected non-small cell lung cancer patients with non-squamous histology. Jpn. J. Clin. Oncol. 2008, 38, 661–669. [Google Scholar] [CrossRef]
- Szoke, T.; Kayser, K.; Baumhakel, J.D.; Trojan, I.; Furak, J.; Tiszlavicz, L.; Horvath, A.; Szluha, K.; Gabius, H.J.; Andre, S. Prognostic significance of endogenous adhesion/growth-regulatory lectins in lung cancer. Oncology 2005, 69, 167–174. [Google Scholar] [CrossRef]
- Szoke, T.; Kayser, K.; Trojan, I.; Kayser, G.; Furak, J.; Tiszlavicz, L.; Baumhakel, J.D.; Gabius, H.J. The role of microvascularization and growth/adhesion-regulatory lectins in the prognosis of non-small cell lung cancer in stage II. Eur. J. Cardiothorac. Surg. 2007, 31, 783–787. [Google Scholar] [CrossRef]
- Puglisi, F.; Minisini, A.M.; Barbone, F.; Intersimone, D.; Aprile, G.; Puppin, C.; Damante, G.; Paron, I.; Tell, G.; Piga, A.; Di Loreto, C. Galectin-3 expression in non-small cell lung carcinoma. Cancer Lett. 2004, 212, 233–239. [Google Scholar] [CrossRef]
- Iurisci, I.; Tinari, N.; Natoli, C.; Angelucci, D.; Cianchetti, E.; Iacobelli, S. Concentrations of galectin-3 in the sera of normal controls and cancer patients. Clin. Cancer Res. 2000, 6, 1389–1393. [Google Scholar]
- Henno, S.; Brichory, F.; Langanay, T.; Desrues, B.; Bidon, N.; Delaval, P.; Ramee, M.P.; Dazord, L.; Caulet-Maugendre, S. Expression of Po66-CBP, a galectin-8, in different types of primary and secondary broncho-pulmonary tumors. Oncol. Rep. 2002, 9, 177–180. [Google Scholar]
- Caulet-Maugendre, S.; Birolleau, S.; Corbineau, H.; Bassen, R.; Desrues, B.; Bidon, N.; Delaval, P.; Ramee, M.P.; Brichory, F.; Dazord, L. Immunohistochemical expression of the intracellular component of galectin-8 in squamous cell metaplasia of the bronchial epithelium in neoplastic and benign processes. Pathol. Res. Pract. 2001, 197, 797–801. [Google Scholar] [CrossRef]
- Konstantinov, K.N.; Robbins, B.A.; Liu, F.T. Galectin-3, a beta-galactoside-binding animal lectin, is a marker of anaplastic large-cell lymphoma. Am. J. Pathol. 1996, 148, 25–30. [Google Scholar]
- Kim, S.J.; Lee, S.J.; Sung, H.J.; Choi, I.K.; Choi, C.W.; Kim, B.S.; Kim, J.S.; Yu, W.; Hwang, H.S.; Kim, I.S. Increased serum 90K and Galectin-3 expression are associated with advanced stage and a worse prognosis in diffuse large B-cell lymphomas. Acta Haematol. 2008, 120, 211–216. [Google Scholar] [CrossRef]
- Bresalier, R.S.; Yan, P.S.; Byrd, J.C.; Lotan, R.; Raz, A. Expression of the endogenous galactose-binding protein galectin-3 correlates with the malignant potential of tumors in the central nervous system. Cancer 1997, 80, 776–787. [Google Scholar] [CrossRef]
- Gordower, L.; Decaestecker, C.; Kacem, Y.; Lemmers, A.; Gusman, J.; Burchert, M.; Danguy, A.; Gabius, H.; Salmon, I.; Kiss, R.; Camby, I. Galectin-3 and galectin-3-binding site expression in human adult astrocytic tumours and related angiogenesis. Neuropathol. Appl. Neurobiol. 1999, 25, 319–330. [Google Scholar] [CrossRef]
- Strik, H.M.; Deininger, M.H.; Frank, B.; Schluesener, H.J.; Meyermann, R. Galectin-3: cellular distribution and correlation with WHO-grade in human gliomas. J. Neurooncol. 2001, 53, 13–20. [Google Scholar] [CrossRef]
- Das, A.; Tan, W.L.; Smith, D.R. Expression of extracellular matrix markers in benign meningiomas. Neuropathology 2003, 23, 275–281. [Google Scholar] [CrossRef]
- Park, S.H.; Min, H.S.; Kim, B.; Myung, J.; Paek, S.H. Galectin-3: a useful biomarker for differential diagnosis of brain tumors. Neuropathology 2008, 28, 497–506. [Google Scholar] [CrossRef]
- Schaffert, C.; Pour, P.M.; Chaney, W.G. Localization of galectin-3 in normal and diseased pancreatic tissue. Int. J. Pancreatol. 1998, 23, 1–9. [Google Scholar]
- Berberat, P.O.; Friess, H.; Wang, L.; Zhu, Z.; Bley, T.; Frigeri, L.; Zimmermann, A.; Buchler, M.W. Comparative analysis of galectins in primary tumors and tumor metastasis in human pancreatic cancer. J. Histochem. Cytochem. 2001, 49, 539–549. [Google Scholar] [CrossRef]
- Shimamura, T.; Sakamoto, M.; Ino, Y.; Shimada, K.; Kosuge, T.; Sato, Y.; Tanaka, K.; Sekihara, H.; Hirohashi, S. Clinicopathological significance of galectin-3 expression in ductal adenocarcinoma of the pancreas. Clin. Cancer Res. 2002, 8, 2570–2575. [Google Scholar]
- Chung, J.C.; Oh, M.J.; Choi, S.H.; Bae, C.D. Proteomic analysis to identify biomarker proteins in pancreatic ductal adenocarcinoma. ANZ J. Surg. 2008, 78, 245–251. [Google Scholar] [CrossRef]
- Gillenwater, A.; Xu, X.C.; el-Naggar, A.K.; Clayman, G.L.; Lotan, R. Expression of galectins in head and neck squamous cell carcinoma. Head Neck 1996, 18, 422–432. [Google Scholar] [CrossRef]
- Piantelli, M.; Iacobelli, S.; Almadori, G.; Iezzi, M.; Tinari, N.; Natoli, C.; Cadoni, G.; Lauriola, L.; Ranelletti, F.O. Lack of expression of galectin-3 is associated with a poor outcome in node-negative patients with laryngeal squamous-cell carcinoma. J. Clin. Oncol. 2002, 20, 3850–3856. [Google Scholar] [CrossRef]
- Plzak, J.; Smetana, K., Jr.; Hrdlickova, E.; Kodet, R.; Holikova, Z.; Liu, F.T.; Dvorankova, B.; Kaltner, H.; Betka, J.; Gabius, H.J. Expression of galectin-3-reactive ligands in squamous cancer and normal epithelial cells as a marker of differentiation. Int. J. Oncol. 2001, 19, 59–64. [Google Scholar]
- Plzak, J.; Betka, J.; Smetana, K., Jr.; Chovanec, M.; Kaltner, H.; Andre, S.; Kodet, R.; Gabius, H.J. Galectin-3 - an emerging prognostic indicator in advanced head and neck carcinoma. Eur. J. Cancer 2004, 40, 2324–2330. [Google Scholar] [CrossRef]
- Betka, J.; Plzak, J.; Smetana, K., Jr.; Gabius, H.J. Galectin-3, an endogenous lectin, as a tool for monitoring cell differentiation in head and neck carcinomas with implications for lectin-glycan functionality. Acta Otolaryngol. 2003, 123, 261–263. [Google Scholar]
- Saussez, S.; Decaestecker, C.; Mahillon, V.; Cludts, S.; Capouillez, A.; Chevalier, D.; Vet, H.K.; Andre, S.; Toubeau, G.; Leroy, X.; Gabius, H.J. Galectin-3 upregulation during tumor progression in head and neck cancer. Laryngoscope 2008, 118, 1583–1590. [Google Scholar] [CrossRef]
- Chovanec, M.; Smetana, K., Jr.; Plzak, J.; Betka, J.; Plzakova, Z.; Stork, J.; Hrdlickova, E.; Kuwabara, I.; Dvorankova, B.; Liu, F.T.; Kaltner, H.; Andre, S.; Gabius, H.J. Detection of new diagnostic markers in pathology by focus on growth-regulatory endogenous lectins. The case study of galectin-7 in squamous epithelia. Prague Med. Rep. 2005, 106, 209–216. [Google Scholar]
- Saussez, S.; Lorfevre, F.; Lequeux, T.; Laurent, G.; Chantrain, G.; Vertongen, F.; Toubeau, G.; Decaestecker, C.; Kiss, R. The determination of the levels of circulating galectin-1 and -3 in HNSCC patients could be used to monitor tumor progression and/or responses to therapy. Oral Oncol. 2008, 44, 86–93. [Google Scholar] [CrossRef]
- Xu, X.C.; el-Naggar, A.K.; Lotan, R. Differential expression of galectin-1 and galectin-3 in thyroid tumors. Potential diagnostic implications. Am. J. Pathol. 1995, 147, 815–822. [Google Scholar]
- Bartolazzi, A.; Gasbarri, A.; Papotti, M.; Bussolati, G.; Lucante, T.; Khan, A.; Inohara, H.; Marandino, F.; Orlandi, F.; Nardi, F.; Vecchione, A.; Tecce, R.; Larsson, O.; Group, T.C.S. Application of an immunodiagnostic method for improving preoperative diagnosis of nodular thyroid lesions. Lancet 2001, 357, 1644–1650. [Google Scholar] [CrossRef]
- Orlandi, F.; Saggiorato, E.; Pivano, G.; Puligheddu, B.; Termine, A.; Cappia, S.; De Giuli, P.; Angeli, A. Galectin-3 is a presurgical marker of human thyroid carcinoma. Cancer Res. 1998, 58, 3015–3020. [Google Scholar]
- Lloyd, R.V. Distinguishing benign from malignant thyroid lesions: galectin 3 as the latest candidate. Endocr. Pathol. 2001, 12, 255–257. [Google Scholar] [CrossRef]
- Aratake, Y.; Umeki, K.; Kiyoyama, K.; Hinoura, Y.; Sato, S.; Ohno, A.; Kuribayashi, T.; Hirai, K.; Nabeshima, K.; Kotani, T. Diagnostic utility of galectin-3 and CD26/DPPIV as preoperative diagnostic markers for thyroid nodules. Diagn. Cytopathol. 2002, 26, 366–372. [Google Scholar] [CrossRef]
- Beesley, M.F.; McLaren, K.M. Cytokeratin 19 and galectin-3 immunohistochemistry in the differential diagnosis of solitary thyroid nodules. Histopathology 2002, 41, 236–243. [Google Scholar] [CrossRef]
- Coli, A.; Bigotti, G.; Zucchetti, F.; Negro, F.; Massi, G. Galectin-3, a marker of well-differentiated thyroid carcinoma, is expressed in thyroid nodules with cytological atypia. Histopathology 2002, 40, 80–87. [Google Scholar] [CrossRef]
- Niedziela, M.; Maceluch, J.; Korman, E. Galectin-3 is not an universal marker of malignancy in thyroid nodular disease in children and adolescents. J. Clin. Endocrinol. Metab. 2002, 87, 4411–4415. [Google Scholar] [CrossRef]
- Oestreicher-Kedem, Y.; Halpern, M.; Roizman, P.; Hardy, B.; Sulkes, J.; Feinmesser, R.; Stern, Y. Diagnostic value of galectin-3 as a marker for malignancy in follicular patterned thyroid lesions. Head Neck 2004, 26, 960–966. [Google Scholar] [CrossRef]
- Mehrotra, P.; Okpokam, A.; Bouhaidar, R.; Johnson, S.J.; Wilson, J.A.; Davies, B.R.; Lennard, T.W.J. Galectin-3 does not reliably distinguish benign from malignant thyroid neoplasms. Histopathology 2004, 45, 493–500. [Google Scholar] [CrossRef]
- Mills, L.J.; Poller, D.N.; Yiangou, C. Galectin-3 is not useful in thyroid FNA. Cytopathology 2005, 16, 132–138. [Google Scholar] [CrossRef]
- Than, T.H.; Swethadri, G.K.; Wong, J.; Ahmad, T.; Jamil, D.; Maganlal, R.K.; Hamdi, M.M.; Abdullah, M.S. Expression of Galectin-3 and Galectin-7 in thyroid malignancy as potential diagnostic indicators. Singapore Med. J. 2008, 49, 333–338. [Google Scholar]
- Liu, Y.Y.; Morreau, H.; Kievit, J.; Romijn, J.A.; Carrasco, N.; Smit, J.W. Combined immunostaining with galectin-3, fibronectin-1, CITED-1, Hector Battifora mesothelial-1, cytokeratin-19, peroxisome proliferator-activated receptor-{gamma}, and sodium/iodide symporter antibodies for the differential diagnosis of non-medullary thyroid carcinoma. Eur. J. Endocrinol. 2008, 158, 375–384. [Google Scholar] [CrossRef]
- Rydlova, M.; Ludvikova, M.; Stankova, I. Potential diagnostic markers in nodular lesions of the thyroid gland: an immunohistochemical study. Biomed. Pap. of the Med. Fac. Univ. Palacký 2008, 152, 53–59. [Google Scholar] [CrossRef]
- Saussez, S.; Glinoer, D.; Chantrain, G.; Pattou, F.; Carnaille, B.; Andre, S.; Gabius, H.J.; Laurent, G. Serum galectin-1 and galectin-3 levels in benign and malignant nodular thyroid disease. Thyroid 2008, 18, 705–712. [Google Scholar] [CrossRef]
- Bartolazzi, A.; Orlandi, F.; Saggiorato, E.; Volante, M.; Arecco, F.; Rossetto, R.; Palestini, N.; Ghigo, E.; Papotti, M.; Bussolati, G.; Martegani, M.P.; Pantellini, F.; Carpi, A.; Giovagnoli, M.R.; Monti, S.; Toscano, V.; Sciacchitano, S.; Pennelli, G.M.; Mian, C.; Pelizzo, M.R.; Rugge, M.; Troncone, G.; Palombini, L.; Chiappetta, G.; Botti, G.; Vecchione, A.; Bellocco, R. Galectin-3-expression analysis in the surgical selection of follicular thyroid nodules with indeterminate fine-needle aspiration cytology: a prospective multicentre study. Lancet Oncol. 2008, 9, 543–549. [Google Scholar]
- Wiseman, S.M.; Melck, A.; Masoudi, H.; Ghaidi, F.; Goldstein, L.; Gown, A.; Jones, S.J.; Griffith, O.L. Molecular phenotyping of thyroid tumors identifies a marker panel for differentiated thyroid cancer diagnosis. Ann. Surg. Oncol. 2008, 15, 2811–2826. [Google Scholar] [CrossRef]
- Griffith, O.L.; Chiu, C.G.; Gown, A.M.; Jones, S.J.; Wiseman, S.M. Biomarker panel diagnosis of thyroid cancer: a critical review. Expert Rev. Anticancer Ther. 2008, 8, 1399–1413. [Google Scholar] [CrossRef]
- Balan, V.; Nangia-Makker, P.; Schwartz, A.G.; Jung, Y.S.; Tait, L.; Hogan, V.; Raz, T.; Wang, Y.; Yang, Z.Q.; Wu, G.S.; Guo, Y.; Li, H.; Abrams, J.; Couch, F.J.; Lingle, W.L.; Lloyd, R.V.; Ethier, S.P.; Tainsky, M.A.; Raz, A. Racial disparity in breast cancer and functional germ line mutation in galectin-3 (rs4644): a pilot study. Cancer Res. 2008, 68, 10045–10050. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Balan, V.; Nangia-Makker, P.; Raz, A. Galectins as Cancer Biomarkers. Cancers 2010, 2, 592-610. https://doi.org/10.3390/cancers2020592
Balan V, Nangia-Makker P, Raz A. Galectins as Cancer Biomarkers. Cancers. 2010; 2(2):592-610. https://doi.org/10.3390/cancers2020592
Chicago/Turabian StyleBalan, Vitaly, Pratima Nangia-Makker, and Avraham Raz. 2010. "Galectins as Cancer Biomarkers" Cancers 2, no. 2: 592-610. https://doi.org/10.3390/cancers2020592
APA StyleBalan, V., Nangia-Makker, P., & Raz, A. (2010). Galectins as Cancer Biomarkers. Cancers, 2(2), 592-610. https://doi.org/10.3390/cancers2020592