A Novel Method for Sentinel Lymph Node Biopsy by Indocyanine Green Fluorescence Technique in Breast Cancer
Abstract
:1. Introduction
2. Results and Discussion
2.1. Patients and Tumor Characteristics
| No. | % | ||
| Age (years) | >50 | 131 | 31.9 |
| <50 | 280 | 68.1 | |
| Site | Area A | 52 | 12.6 |
| Area B | 15 | 3.7 | |
| Area C | 136 | 33.1 | |
| Area D | 36 | 8.8 | |
| Area E | 6 | 1.5 | |
| Two areas | 149 | 36.2 | |
| More than two areas | 17 | 4.1 | |
| Tumor size | Tis | 54 | 13.1 |
| T1 | 215 | 52.3 | |
| T2 | 112 | 27.3 | |
| T3 | 12 | 2.9 | |
| Missed | 18 | 4.4 | |
| Histology | DCIS | 54 | 13.1 |
| IDC | 357 | 86.9 | |
| Grade | 0 | 26 | 6.3 |
| 1 | 153 | 37.2 | |
| 2 | 73 | 17.8 | |
| 3 | 79 | 19.2 | |
| Missing | 80 | 19.5 | |
| ER | Negative | 104 | 25.3 |
| Positive | 169 | 41.1 | |
| Missing | 138 | 33.6 | |
| PR | Negative | 104 | 25.3 |
| Positive | 169 | 41.1 | |
| Missing | 138 | 33.6 | |
| HER2/neu | Negative | 212 | 51.6 |
| IHC2+ | 13 | 3.2 | |
| IHC3+ | 28 | 6.35 | |
| Missing | 158 | 38.4 |
2.2. The Number of SLNs Removed and the Percentage of SLNs Involved
| No. of SLNs removed | Kyoto University Hospital | Shinko Hospital | Kobe City Medical Center | Total (%) |
| 1 | 7 | 36 | 8 84484 | 127 (30.1) |
| 2 | 6 | 26 | 89 | 121 (29.4) |
| 3 | 4 | 13 | 81 | 98 (23.9) |
| 4 | 12 | 1 | 30 | 43 (11) |
| 5 | 8 | 0 | 7 | 15 (3.7) |
| 6 | 3 | 0 | 0 | 3 (0.7) |
| 9 | 1 | 0 | 0 | 1 (0.2) |
| Total | 41 | 76 | 291 | 408 |
| No. of SLNs involved | No. of patients | % |
| 0 | 369 | 90.5 |
| 1 | 30 | 7.3 |
| 2 | 4 | 1 |
| 3 | 5 | 1.2 |
| Total | 408 | 100 |
2.3. Discussion
3. Experimental Section
3.1. Patients
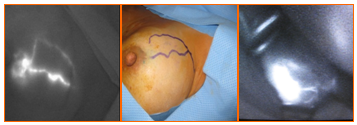
3.2. ICG Fluorescence Technique


4. Conclusions
References
- Veronesi, U.; Paganelli, G.; Viale, G.; Luini, A.; Zurrida, S.; Galimberti, V.; Intra, M.; Veronesi, P.; Mainsonneuve, P.; Gatti, G.; Massazol, G.; De Cicco, C.; Manfredi, G.; Fernández, J.R. Sentinel-lymph-node biopsy as a staging procedure in breast cancer: update of a randomised control study. Lancet Oncol. 2006, 7, 983–990. [Google Scholar] [CrossRef]
- Lyman, G.H.; Giuliano, A.E.; Somerfield, M.R.; Benson, A.B., III; Bodurka, D.C.; Burstein, H.J.; Cochran, A.J.; Cody, H.S., III; Edge, S.B.; Galper, S.; Hayman, J.A.; Kim, T.Y.; Perkins, C.L.; Podoloff, D.A.; Sivasubramaniam, V.H.; Turner, R.R.; Wahl, R.; Weaver, D.L.; Wolff, A.C.; Winer, E.P. American Society of Clinical Oncology Guideline Recommendations for sentinel lymph node biopsy in early-stage breast cancer. J. Clin. Oncol. 2005, 23, 7703–7720. [Google Scholar] [CrossRef]
- Kitai, T.; Inomoto, T.; Miwa, M.; Shikayama, T. Fluorescence navigation with indocyanine green for detecting sentinel lymph nodes in breast cancer. Breast Cancer 2005, 12, 211–215. [Google Scholar] [CrossRef]
- Tagaya, N.; Yamazaki, R.; Nakagawa, A.; Abe, A.; Hamada, K.; Kubota, K.; Oyama, T. Intraoperative identification of sentinel lymph nodes by near-infrared fluorescence imaging in patients with breast cancer. Am. J. Surg. 2008, 195, 850–853. [Google Scholar] [CrossRef]
- Veronesi, U.; Paganelli, G.; Viale, G.; Luini, A.; Zurrida, S.; Galimberti, V.; Intra, M.; Veronesi, P.; Robertson, C.; Maisonneuve, P.; Renne, G.; De Cicco, C.; De Lucia, F.; Gennari, R.A. randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N. Eng. J. Med. 2003, 349, 546–553. [Google Scholar] [CrossRef]
- Krag, D.N.; Weaver, D.L.; Alex, J.C.; Fairbank, J.T. Surgical resection and radio-localization of the sentinel lymph node in breast cancer using gamma probe. Surg. Oncol. 1993, 2, 335–359. [Google Scholar] [CrossRef]
- Giuliano, A.E.; Kirgan, D.M.; Guenther, J.M.; Morton, D.L. Lymphatic mapping and sentinel lymphadecectomy for breast cancer. Ann. Surg. 1994, 220, 391–401. [Google Scholar] [CrossRef]
- Rubio, I.T.; Klimberg, V.S. Techniques of sentinel lymph node biopsy. Semin. Surg. Oncol. 2001, 20, 214–223. [Google Scholar] [CrossRef]
- Giuliano, A.E. Sentinel lymphadenectomy in primary breast carcinoma: an alternative to routine axillary dissection. J. Surg. Oncol. 1996, 62, 75–77. [Google Scholar] [CrossRef]
- Linehan, D.C.; Hill, A.D.; Tran, K.N.; Yeung, H.; Yeh, S.D.; Borgen, P.I.; Cody, H.S., III. Sentinel lymph node biopsy in breast cancer: unfiltered radioisotope is superior to filtered. J. Am. Coll. Surg. 1999, 188, 377–381. [Google Scholar] [CrossRef]
- Bass, S.S.; Cox, C.E.; Ku, N.N.; Berman, C.; Reintgen, D.S. The role of sentinel lymph node biopsy in breast cancer. J. Am. Coll. Surg. 1999, 189, 183–194. [Google Scholar] [CrossRef]
- Gipponi, M.; Bassetti, C.; Canavese, G.; Catturich, A.; Di Somma, C.; Vecchio, C.; Nicolò, G.; Schenone, F.; Tomei, D.; Cafiero, F. Sentinel lymph node as a new marker for therapeutic planning in breast cancer patients. J. Surg. Oncol. 2004, 85, 102–111. [Google Scholar] [CrossRef]
- Chung, A.; Yu, J.; Stempel, M.; Patil, S.; Cody, H.; Montgomery, L. Is the “10% rule” equally valid for all subsets of sentinel-node-positive breast cancer patients? Ann. Surg. Oncol. 2008, 15, 2728–2733. [Google Scholar] [CrossRef]
- Hojo, T.; Nagao, T.; Kikuyama, M.; Akashi, S.; Kinoshita, T. Evaluation of sentinel node biopsy combined fluorescent and dye method and lymph flow for breast cancer. Breast 2010, in press. [Google Scholar]
- Murawa, D.; Hirche, C.; Dresel, S.; Hunerbein, M. Sentinel lymph node biopsy in breast cancer guided by indocyanine green fluorescence. Br. J. Surg. 2009, 96, 1289–1294. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sugie, T.; Kassim, K.A.; Takeuchi, M.; Hashimoto, T.; Yamagami, K.; Masai, Y.; Toi, M. A Novel Method for Sentinel Lymph Node Biopsy by Indocyanine Green Fluorescence Technique in Breast Cancer. Cancers 2010, 2, 713-720. https://doi.org/10.3390/cancers2020713
Sugie T, Kassim KA, Takeuchi M, Hashimoto T, Yamagami K, Masai Y, Toi M. A Novel Method for Sentinel Lymph Node Biopsy by Indocyanine Green Fluorescence Technique in Breast Cancer. Cancers. 2010; 2(2):713-720. https://doi.org/10.3390/cancers2020713
Chicago/Turabian StyleSugie, Tomoharu, Kassim Abdelazeem Kassim, Megumi Takeuchi, Takashi Hashimoto, Kazuhiko Yamagami, Yoshikazu Masai, and Masakazu Toi. 2010. "A Novel Method for Sentinel Lymph Node Biopsy by Indocyanine Green Fluorescence Technique in Breast Cancer" Cancers 2, no. 2: 713-720. https://doi.org/10.3390/cancers2020713
APA StyleSugie, T., Kassim, K. A., Takeuchi, M., Hashimoto, T., Yamagami, K., Masai, Y., & Toi, M. (2010). A Novel Method for Sentinel Lymph Node Biopsy by Indocyanine Green Fluorescence Technique in Breast Cancer. Cancers, 2(2), 713-720. https://doi.org/10.3390/cancers2020713