The Problems of Radiofrequency Ablation as an Approach for Advanced Unresectable Ductal Pancreatic Carcinoma
Abstract
:1. Introduction

2. Search Strategy
3. Data Extraction
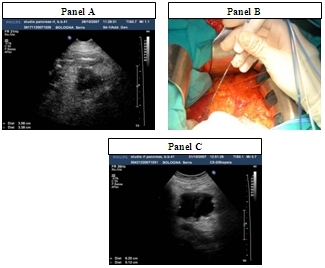
4. Results
| Reference | No. of patients | Gender | Age (year) | Site of tumors | Tumor size (cm) | Extension of disease | |||
|---|---|---|---|---|---|---|---|---|---|
| FemalesNo. (%) | MalesNo. (%) | Median (range) | HeadNo. (%) | Body-tail No. (%) | Median (range) | Locally advancedNo. (%) | MetastasesNo. (%) | ||
| Matsui [63] | 20 | 8 (40) | 12 (60) | 59 (45–77) | 11 (55) | 9 (45) | 5.3 (3–10) | 9 (45) | 11 (55) |
| Date [30] | 1 | – | 1 | 58 | 1 | – | 3 | – | 1 |
| Varshney [36] | 3 | 2 (66.7) | 1 (33.3) | 60 (48–66) | 3 (100) | – | 6.5 (5–8) | 3 (100) | – |
| Hadjicostas [64] | 4 | 2 (50) | 2 (50) | 70 (59–79) | 3 (75) | 1 (25) | 8.5 (3–12) | 4 (100) | – |
| Wu [39] | 16 | 6 (37.5) | 10 (62.5) | 67 (42–89) | 8 (50) | 8 (50) | NR | 11 (68.7) | 5 (31.3) |
| Spiliotis [41] | 12 | 6 (50) | 6(50) | 66.5 (61–79) | 11 (91.7) | 1 (8.3) | 3.7 (3–10) | 8 (66.7) | 4 (33.3) |
| Girelli [62] | 50 | 23 (46) | 27 (54) | 64.5 (54.5–74) | 34 (68) | 16 (32) | 4 (3–5) | 50 (100) | – |
| Overall | 106 | 47 (44.3) | 59 (56.7) | 64.5 (58–70) | 71 (66.9) | 35 (33.1) | 4.6 (3–12) | 85 (80.1) | 21 (19.9) |
| Reference | No. of patients | Approach | Type of associated procedures | No. of passes/size | RFA parameters | ||
|---|---|---|---|---|---|---|---|
| Surgical No. (%) | Radiological No. (%) | No. (%) | Ratio | Temperature °C median and (range) | Time (min) median and (range) | ||
| Matsui [63] | 20 | 20 (100) | – | – | NR | 50 | 15 |
| Date [30] | 1 | 1 (100) | – | 1 DB (100) | 1 | 90 | 10 |
| Varshney [36] | 3 | 2 (66.7) | 1 (33.3) | – | NR | NR | NR |
| Hadjicostas [64] | 4 | 4 (100) | – | 4 CJ * (100) | 0.36 | NR | NR |
| Wu [39] | 16 | 16 (100) | – | – | NR | 30 ** | 12 ** |
| Spiliotis [41] | 12 | 12 (100) | – | 12 DB (100) | 0.5 | 90 | 6.5 (2–7) |
| Girelli [62] | 50 | 50 (100) | – | 19 DB; 8 GB; 3 BB; 1 PJ (62) | NR | 97.5 (90–105) | NR |
| Overall | 106 | 105 (95.2) | 1 (4.8) | 48 (45.3) | 0.5 (0.36–1) | 90 (30–105) | 11 (2–15) |
| Reference | No. of patients | Morbidity No. of cases (%) | Reoperation No. of cases (%) | Mortality No. of cases (%) | Crude Survival in months Median and (range) |
|---|---|---|---|---|---|
| Matsui [63] | 20 | 3 (15) | 1 (5) | 3 (15) | 3 |
| Date [30] | 1 | 1 (100) | – | – | 3 |
| Varshney [36] | 3 | 2 (66.7) | – | – | 9 (1–11) |
| Hadjicostas [64] | 4 | – | – | – | 6.5 (3–12 |
| Wu [39] | 16 | 7 (43.7) | – | 4 (25) | NR |
| Spiliotis [41] | 12 | 4 (33.3) | – | – | 33 (6–39) |
| Girelli [62] | 50 | 13 (26) | 3 (6) | 1 (2) | NR |
| Overall | 106 | 30 (28.3) | 4 (3.7) | 7 (7.5) | 6.5 (1–33) |
| Complication | No. of complication | (%) |
|---|---|---|
| Gastro-intestinal hemorrhage | 8 | 22.9 |
| Pancreatic fistula | 5 | 14.3 |
| Biliary leak | 5 | 14.3 |
| Portal vein thrombosis | 4 | 11.4 |
| Pseudocyst | 3 | 8.6 |
| Sepsis | 2 | 5.7 |
| Polyuria | 1 | 2.9 |
| Ascites | 1 | 2.9 |
| Pmeumonia | 1 | 2.9 |
| Liver failure | 1 | 2.9 |
| Anastomotic ulcer | 1 | 2.9 |
| Severe acute pancreatitis | 1 | 2.9 |
| Renal failure | 1 | 2.9 |
| Delayed gastric emptying | 1 | 2.9 |
| Overall | 35 | 100.0 |
5. Discussion
6. Conclusions
References
- Wagner, M.; Redaelli, C.; Lietz, M.; Seiler, A.; Friess, H.; Buchler, W. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br. J. Surg. 2004, 91, 586–594. [Google Scholar]
- Neoptolemos, J.P.; Stocken, D.D.; Friess, H.; Bassi, C.; Dunn, J.A.; Hickey, H.; Beger, H.; Fernandez-Cruz, L.; Dervenis, C.; Lacaine, F.; Falconi, M.; Pederzoli, P.; Pap, A.; Spooner, D.; Kerr, D.J.; Büchler, M.W. European Study Group for Pancreatic Cancer. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N. Engl. J. Med. 2004, 350, 1200–1210. [Google Scholar] [CrossRef]
- Verslype, C.; Van Cutsem, E.; Dicato, M.; Cascinu, S.; Cunningham, D.; Diaz-Rubio, E.; Glimelius, B.; Haller, D.; Haustermans, K.; Heinemann, V.; Hoff, P.; Johnston, P.G.; Kerr, D.; Labianca, R.; Louvet, C.; Minsky, B.; Moore, M.; Nordlinger, B.; Pedrazzoli, S.; Roth, A.; Rothenberg, M.; Rougier, P.; Schmoll, H.J.; Tabernero, J.; Tempero, M.; van de Velde, C.; Van Laethem, J.L; Zalcberg, J. The management of pancreatic cancer. Current expert opinion and recommendations derived from the 8th World Congress on Gastrointestinal Cancer, Barcelona, 2006. Ann. Oncol. 2007, 18, 1–10. [Google Scholar]
- Siriwardana, H.P.; Siriwardena, A.K. Systematic review of outcome of synchronous portal-superior mesenteric vein resection during pancreatectomy for cancer. Br. J. Surg. 2006, 93, 662–673. [Google Scholar] [CrossRef]
- Yip, D.; Karapetis, C.; Strickland, A.; Steer, C.B.; Goldstein, D. Chemotherapy and radiotherapy for inoperable advanced pancreatic cancer. Cochrane Database of Syst. Rev. 2006, 3, CD002093. [Google Scholar]
- Jansen, M.C.; van Hillegersberg, R.; Chamuleau, R.A.; van Delden, O.M.; Gouma, D.J.; van Gulik, T.M. Outcome of regional and local ablative therapies for hepatocellular carcinoma: a collective review. Surg. Oncol. 2005, 31, 331–347. [Google Scholar] [CrossRef]
- Simon, C.J.; Duppy, D.E. Current role of image-guided therapies in lung cancer. Anticancer Ther. 2005, 5, 657–666. [Google Scholar] [CrossRef]
- Boss, A.; Clasen, S.; Kuczyk, M.; Anastasiadis, A.; Schmidt, D.; Graf, H.; Schick, F.; Claussen, C.D.; Pereira, P.L. Magnetic resonance-guided percutaneous radiofrequency ablation of renal cell carcinomas: a pilot clinically study. Invest. Radiol. 2005, 4, 583–590. [Google Scholar]
- Wood, B.J.; Abraham, J.; Hvizda, J.L.; Alexander, H.R.; Fojo, T. Radiofrequency ablation of adrenal tumors and adrenocortical carcinoma metastases. Cancer 2003, 97, 554–560. [Google Scholar] [CrossRef]
- Shariat, S.F.; Raptidis, G.; Masatoschi, M.; Bergamaschi, F.; Slawin, K.M. Pilot study of radiofrequency interstitial tumor ablation (RITA) for the treatment of radio-current prostate cancer. Prostate 2005, 65, 260–267. [Google Scholar]
- Martel, J.; Bueno, A.; Ortiz, E. Percutaneous radiofrequency treatment of osteoid osteoma using cool tip electrode. Eur. J. Radiol. 2005, 56, 403–408. [Google Scholar] [CrossRef]
- Chiou, Y.Y.; Hwang, J.I.; Chou, Y.H.; Wang, H.K.; Chiang, J.H.; Chang, C.Y. Percutaneous ultrasound-guided radiofrequency ablation of the intrahepatic cholangiocarcinoma. Kaohsiung J. Med. Sci. 2005, 21, 304–309. [Google Scholar] [CrossRef]
- Pezzilli, R.; Ricci, C.; Serra, C.; Casadei, R.; Monari, F.; D’Ambra, M.; Minni, F. Radiofrequency ablation for advanced ductal pancreatic carcinoma: is this approach beneficial for our patients? A systematic review. Pancreas 2010, in press. [Google Scholar]
- Krinsky, M.L.; Binmoeller, K.F. EUS–guided investigational therapy for pancreatic cancer. Gastrointest. Endosc. 2000, 52 (Suppl. 6), S35–S38. [Google Scholar] [CrossRef]
- Fosh, B.G.; Finch, J.G.; Anthony, A.A.; Texler, M.; Maddern, G.J. Electrolytic ablation of the rat pancreas: a feasibility trial. BMC Gastroenterol. 2001, 1, 9. [Google Scholar] [CrossRef] [Green Version]
- Scott, A.; Hinwood, D.; Donnelly, R. Radio-frequency ablation for symptom control in a patient with metastatic pancreatic insulinoma. Clin. Endocrinol. (Oxf.) 2002, 56, 557–559. [Google Scholar] [CrossRef]
- Hellman, P.; Ladjevardi, S.; Skogseid, B.; Akerström, G.; Elvin, A. Radiofrequency tissue ablation using cooled tip for liver metastases of endocrine tumors. World J. Surg. 2002, 26, 1052–1056. [Google Scholar]
- Li, M.L.; Norton, J.A. Gastrinoma. Curr. Treat. Options Oncol. 2001, 2, 337–346. [Google Scholar] [CrossRef]
- Wemyss-Holden, S.A.; Court, F.G.; Morrison, C.P.; Teague, B.D.; Burrell, A.; Morales, D.R.; Rodgers, N.; Anthony, A.A.; Metcalfe, M.S.; Dennison, A.R.; Maddern, G.J. Palliation of pancreatic cancer using electrolytic ablation. Surg. Endosc. 2003, 17, 207–211. [Google Scholar] [CrossRef]
- Varshney, S.; Sharma, S.; Pamecha, V.; Sewkani, A.; Jhawar, L.; Narkhede, V.; Tewari, V.; Patel, K. Radiofrequency tissue ablation: an early Indian experience. Indian J. Gastroenterol. 2003, 22, 91–93. [Google Scholar]
- Henn, A.R.; Levine, E.A.; McNulty, W.; Zagoria, R.J. Percutaneous radiofrequency ablation of hepatic metastases for symptomatic relief of neuroendocrine syndromes. Am. J. Roentgenol. 2003, 181, 1005–1010. [Google Scholar] [CrossRef]
- Deol, Z.K.; Frezza, E.; DeJong, S.; Pickleman, J. Solitary hepatic gastrinoma treated with laparoscopic radiofrequency ablation. JSLS 2003, 7, 285–289. [Google Scholar]
- Elias, D.; Baton, O.; Sideris, L.; Lasser, P.; Pocard, M. Necrotizing pancreatitis after radiofrequency destruction of pancreatic tumours. Eur. J. Surg. Oncol. 2004, 30, 85–87. [Google Scholar] [CrossRef]
- Komoto, I.; Shimada, Y.; Fujimoto, K.; Itami, A.; Kawamura, J.; Doi, R.; Imamura, M. Diagnosis of and therapy for endocrine tumors of the pancreas. Nippon Naika Gakkai Zasshi 2004, 93, 77–83. [Google Scholar] [CrossRef]
- Scherübl, H.; Schaaf, L.; Raue, F.; Faiss, S.; Zeitz, M. Hereditary neuroendocrine gastroenteropancreatic tumors and multiple endocrine neoplasia type 1. Dtsch. Med. Wochenschr. 2004, 129, 689–692. [Google Scholar] [CrossRef]
- Frezza, E.E. Laparoscopic radiofrequency ablation of solitary hepatic gastrinoma metastases. Dig. Dis. Sci. 2004, 49, 224–227. [Google Scholar] [CrossRef]
- Thomas, K.T.; Bream, P.R., Jr.; Berlin, J.; Meranze, S.G.; Wright, J.K.; Chari, R.S. Use of percutaneous drainage to treat hepatic abscess after radiofrequency ablation of metastatic pancreatic adenocarcinoma. Am. Surg. 2004, 70, 496–499. [Google Scholar]
- Kouvaraki, M.A.; Solorzano, C.C.; Shapiro, S.E.; Yao, J.C.; Perrier, N.D.; Lee, J.E.; Evans, D.B. Surgical treatment of non-functioning pancreatic islet cell tumors. J. Surg. Oncol. 2005, 89, 170–185. [Google Scholar] [CrossRef]
- Ramage, J.K.; Davies, A.H.; Ardill, J.; Bax, N.; Caplin, M.; Grossman, A.; Hawkins, R.; McNicol, A.M.; Reed, N.; Sutton, R.; Thakker, R.; Aylwin, S.; Breen, D.; Britton, K.; Buchanan, K.; Corrie, P.; Gillams, A.; Lewington, V.; McCance, D.; Meeran, K.; Watkinson, A.; UKNETwork for Neuroendocrine Tumours. Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours. Gut 2005, 54 (Suppl. 4), iv1–16. [Google Scholar] [CrossRef]
- Date, R.S.; Siriwardena, A.K. Radiofrequency ablation of the pancreas. II: Intra-operative ablation of non-resectable pancreatic cancer. A description of technique and initial outcome. JOP 2005, 6, 588–592. [Google Scholar]
- Moug, S.J.; Leen, E.; Horgan, P.G.; Imrie, C.W. Radiofrequency ablation has a valuable therapeutic role in metastatic VIPoma. Pancreatology 2006, 6, 155–159. [Google Scholar] [CrossRef]
- Veenendaal, L.M.; Borel Rinkes, I.H.; van Hillegersberg, R. Multipolar radiofrequency ablation of large hepatic metastases of endocrine tumours. Eur. J. Gastroenterol. Hepatol. 2006, 18, 89–92. [Google Scholar] [CrossRef]
- House, M.G.; Cameron, J.L.; Lillemoe, K.D.; Schulick, R.D.; Choti, M.A.; Hansel, D.E.; Hruban, R.H.; Maitra, A.; Yeo, C.J. Differences in survival for patients with resectable versus unresectable metastases from pancreatic islet cell cancer. J. Gastrointest. Surg. 2006, 10, 138–145. [Google Scholar] [CrossRef]
- Siriwardena, A.K. Radiofrequency ablation for locally advanced cancer of the pancreas. JOP 2006, 7, 1–4. [Google Scholar]
- Delis, S.; Bakoyiannis, A.; Giannakou, N.; Tsigka, A.; Avgerinos, C.; Dervenis, C. Asymptomatic calcitonin-secreting tumor of the pancreas.A case report. JOP 2006, 7, 70–73. [Google Scholar]
- Varshney, S.; Sewkani, A; Sharma, S.; Kapoor, S.; Naik, S.; Sharma, A.; Patel, K. Radiofrequency ablation of unresectable pancreatic carcinoma: feasibility, efficacy and safety. JOP 2006, 7, 74–78. [Google Scholar]
- Hodul, P.; Malafa, M.; Choi, J.; Kvols, L. The role of cytoreductive hepatic surgery as an adjunct to the management of metastatic neuroendocrine carcinomas. Cancer Contr. 2006, 13, 61–71. [Google Scholar]
- Date, R.S. Current status of local ablative techniques in the treatment of pancreatic cancer. Pancreas 2006, 33, 198–199. [Google Scholar] [CrossRef]
- Wu, Y.; Tang, Z.; Fang, H.; Gao, S.; Chen, J.; Wang, Y.; Yan, H. High operative risk of cool-tip radiofrequency ablation for unresectable pancreatic head cancer. J. Surg. Oncol. 2006, 94, 392–395. [Google Scholar] [CrossRef]
- Musunuru, S.; Chen, H.; Rajpal, S.; Stephani, N.; McDermott, J.C.; Holen, K.; Rikkers, L.F.; Weber, S.M. Metastatic neuroendocrine hepatic tumors: resection improves survival. Arch. Surg. 2006, 141, 1000–1004. [Google Scholar] [CrossRef]
- Spiliotis, J.D.; Datsis, A.C.; Michalopoulos, N.V.; Kekelos, S.P.; Vaxevanidou, A.; Rogdakis, A.G.; Christopoulou, A.N. Radiofrequency ablation combined with palliative surgery may prolong survival of patients with advanced cancer of the pancreas. Langenbecks Arch. Surg. 2007, 392, 55–60. [Google Scholar] [CrossRef]
- Spiliotis, J.D.; Datsis, A.C.; Michalopoulos, N.V.; Kekelos, S.P.; Vaxevanidou, A.; Rogdakis, A.G.; Christopoulou, A.N. High operative risk of cool-tip radiofrequency ablation for unresectable pancreatic head cancer. J. Surg. Oncol. 2007, 96, 89–90. [Google Scholar] [CrossRef]
- Yalcin, S.; Oyan, B.; Bayraktar, Y. Current medical treatment of pancreatic neuroendocrine tumors. Hepatogastroenterology 2007, 54, 278–284. [Google Scholar]
- Alberts, S.R.; Gores, G.J.; Kim, G.P.; Roberts, L.R.; Kendrick, M.L.; Rosen, C.B.; Chari, S.T.; Martenson, J.A. Treatment options for hepatobiliary and pancreatic cancer. Mayo Clin. Proc. 2007, 82, 628–637. [Google Scholar] [CrossRef]
- Boujaoude, J. Role of endoscopic ultrasound in diagnosis and therapy of pancreatic adenocarcinoma. World J. Gastroenterol. 2007, 13, 3662–3666. [Google Scholar]
- Carrafiello, G.; Laganà, D.; Ianniello, A.; Dionigi, G.; Novario, R.; Recaldini, C.; Mangini, M.; Cuffari, S.; Fugazzola, C. Post-radiofrequency ablation syndrome after percutaneous radiofrequency of abdominal tumours: one centre experience and review of published works. Australas. Radiol. 2007, 51, 550–554. [Google Scholar] [CrossRef]
- Hah, J.O.; Park, W.K.; Lee, N.H.; Choi, J.H. Preoperative chemotherapy and intraoperative radiofrequency ablation for unresectable solid pseudopapillary tumor of the pancreas. J. Pediatr. Hematol. Oncol. 2007, 29, 851–853. [Google Scholar] [CrossRef]
- Yusuf, T.E.; Matthes, K.; Brugge, W.R. EUS-guided photodynamic therapy with verteporfin for ablation of normal pancreatic tissue: a pilot study in a porcine model (with video). Gastrointest. Endosc. 2008, 67, 957–961. [Google Scholar] [CrossRef]
- Carrafiello, G.; Laganà, D.; Recaldini, C.; Dionigi, G.; Boni, L.; Bacuzzi, A.; Fugazzola, C. Radiofrequency ablation of a pancreatic metastasis from renal cell carcinoma: case report. Surg. Laparosc. Endosc. Percutan. Tech. 2008, 18, 64–66. [Google Scholar] [CrossRef]
- Eriksson, J.; Stålberg, P.; Nilsson, A.; Krause, J.; Lundberg, C.; Skogseid, B.; Granberg, D.; Eriksson, B.; Akerström, G.; Hellman, P. Surgery and radiofrequency ablation for treatment of liver metastases from midgut and foregut carcinoids and endocrine pancreatic tumors. World J. Surg. 2008, 32, 930–938. [Google Scholar] [CrossRef]
- Yan, B.M.; Van Dam, J. Endoscopic ultrasound-guided intratumoural therapy for pancreatic cancer. Can. J. Gastroenterol. 2008, 22, 405–410. [Google Scholar]
- Wolfsen, H.C. Photodynamic therapy for pancreatic cancer: let's get serious. Gastrointest. Endosc. 2008, 67, 961–963. [Google Scholar] [CrossRef]
- Brugge, W.R. Management and outcomes of pancreatic cystic lesions. Dig. Liver Dis. 2008, 40, 854–859. [Google Scholar] [CrossRef]
- Atwell, T.D.; Lloyd, R.V.; Nagorney, D.M.; Fidler, J.L.; Andrews, J.C.; Reading, C.C. Peritumoral steatosis associated with insulinomas: appearance at imaging. Abdom. Imaging. 2008, 33, 571–574. [Google Scholar] [CrossRef]
- Tang, Z.; Wu, Y.L.; Fang, H.Q.; Xu, J.; Mo, G.Q.; Chen, X.M.; Gao, S.L.; Li, J.T.; Liu, Y.B.; Wang, Y. Treatment of unresectable pancreatic carcinoma by radiofrequency ablation with 'cool-tip needle': report of 18 cases. Zhonghua Yi Xue Za Zhi 2008, 88, 391–394. [Google Scholar]
- Knigge, U.; Hansen, C.P.; Stadil, F. Interventional treatment of neuroendocrine liver metastases. Surgeon 2008, 6, 232–239. [Google Scholar] [CrossRef]
- Hlavsa, J.; Kala, Z.; Válek, V.; Mikulica, M.; Man, M.; Procházka, V.; Kiss, I. Radiofrequency ablation (RFA) of pancreatic tumors. Rozhl. Chir. 2008, 87, 462–466. [Google Scholar]
- Brugge, W.R. EUS-guided tumor ablation with heat, cold, microwave, or radiofrequency: will there be a winner? Gastrointest. Endosc. 2009, 69 (Suppl. 2), S212–216. [Google Scholar] [CrossRef]
- Abood, G.J.; Go, A.; Malhotra, D.; Shoup, M. The surgical and systemic management of neuroendocrine tumors of the pancreas. Surg. Clin. North Am. 2009, 89, 249–266. [Google Scholar] [CrossRef]
- Davies, K.; Conlon, K.C. Neuroendocrine tumors of the pancreas. Curr. Gastroenterol. Rep. 2009, 11, 119–127. [Google Scholar] [CrossRef]
- Ashida, R.; Chang, K.J. Interventional EUS for the treatment of pancreatic cancer. J. Hepatobiliary Pancreat. Surg. 2009, 16, 592–597. [Google Scholar] [CrossRef]
- Girelli, R.; Frigerio, I.; Salvia, R.; Barbi, E.; Tinazzi Martini, P.; Bassi, C. Feasibility and safety of radiofrequency ablation for locally advanced pancreatic cancer. Br. J. Surg. 2010, 97, 220–225. [Google Scholar] [CrossRef]
- Matsui, Y.; Nakagawa, A.; Kamiyama, Y.; Yamamoto, K.; Kubo, N.; Nakase, Y. Selective thermocoagulation of unresectable pancreatic cancers by using radiofrequency capacitive heating. Pancreas 2000, 20, 14–20. [Google Scholar] [CrossRef]
- Hadjicostas, P.; Malakounides, N.; Varianos, C.; Kitiris, E.; Lerni, F.; Symeonides, P. Radiofrequency ablation in pancreatic cancer. HPB 2006, 8, 61–64. [Google Scholar] [CrossRef]
- Giovannini, M.; Moutardier, V.; Danisi, C.; Bories, E.; Pesenti, C.; Delpéro, J.R. Treatment of hepatocellular carcinoma using percutaneous radiofrequency thermoablation: results and outcomes in 56 patients. J. Gastrointest. Surg. 2003, 7, 791–796. [Google Scholar] [CrossRef]
- Vivarelli, M.; Guglielmi, A.; Ruzzenente, A.; Cucchetti, A.; Bellusci, R.; Cordiano, C.; Cavallari, A. Surgical resection versus percutaneous radiofrequency ablation in the treatment of hepatocellular carcinoma on cirrhotic liver. Ann. Surg. 2004, 240, 102–107. [Google Scholar] [CrossRef]
- Giovannini, M. Concentration-dependent ablation of pancreatic tissue by EUS-guided ethanol injection. Gastrointest. Endosc. 2007, 65, 278–280. [Google Scholar] [CrossRef]
- Casadei, R.; Ricci, C.; Pezzilli, R.; Serra, C.; Calculli, L.; Morselli-Labate, A.M.; Santini, D.; Minni, F. A prospective study on radiofrequency ablation for locally advanced pancreatic cancer. HBP Dis. Int. 2010, 9, 306–311. [Google Scholar]
- Pezzilli, R.; Ricci, C.; Casadei, R.; Serra, C.; Calculli, L.; Antonacci, N.; Santini, D.; Minni, F. Radiofrequency ablation of pancreatic cancer: a new attractive approach or another unsuccesful technique for the treatment of pancreatic adenocarcinoma? A systematic review. Cancer Ther. 2008, 6, 741–744. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pezzilli, R.; Ricci, C.; Serra, C.; Casadei, R.; Monari, F.; D’Ambra, M.; Corinaldesi, R.; Minni, F. The Problems of Radiofrequency Ablation as an Approach for Advanced Unresectable Ductal Pancreatic Carcinoma. Cancers 2010, 2, 1419-1431. https://doi.org/10.3390/cancers2031419
Pezzilli R, Ricci C, Serra C, Casadei R, Monari F, D’Ambra M, Corinaldesi R, Minni F. The Problems of Radiofrequency Ablation as an Approach for Advanced Unresectable Ductal Pancreatic Carcinoma. Cancers. 2010; 2(3):1419-1431. https://doi.org/10.3390/cancers2031419
Chicago/Turabian StylePezzilli, Raffaele, Claudio Ricci, Carla Serra, Riccardo Casadei, Francesco Monari, Marielda D’Ambra, Roberto Corinaldesi, and Francesco Minni. 2010. "The Problems of Radiofrequency Ablation as an Approach for Advanced Unresectable Ductal Pancreatic Carcinoma" Cancers 2, no. 3: 1419-1431. https://doi.org/10.3390/cancers2031419