Formulations for Intranasal Delivery of Pharmacological Agents to Combat Brain Disease: A New Opportunity to Tackle GBM?
Abstract
:1. Introduction to Glioblastoma
1.1. Epidemiology
1.2. Standard of Care
1.3. Shortcomings of Standard Care and Novel Treatments
1.3.1. Tumor Vaccines
1.3.2. Local Administration
2. Intranasal Administration
3. Anatomy Relevant for Nose-to-Brain Transport
3.1. Macroscopical Anatomy
3.1.1. Olfactory Pathway/Olfactory Region (Figure 1A)

3.1.2. Trigeminal Pathway/Respiratory Region (Figure 1B)
3.1.3. Other Possible Pathways (Figure 1A,B)
3.2. Microscopical Anatomy (Figure 1, Figure 2)

3.2.1. Nasal Mucosa and Mucus
3.2.2. Tight Junctions (TJs)/Paracellular Transport between Nasal Mucosa
3.2.3. Endocytosis/Transcellular Transport across Nasal Mucosa
3.2.4. Organization Nerves/Filia Olfactoria (Figure 1A)
4. Pre-Clinical and Clinical Evidence of Nose-to-Brain Transport for GBM
| Compound | Intranasal dose | Plasma concentration | CSF concentration | GBM model | Efficacy | Ref. |
|---|---|---|---|---|---|---|
| Animals | ||||||
| Methotrexate | 3.2 mg/kg | 345 ± 58 ng/mL | 1,278 ± 393 ng/mL | - | - | [55] |
| Methotrexate | 2.5 mg | 1 µg/mL | 12.54 ± 1.54 µg/mL (+AZA) | 9L rat glioma | Decreased tumor weight | [56] |
| 5-fluorouracil | 26.7 nmol | 2.4 fmol/mL | 6 fmol/mL (+AZA) | - | - | [57] |
| GRN163 | 0.65 µmol | - | - | U-521 MG rat glioma | Increased median survival from 35 days to 75.5 days | [58] |
| Vascular Stomatitis Virus | 2.5 × 107 PFU | - | - | U87 MG glioma | Selective infection and killing of olfactory bulb tumor | [59] |
| Neural Stem and Progenitor cells | 3 × 105 cells | - | - | U87 MG, NCE-G55T2, GL261 | Rapid, targeted migration of cells towards intracerebral glioma | [60] |
| Human | ||||||
| Monoterpene perillyl alcohol | 440 mg/day | - | - | Recurrent GBM patients with at least 3 relapses | Increased median survival from 2.3 to 5.9 months | [61] |
4.1. Intranasal Administration for GBM
4.1.1. Animal Models
4.1.2. Clinical Setting
4.1.3. Possible Pitfalls
5. Improvement of the Nose-to-Brain Pathway through Formulations
5.1. Via Nanoparticles
5.1.1. Polymer-Based Nanoparticles
| Formulation compound | Structure | Formulation |
|---|---|---|
| Polymer based | ||
| Chitosan (CS) | 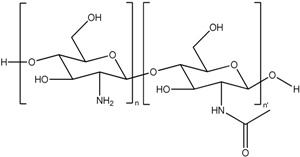 | Nanoparticle |
| [82,83,84,85,86,87] | ||
| Gel | ||
| [88,89,90] | ||
| Maltodextrin | 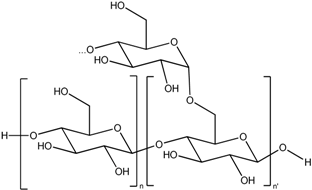 | Nanoparticle |
| [91] | ||
| Poly ethylene glycol (PEG) |  | Nanoparticle |
| [92,93,94,95,96,97,98,99,100,101] | ||
| Gel | ||
| [102] | ||
| Poly lactic acid (PLA) |  | Nanoparticle |
| [93,96,97,98,99,100] | ||
| Polylactic-co-glycolic acid (PLGA) | 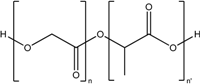 | Nanoparticle |
| [103,104] | ||
| PAMAM dendrimer |  | Nanoparticle |
| [89,105] | ||
| Poloxamer |  | Gel |
| [106] | ||
| Lipid based | ||
| Glycerol monocaprate (CapmulTM) |  | Emulsion |
| [107,108] | ||
| Mixture of mono-, di-, and triglycerides and mono- and di- fatty esters of PEG (LabrafilTM) | 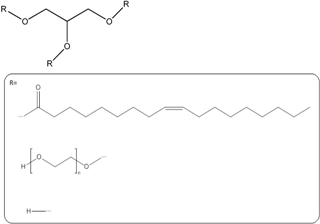 | Emulsion |
| [109] | ||
| Palmitate |  | Solid lipid particles |
| [110] | ||
| Glycerol monostearate | 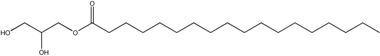 | Lipid particles |
| [111] | ||
| Phospholipids | 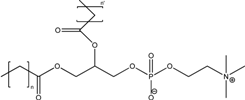 | |
| Lipid nanovesicles | ||
| [112] | ||
| Liposomes | ||
| [113,114,115,116] | ||
5.1.2. Lipid Based Nanosized Formulations
5.1.2.1. Via (nano)Emulsions
5.1.2.2. Via Solid Lipid Nanoparticles
5.1.2.3. Via Liposomes
5.2. Functionalization of the Nanoparticle Surface by Ligands
5.3. Via Gels
5.4. Via Indirect Enhancers/Devices (Figure 3)

6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Lefranc, F.; Sadeghi, N.; Camby, I.; Metens, T.; Dewitte, O.; Kiss, R. Present and potential future issues in glioblastoma treatment. Expert Rev. Anticancer Ther. 2006, 6, 719–732. [Google Scholar] [CrossRef]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Scheithauer, B.W.; Kleihues, P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007, 114, 97–109. [Google Scholar] [CrossRef]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-Year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Stummer, W.; Tonn, J.C.; Mehdorn, H.M.; Nestler, U.; Franz, K.; Goetz, C.; Bink, A.; Pichlmeier, U. Counterbalancing risks and gains from extended resections in malignant glioma surgery: A supplemental analysis from the randomized 5-aminolevulinic acid glioma resection study. J. Neurosurg. 2011, 114, 613–623. [Google Scholar] [CrossRef]
- Burnet, N.G.; Jefferies, S.J.; Benson, R.J.; Hunt, D.P.; Treasure, F.P. Years of life lost (YLL) from cancer is an important measure of population burden and should be considered when allocating research funds. Br. J. Cancer 2005, 92, 241–245. [Google Scholar]
- Del Vecchio, C.A.; Wong, A.J. Rindopepimut, a 14-mer injectable peptide vaccine against EGFRvIII for the potential treatment of glioblastoma multiforme. Curr. Opin. Mol. Ther. 2010, 12, 741–754. [Google Scholar]
- Van Gool, S.; Maes, W.; Ardon, H.; Verschuere, T.; van Cauter, S.; de Vleeschouwer, S. Dendritic cell therapy of high-grade gliomas. Brain Pathol. 2009, 19, 694–712. [Google Scholar] [CrossRef]
- De Vleeschouwer, S.; Ardon, H.; van Calenbergh, F.; Sciot, R.; Wilms, G.; van Loon, J.; Goffin, J.; van Gool, S. Stratification according to HGG-IMMUNO RPA model predicts outcome in a large group of patients with relapsed malignant glioma treated by adjuvant postoperative dendritic cell vaccination. Cancer Immunol. Immunother. 2012, 61, 2105–2112. [Google Scholar] [CrossRef]
- Van Gool, S.; de Vleeschouwer, S. Should dendritic cell-based tumor vaccination be incorporated into standard therapy for newly diagnosed glioblastoma patients? Expert Rev. Neurother. 2012, 12, 1173–1176. [Google Scholar] [CrossRef]
- Ardon, H.; van Gool, S.W.; Verschuere, T.; Maes, W.; Fieuws, S.; Sciot, R.; Wilms, G.; Demaerel, P.; Goffin, J.; van Calenbergh, F.; et al. Integration of autologous dendritic cell-based immunotherapy in the standard of care treatment for patients with newly diagnosed glioblastoma: Results of the HGG-2006 phase I/II trial. Cancer Immunol. Immunother. 2012, 61, 2033–2044. [Google Scholar] [CrossRef]
- Pardridge, W.M. Blood-brain barrier delivery. Drug Discov. Today 2007, 12, 54–61. [Google Scholar] [CrossRef]
- Varenika, V.; Dickinson, P.; Bringas, J.; LeCouteur, R.; Higgins, R.; Park, J.; Fiandaca, M.; Berger, M.; Sampson, J.; Bankiewicz, K. Detection of infusate leakage in the brain using real-time imaging of convection-enhanced delivery. J. Neurosurg. 2008, 109, 874–880. [Google Scholar] [CrossRef]
- Debinski, W. Local treatment of brain tumors with targeted chimera cytotoxic proteins. Cancer Invest. 2002, 20, 801–809. [Google Scholar] [CrossRef]
- Laske, D.W.; Youle, R.J.; Oldfield, E.H. Tumor regression with regional distribution of the targeted toxin TF-CRM107 in patients with malignant brain tumors. Nat. Med. 1997, 3, 1362–1368. [Google Scholar] [CrossRef]
- Kunwar, S.; Prados, M.D.; Chang, S.M.; Berger, M.S.; Lang, F.F.; Piepmeier, J.M.; Sampson, J.H.; Ram, Z.; Gutin, P.H.; Gibbons, R.D.; et al. Direct intracerebral delivery of cintredekin besudotox (IL13-PE38QQR) in recurrent malignant glioma: A report by the Cintredekin Besudotox Intraparenchymal Study Group. J. Clin. Oncol. 2007, 25, 837–844. [Google Scholar] [CrossRef]
- Mardor, Y.; Roth, Y.; Lidar, Z.; Jonas, T.; Pfeffer, R.; Maier, S.E.; Faibel, M.; Nass, D.; Hadani, M.; Orenstein, A.; et al. Monitoring response to convection-enhanced taxol delivery in brain tumor patients using diffusion-weighted magnetic resonance imaging. Cancer Res. 2001, 61, 4971–4973. [Google Scholar]
- Debinski, W.; Tatter, S.B. Convection-enhanced delivery for the treatment of brain tumors. Expert Rev. Neurother. 2009, 9, 1519–1527. [Google Scholar] [CrossRef]
- Bobo, R.H.; Laske, D.W.; Akbasak, A.; Morrison, P.F.; Dedrick, R.L.; Oldfield, E.H. Convection-enhanced delivery of macromolecules in the brain. Proc. Natl. Acad. Sci. USA 1994, 91, 2076–2080. [Google Scholar] [CrossRef]
- Groothuis, D.R. The blood-brain and blood-tumor barriers: A review of strategies for increasing drug delivery. Neuro-oncology 2002, 2, 45–59. [Google Scholar]
- Thorne, R.G.; Emory, C.R.; Ala, T.A.; Frey, W.H., 2nd. Quantitative analysis of the olfactory pathway for drug delivery to the brain. Brain Res. 1995, 692, 278–282. [Google Scholar] [CrossRef]
- Shipley, M.T. Transport of molecules from nose to brain: Transneuronal anterograde and retrograde labeling in the rat olfactory system by wheat germ agglutinin-horseradish peroxidase applied to the nasal epithelium. Brain Res. Bull. 1985, 15, 129–142. [Google Scholar] [CrossRef]
- Illum, L. Transport of drugs from the nasal cavity to the central nervous system. Eur. J. Pharm. Sci. 2000, 11, 1–18. [Google Scholar] [CrossRef]
- Pajouhesh, H.; Lenz, G.R. Medicinal chemical properties of successful central nervous system drugs. NeuroRx 2005, 2, 541–553. [Google Scholar] [CrossRef]
- Reese, T.S.; Karnovsky, M.J. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J. Cell Biol. 1967, 34, 207–217. [Google Scholar] [CrossRef]
- Miller, D.S. Regulation of P-glycoprotein and other ABC drug transporters at the blood-brain barrier. Trends Pharmacol. Sci. 2010, 31, 246–254. [Google Scholar] [CrossRef]
- Lochhead, J.J.; Thorne, R.G. Intranasal delivery of biologics to the central nervous system. Adv. Drug Deliver. Rev. 2012, 64, 614–628. [Google Scholar] [CrossRef]
- Dhuria, S.V.; Hanson, L.R.; Frey, W.H., 2nd. Intranasal delivery to the central nervous system: Mechanisms and experimental considerations. J. Pharm. Sci. 2010, 99, 1654–1673. [Google Scholar]
- Jansson, B.; Bjork, E. Visualization of in vivo olfactory uptake and transfer using fluorescein dextran. J. Drug Target. 2002, 10, 379–386. [Google Scholar] [CrossRef]
- Leopold, D.A. The relationship between nasal anatomy and human olfaction. Laryngoscope 1988, 98, 1232–1238. [Google Scholar] [CrossRef]
- Caggiano, M.; Kauer, J.S.; Hunter, D.D. Globose basal cells are neuronal progenitors in the olfactory epithelium: A lineage analysis using a replication-incompetent retrovirus. Neuron 1994, 13, 339–352. [Google Scholar] [CrossRef]
- Carmichael, S.T.; Clugnet, M.C.; Price, J.L. Central olfactory connections in the macaque monkey. J. Comp. Neurol. 1994, 346, 403–434. [Google Scholar] [CrossRef]
- Lledo, P.M.; Gheusi, G.; Vincent, J.D. Information processing in the mammalian olfactory system. Physiol. Rev. 2005, 85, 281–317. [Google Scholar] [CrossRef]
- Jafek, B.W. Ultrastructure of human nasal mucosa. Laryngoscope 1983, 93, 1576–1599. [Google Scholar] [CrossRef]
- Johnson, N.J.; Hanson, L.R.; Frey, W.H. Trigeminal pathways deliver a low molecular weight drug from the nose to the brain and orofacial structures. Mol. Pharm. 2010, 7, 884–893. [Google Scholar] [CrossRef]
- Bojsen-Moller, F. Demonstration of terminalis, olfactory, trigeminal and perivascular nerves in the rat nasal septum. J. Comp. Neurol. 1975, 159, 245–256. [Google Scholar] [CrossRef]
- Fuss, S.H.; Omura, M.; Mombaerts, P. The Grueneberg ganglion of the mouse projects axons to glomeruli in the olfactory bulb. Eur. J. Neurosci. 2005, 22, 2649–2654. [Google Scholar] [CrossRef]
- DeSesso, J.M. The relevance to humans of animal models for inhalation studies of cancer in the nose and upper airways. Qual. Assur. 1993, 2, 213–231. [Google Scholar]
- Pollock, H.; Hutchings, M.; Weller, R.O.; Zhang, E.T. Perivascular spaces in the basal ganglia of the human brain: Their relationship to lacunes. J. Anat. 1997, 191, 337–346. [Google Scholar]
- Hadaczek, P.; Yamashita, Y.; Mirek, H.; Tamas, L.; Bohn, M.C.; Noble, C.; Park, J.W.; Bankiewicz, K. The “perivascular pump” driven by arterial pulsation is a powerful mechanism for the distribution of therapeutic molecules within the brain. Mol. Ther. 2006, 14, 69–78. [Google Scholar] [CrossRef]
- Johnston, M.; Zakharov, A.; Papaiconomou, C.; Salmasi, G.; Armstrong, D. Evidence of connections between cerebrospinal fluid and nasal lymphatic vessels in humans, non-human primates and other mammalian species. Cerebrospinal Fluid Res. 2004, 1, e2. [Google Scholar]
- Walter, B.A.; Valera, V.A.; Takahashi, S.; Ushiki, T. The olfactory route for cerebrospinal fluid drainage into the peripheral lymphatic system. Neuropathol. Appl. Neurobiol. 2006, 32, 388–396. [Google Scholar] [CrossRef]
- Yoffey, J.M.; Drinker, C.K. The Lymphatic Pathway from the Nose and Pharynx: The Absorption of Dyes. J. Exp. Med. 1938, 68, 629–640. [Google Scholar] [CrossRef]
- Kaliner, M.; Marom, Z.; Patow, C.; Shelhamer, J. Human respiratory mucus. J. Allergy Clin. Immunol. 1984, 73, 318–323. [Google Scholar] [CrossRef]
- Khutoryanskiy, V.V. Advances in mucoadhesion and mucoadhesive polymers. Macromol. Biosci. 2011, 11, 748–764. [Google Scholar] [CrossRef]
- Moran, D.T.; Rowley, J.C., 3rd; Jafek, B.W.; Lovell, M.A. The fine structure of the olfactory mucosa in man. J. Neurocytol. 1982, 11, 721–746. [Google Scholar] [CrossRef]
- Schipper, N.G.; Verhoef, J.C.; Merkus, F.W. The nasal mucociliary clearance: Relevance to nasal drug delivery. Pharm. Res. 1991, 8, 807–814. [Google Scholar] [CrossRef]
- Van Itallie, C.M.; Anderson, J.M. Claudins and epithelial paracellular transport. Annu. Rev. Physiol. 2006, 68, 403–429. [Google Scholar] [CrossRef]
- Altner, H.; Altner-Kolnberger, I. Freeze-fracture and tracer experiments on the permeability of the zonulae occludentes in the olfactory mucosa of vertebrates. Cell Tissue Res. 1974, 154, 51–59. [Google Scholar]
- Conner, S.D.; Schmid, S.L. Regulated portals of entry into the cell. Nature 2003, 422, 37–44. [Google Scholar] [CrossRef]
- Baker, H.; Spencer, R.F. Transneuronal transport of peroxidase-conjugated wheat germ agglutinin (WGA-HRP) from the olfactory epithelium to the brain of the adult rat. Exp. Brain Res. 1986, 63, 461–473. [Google Scholar] [CrossRef]
- Doty, R.L. The olfactory vector hypothesis of neurodegenerative disease: Is it viable? Ann. Neurol. 2008, 63, 7–15. [Google Scholar] [CrossRef]
- De Lorenzo, A.J. Electron microscopy of the olfactory and gustatory pathways. Ann. Oto. Rhinol. Laryn. 1960, 69, 410–420. [Google Scholar]
- Morrison, E.E.; Costanzo, R.M. Morphology of olfactory epithelium in humans and other vertebrates. Microsc. Res. Tech. 1992, 23, 49–61. [Google Scholar] [CrossRef]
- Mistry, A.; Stolnik, S.; Illum, L. Nanoparticles for direct nose-to-brain delivery of drugs. Int. J. Pharm. 2009, 379, 146–157. [Google Scholar] [CrossRef]
- Wang, F.; Jiang, X.; Lu, W. Profiles of methotrexate in blood and CSF following intranasal and intravenous administration to rats. Int. J. Pharm. 2003, 263, 1–7. [Google Scholar] [CrossRef]
- Shingaki, T.; Inoue, D.; Furubayashi, T.; Sakane, T.; Katsumi, H.; Yamamoto, A.; Yamashita, S. Transnasal delivery of methotrexate to brain tumors in rats: A new strategy for brain tumor chemotherapy. Mol. Pharm. 2010, 7, 1561–1568. [Google Scholar] [CrossRef]
- Shingaki, T.; Hidalgo, I.J.; Furubayashi, T.; Katsumi, H.; Sakane, T.; Yamamoto, A.; Yamashita, S. The transnasal delivery of 5-fluorouracil to the rat brain is enhanced by acetazolamide (the inhibitor of the secretion of cerebrospinal fluid). Int. J. Pharm. 2009, 377, 85–91. [Google Scholar] [CrossRef]
- Hashizume, R.; Ozawa, T.; Gryaznov, S.M.; Bollen, A.W.; Lamborn, K.R.; Frey, W.H., 2nd; Deen, D.F. New therapeutic approach for brain tumors: Intranasal delivery of telomerase inhibitor GRN163. Neuro-oncology 2008, 10, 112–120. [Google Scholar] [CrossRef]
- Ozduman, K.; Wollmann, G.; Piepmeier, J.M.; van den Pol, A.N. Systemic vesicular stomatitis virus selectively destroys multifocal glioma and metastatic carcinoma in brain. J. Neurosci. 2008, 28, 1882–1893. [Google Scholar] [CrossRef]
- Reitz, M.; Demestre, M.; Sedlacik, J.; Meissner, H.; Fiehler, J.; Kim, S.U.; Westphal, M.; Schmidt, N.O. Intranasal delivery of neural stem/progenitor cells: A noninvasive passage to target intracerebral glioma. Stem Cells Transl. Med. 2012, 1, 866–873. [Google Scholar] [CrossRef]
- Da Fonseca, C.O.; Simao, M.; Lins, I.R.; Caetano, R.O.; Futuro, D.; Quirico-Santos, T. Efficacy of monoterpene perillyl alcohol upon survival rate of patients with recurrent glioblastoma. J. Cancer Res. Clin. Oncol. 2011, 137, 287–293. [Google Scholar] [CrossRef]
- Rall, D.P. Conference on obstacles to the control of acute leukemia. Experimental studies of the blood-brain barrier. Cancer Res. 1965, 25, 1572–1577. [Google Scholar]
- Poortmans, P.M.; Kluin-Nelemans, H.C.; Haaxma-Reiche, H.; van’t Veer, M.; Hansen, M.; Soubeyran, P.; Taphoorn, M.; Thomas, J.; van den Bent, M.; Fickers, M.; et al. High-dose methotrexate-based chemotherapy followed by consolidating radiotherapy in non-AIDS-related primary central nervous system lymphoma: European Organization for Research and Treatment of Cancer Lymphoma Group Phase II Trial 20962. J. Clin. Oncol. 2003, 21, 4483–4488. [Google Scholar] [CrossRef]
- Sakane, T.; Yamashita, S.; Yata, N.; Sezaki, H. Transnasal delivery of 5-fluorouracil to the brain in the rat. J. Drug Target. 1999, 7, 233–240. [Google Scholar] [CrossRef]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Wang, D.; Gao, Y.; Yun, L. Study on brain targeting of raltitrexed following intranasal administration in rats. Cancer Chemother. Pharmacol. 2006, 57, 97–104. [Google Scholar] [CrossRef]
- Le, S.; Zhu, J.J.; Anthony, D.C.; Greider, C.W.; Black, P.M. Telomerase activity in human gliomas. Neurosurgery 1998, 42, 1120–1125. [Google Scholar] [CrossRef]
- Ozawa, T.; Gryaznov, S.M.; Hu, L.J.; Pongracz, K.; Santos, R.A.; Bollen, A.W.; Lamborn, K.R.; Deen, D.F. Antitumor effects of specific telomerase inhibitor GRN163 in human glioblastoma xenografts. Neuro-oncology 2004, 6, 218–226. [Google Scholar] [CrossRef]
- Rainov, N.G.; Ren, H. Oncolytic viruses for treatment of malignant brain tumours. Acta Neurochir. Suppl. 2003, 88, 113–123. [Google Scholar]
- Aboody, K.S.; Najbauer, J.; Danks, M.K. Stem and progenitor cell-mediated tumor selective gene therapy. Gene Ther. 2008, 15, 739–752. [Google Scholar] [CrossRef]
- Da Fonseca, C.O.; Schwartsmann, G.; Fischer, J.; Nagel, J.; Futuro, D.; Quirico-Santos, T.; Gattass, C.R. Preliminary results from a phase I/II study of perillyl alcohol intranasal administration in adults with recurrent malignant gliomas. Surg. Neurol. 2008, 70, 259–267. [Google Scholar] [CrossRef]
- Da Fonseca, C.O.; Landeiro, J.A.; Clark, S.S.; Quirico-Santos, T.; da Costa Carvalho Mda, G.; Gattass, C.R. Recent advances in the molecular genetics of malignant gliomas disclose targets for antitumor agent perillyl alcohol. Surg. Neurol. 2006, 65, S1:2–S1:9. [Google Scholar]
- Sarkar, M.A. Drug metabolism in the nasal mucosa. Pharm.Res. 1992, 9, 1–9. [Google Scholar] [CrossRef]
- Bernkop-Schnurch, A.; Dunnhaupt, S. Chitosan-based drug delivery systems. Eur. J. Pharm. Biopharm. 2012, 81, 463–469. [Google Scholar] [CrossRef]
- Garcia-Fuentes, M.; Alonso, M.J. Chitosan-based drug nanocarriers: Where do we stand? J. Control. Release 2012, 161, 496–504. [Google Scholar] [CrossRef]
- Kang, M.L.; Cho, C.S.; Yoo, H.S. Application of chitosan microspheres for nasal delivery of vaccines. Biotechnol. Adv. 2009, 27, 857–865. [Google Scholar] [CrossRef]
- Soane, R.J.; Frier, M.; Perkins, A.C.; Jones, N.S.; Davis, S.S.; Illum, L. Evaluation of the clearance characteristics of bioadhesive systems in humans. Int. J. Pharm. 1999, 178, 55–65. [Google Scholar] [CrossRef]
- Soane, R.J.; Hinchcliffe, M.; Davis, S.S.; Illum, L. Clearance characteristics of chitosan based formulations in the sheep nasal cavity. Int. J. Pharm. 2001, 217, 183–191. [Google Scholar] [CrossRef]
- Illum, L. Chitosan and its use as a pharmaceutical excipient. Pharm. Res. 1998, 15, 1326–1331. [Google Scholar] [CrossRef]
- Amidi, M.; Mastrobattista, E.; Jiskoot, W.; Hennink, W.E. Chitosan-based delivery systems for protein therapeutics and antigens. Adv. Drug Deliver. Rev. 2010, 62, 59–82. [Google Scholar] [CrossRef]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J. Control. Release 2004, 100, 5–28. [Google Scholar] [CrossRef]
- Wang, X.; Chi, N.; Tang, X. Preparation of estradiol chitosan nanoparticles for improving nasal absorption and brain targeting. Eur. J. Pharm. Biopharm. 2008, 70, 735–740. [Google Scholar] [CrossRef]
- Al-Ghananeem, A.M.; Saeed, H.; Florence, R.; Yokel, R.A.; Malkawi, A.H. Intranasal drug delivery of didanosine-loaded chitosan nanoparticles for brain targeting; an attractive route against infections caused by AIDS viruses. J. Drug Target. 2010, 18, 381–388. [Google Scholar] [CrossRef]
- Alam, S.; Khan, Z.I.; Mustafa, G.; Kumar, M.; Islam, F.; Bhatnagar, A.; Ahmad, F.J. Development and evaluation of thymoquinone-encapsulated chitosan nanoparticles for nose-to-brain targeting: A pharmacoscintigraphic study. Int. J. Nanomedicine 2012, 7, 5705–5718. [Google Scholar]
- Fazil, M.; Md, S.; Haque, S.; Kumar, M.; Baboota, S.; Sahni, J.K.; Ali, J. Development and evaluation of rivastigmine loaded chitosan nanoparticles for brain targeting. Eur. J. Pharm. Sci. 2012, 47, 6–15. [Google Scholar] [CrossRef]
- Md, S.; Khan, R.A.; Mustafa, G.; Chuttani, K.; Baboota, S.; Sahni, J.K.; Ali, J. Bromocriptine loaded chitosan nanoparticles intended for direct nose to brain delivery: Pharmacodynamic, pharmacokinetic and scintigraphy study in mice model. Eur. J. Pharm. Sci. 2012, 48, 393–405. [Google Scholar]
- Mistry, A.; Glud, S.Z.; Kjems, J.; Randel, J.; Howard, K.A.; Stolnik, S.; Illum, L. Effect of physicochemical properties on intranasal nanoparticle transit into murine olfactory epithelium. J. Drug Target. 2009, 17, 543–552. [Google Scholar] [CrossRef]
- Charlton, S.; Jones, N.S.; Davis, S.S.; Illum, L. Distribution and clearance of bioadhesive formulations from the olfactory region in man: Effect of polymer type and nasal delivery device. Eur. J. Pharm. Sci. 2007, 30, 295–302. [Google Scholar] [CrossRef]
- Perez, A.P.; Mundina-Weilenmann, C.; Romero, E.L.; Morilla, M.J. Increased brain radioactivity by intranasal P-labeled siRNA dendriplexes within in situ-forming mucoadhesive gels. Int. J. Nanomedicine 2012, 7, 1373–1385. [Google Scholar]
- Khan, S.; Patil, K.; Bobade, N.; Yeole, P.; Gaikwad, R. Formulation of intranasal mucoadhesive temperature-mediated in situ gel containing ropinirole and evaluation of brain targeting efficiency in rats. J. Drug Target. 2010, 18, 223–234. [Google Scholar] [CrossRef]
- Betbeder, D.; Sperandio, S.; Latapie, J.P.; de Nadai, J.; Etienne, A.; Zajac, J.M.; Frances, B. Biovector nanoparticles improve antinociceptive efficacy of nasal morphine. Pharm. Res. 2000, 17, 743–748. [Google Scholar] [CrossRef]
- Lai, S.K.; O’Hanlon, D.E.; Harrold, S.; Man, S.T.; Wang, Y.Y.; Cone, R.; Hanes, J. Rapid transport of large polymeric nanoparticles in fresh undiluted human mucus. Proc. Natl. Acad. Sci. USA 2007, 104, 1482–1487. [Google Scholar]
- Zhang, Q.Z.; Zha, L.S.; Zhang, Y.; Jiang, W.M.; Lu, W.; Shi, Z.Q.; Jiang, X.G.; Fu, S.K. The brain targeting efficiency following nasally applied MPEG-PLA nanoparticles in rats. J. Drug Target. 2006, 14, 281–290. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Lai, S.K.; Suk, J.S.; Pace, A.; Cone, R.; Hanes, J. Addressing the PEG mucoadhesivity paradox to engineer nanoparticles that “slip” through the human mucus barrier. Angew. Chem. Int. Ed. Engl. 2008, 47, 9726–9729. [Google Scholar] [CrossRef]
- Jain, R.; Nabar, S.; Dandekar, P.; Patravale, V. Micellar nanocarriers: Potential nose-to-brain delivery of zolmitriptan as novel migraine therapy. Pharm. Res. 2010, 27, 655–664. [Google Scholar] [CrossRef]
- Gao, X.; Tao, W.; Lu, W.; Zhang, Q.; Zhang, Y.; Jiang, X.; Fu, S. Lectin-conjugated PEG-PLA nanoparticles: Preparation and brain delivery after intranasal administration. Biomaterials 2006, 27, 3482–3490. [Google Scholar] [CrossRef]
- Gao, X.; Wu, B.; Zhang, Q.; Chen, J.; Zhu, J.; Zhang, W.; Rong, Z.; Chen, H.; Jiang, X. Brain delivery of vasoactive intestinal peptide enhanced with the nanoparticles conjugated with wheat germ agglutinin following intranasal administration. J. Control. Release 2007, 121, 156–167. [Google Scholar] [CrossRef]
- Liu, Q.; Shao, X.; Chen, J.; Shen, Y.; Feng, C.; Gao, X.; Zhao, Y.; Li, J.; Zhang, Q.; Jiang, X. In vivo toxicity and immunogenicity of wheat germ agglutinin conjugated poly(ethylene glycol)-poly(lactic acid) nanoparticles for intranasal delivery to the brain. Toxicol. Appl. Pharmacol. 2011, 251, 79–84. [Google Scholar] [CrossRef]
- Gao, X.; Chen, J.; Tao, W.; Zhu, J.; Zhang, Q.; Chen, H.; Jiang, X. UEA I-bearing nanoparticles for brain delivery following intranasal administration. Int. J. Pharm. 2007, 340, 207–215. [Google Scholar] [CrossRef]
- Park, Y.J.; Chang, L.C.; Liang, J.F.; Moon, C.; Chung, C.P.; Yang, V.C. Nontoxic membrane translocation peptide from protamine, low molecular weight protamine (LMWP), for enhanced intracellular protein delivery: In vitro and in vivo study. FASEB J. 2005, 19, 1555–1557. [Google Scholar]
- Liu, Z.; Jiang, M.; Kang, T.; Miao, D.; Gu, G.; Song, Q.; Yao, L.; Hu, Q.; Tu, Y.; Pang, Z.; et al. Lactoferrin-modified PEG-co-PCL nanoparticles for enhanced brain delivery of NAP peptide following intranasal administration. Biomaterials 2013, 34, 3870–3881. [Google Scholar] [CrossRef]
- Jayachandra Babu, R.; Dayal, P.P.; Pawar, K.; Singh, M. Nose-to-brain transport of melatonin from polymer gel suspensions: A microdialysis study in rats. J. Drug Target. 2011, 19, 731–740. [Google Scholar] [CrossRef]
- Seju, U.; Kumar, A.; Sawant, K.K. Development and evaluation of olanzapine-loaded PLGA nanoparticles for nose-to-brain delivery: In vitro and in vivo studies. Acta Biomater. 2011, 7, 4169–4176. [Google Scholar] [CrossRef]
- Md, S.; Ali, M.; Baboota, S.; Sahni, J.K.; Bhatnagar, A.; Ali, J. Preparation, characterization, in vivo biodistribution and pharmacokinetic studies of donepezil-loaded PLGA nanoparticles for brain targeting. Drug Dev. Ind. Pharm. 2013. [Google Scholar] [CrossRef]
- Kim, I.D.; Shin, J.H.; Kim, S.W.; Choi, S.; Ahn, J.; Han, P.L.; Park, J.S.; Lee, J.K. Intranasal delivery of HMGB1 siRNA confers target gene knockdown and robust neuroprotection in the postischemic brain. Mol. Ther. 2012, 20, 829–839. [Google Scholar] [CrossRef]
- Zaki, N.M.; Awad, G.A.; Mortada, N.D.; Abd Elhady, S.S. Enhanced bioavailability of metoclopramide HCl by intranasal administration of a mucoadhesive in situ gel with modulated rheological and mucociliary transport properties. Eur. J. Pharm. Sci. 2007, 32, 296–307. [Google Scholar] [CrossRef]
- Kumar, M.; Misra, A.; Babbar, A.K.; Mishra, A.K.; Mishra, P.; Pathak, K. Intranasal nanoemulsion based brain targeting drug delivery system of risperidone. Int. J. Pharm. 2008, 358, 285–291. [Google Scholar] [CrossRef]
- Kumar, M.; Misra, A.; Mishra, A.K.; Mishra, P.; Pathak, K. Mucoadhesive nanoemulsion-based intranasal drug delivery system of olanzapine for brain targeting. J. Drug Target. 2008, 16, 806–814. [Google Scholar] [CrossRef]
- Jogani, V.V.; Shah, P.J.; Mishra, P.; Mishra, A.K.; Misra, A.R. Intranasal mucoadhesive microemulsion of tacrine to improve brain targeting. Alzheimer Dis. Assoc. Disorder 2008, 22, 116–124. [Google Scholar] [CrossRef]
- Eskandari, S.; Varshosaz, J.; Minaiyan, M.; Tabbakhian, M. Brain delivery of valproic acid via intranasal administration of nanostructured lipid carriers: In vivo pharmacodynamic studies using rat electroshock model. Int. J. Nanomedicine 2011, 6, 363–371. [Google Scholar]
- Joshi, A.S.; Patel, H.S.; Belgamwar, V.S.; Agrawal, A.; Tekade, A.R. Solid lipid nanoparticles of ondansetron HCl for intranasal delivery: Development, optimization and evaluation. J. Mater. Sci. Mater. Med. 2012, 23, 2163–2175. [Google Scholar] [CrossRef]
- Salama, H.A.; Mahmoud, A.A.; Kamel, A.O.; Abdel Hady, M.; Awad, G.A. Phospholipid based colloidal poloxamer-nanocubic vesicles for brain targeting via the nasal route. Colloids Surf. B Biointerfaces 2012, 100, 146–154. [Google Scholar] [CrossRef]
- Arumugam, K.; Subramanian, G.S.; Mallayasamy, S.R.; Averineni, R.K.; Reddy, M.S.; Udupa, N. A study of rivastigmine liposomes for delivery into the brain through intranasal route. Acta Pharm. 2008, 58, 287–297. [Google Scholar]
- Migliore, M.M.; Vyas, T.K.; Campbell, R.B.; Amiji, M.M.; Waszczak, B.L. Brain delivery of proteins by the intranasal route of administration: A comparison of cationic liposomes versus aqueous solution formulations. J. Pharm. Sci. 2010, 99, 1745–1761. [Google Scholar]
- Priprem, A.; Watanatorn, J.; Sutthiparinyanont, S.; Phachonpai, W.; Muchimapura, S. Anxiety and cognitive effects of quercetin liposomes in rats. Nanomedicine 2008, 4, 70–78. [Google Scholar] [CrossRef]
- Yang, Z.Z.; Zhang, Y.Q.; Wang, Z.Z.; Wu, K.; Lou, J.N.; Qi, X.R. Enhanced brain distribution and pharmacodynamics of rivastigmine by liposomes following intranasal administration. Int. J. Pharm. 2013, 452, 344–354. [Google Scholar] [CrossRef]
- Kolsch, H.; Rao, M.L. Neuroprotective effects of estradiol-17beta: Implications for psychiatric disorders. Arch. Womens Ment. Health 2002, 5, 105–110. [Google Scholar] [CrossRef]
- Mansour, M.A.; Nagi, M.N.; El-Khatib, A.S.; Al-Bekairi, A.M. Effects of thymoquinone on antioxidant enzyme activities, lipid peroxidation and DT-diaphorase in different tissues of mice: A possible mechanism of action. Cell Biochem. Funct. 2002, 20, 143–151. [Google Scholar] [CrossRef]
- Lim, J.H.; Kim, S.S.; Boo, D.H.; No, H.; Kang, B.Y.; Kim, E.M.; Hwang, O.; Choi, H.J. Protective effect of bromocriptine against BH4-induced Cath.a cell death involving up-regulation of antioxidant enzymes. Neurosci. Lett. 2009, 451, 185–189. [Google Scholar] [CrossRef]
- Tafaghodi, M.; Abolghasem Sajadi Tabassi, S.; Jaafari, M.R.; Zakavi, S.R.; Momen-Nejad, M. Evaluation of the clearance characteristics of various microspheres in the human nose by gamma-scintigraphy. Int. J. Pharm. 2004, 280, 125–135. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Mattiuz, E.; Franklin, R.; Gillespie, T.; Murphy, A.; Bernstein, J.; Chiu, A.; Hotten, T.; Kassahun, K. Disposition and metabolism of olanzapine in mice, dogs, and rhesus monkeys. Drug Metab. Dispos. 1997, 25, 573–583. [Google Scholar]
- Berger, G.; Kogan, T.; Skutelsky, E.; Ophir, D. Glycoconjugate expression in normal human inferior turbinate mucosa: A lectin histochemical study. Am. J. Rhinol. 2005, 19, 97–103. [Google Scholar]
- Lundh, B.; Brockstedt, U.; Kristensson, K. Lectin-binding pattern of neuroepithelial and respiratory epithelial cells in the mouse nasal cavity. Histochem. J. 1989, 21, 33–43. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, C.; Liu, Q.; Shao, X.; Feng, C.; Shen, Y.; Zhang, Q.; Jiang, X. Solanum tuberosum lectin-conjugated PLGA nanoparticles for nose-to-brain delivery: In vivo and in vitro evaluations. J. Drug Target. 2012, 20, 174–184. [Google Scholar] [CrossRef]
- Elfinger, M.; Maucksch, C.; Rudolph, C. Characterization of lactoferrin as a targeting ligand for nonviral gene delivery to airway epithelial cells. Biomaterials 2007, 28, 3448–3455. [Google Scholar] [CrossRef]
- Jobling, M.G.; Holmes, R.K. Mutational analysis of ganglioside GM(1)-binding ability, pentamer formation, and epitopes of cholera toxin B (CTB) subunits and CTB/heat-labile enterotoxin B subunit chimeras. Infect. Immun. 2002, 70, 1260–1271. [Google Scholar] [CrossRef]
- Wan, X.M.; Chen, Y.P.; Xu, W.R.; Yang, W.J.; Wen, L.P. Identification of nose-to-brain homing peptide through phage display. Peptides 2009, 30, 343–350. [Google Scholar] [CrossRef]
- Charlton, S.T.; Davis, S.S.; Illum, L. Evaluation of bioadhesive polymers as delivery systems for nose to brain delivery: In vitro characterisation studies. J. Control. Release 2007, 118, 225–234. [Google Scholar] [CrossRef]
- Graff, C.L.; Pollack, G.M. P-Glycoprotein attenuates brain uptake of substrates after nasal instillation. Pharm. Res. 2003, 20, 1225–1230. [Google Scholar] [CrossRef]
- Charlton, S.T.; Davis, S.S.; Illum, L. Evaluation of effect of ephedrine on the transport of drugs from the nasal cavity to the systemic circulation and the central nervous system. J. Drug Target. 2007, 15, 370–377. [Google Scholar] [CrossRef]
- Dhuria, S.V.; Hanson, L.R.; Frey, W.H., 2nd. Novel vasoconstrictor formulation to enhance intranasal targeting of neuropeptide therapeutics to the central nervous system. J. Pharmacol. Exp. Ther. 2009, 328, 312–320. [Google Scholar] [CrossRef]
- Celebisoy, N.; Gokcay, F.; Sirin, H.; Akyurekli, O. Treatment of idiopathic intracranial hypertension: Topiramate vs. acetazolamide, an open-label study. Acta Neurol. Scand. 2007, 116, 322–327. [Google Scholar] [CrossRef]
- Kosfeld, M.; Heinrichs, M.; Zak, P.J.; Fischbacher, U.; Fehr, E. Oxytocin increases trust in humans. Nature 2005, 435, 673–676. [Google Scholar] [CrossRef]
- Craft, S.; Baker, L.D.; Montine, T.J.; Minoshima, S.; Watson, G.S.; Claxton, A.; Arbuckle, M.; Callaghan, M.; Tsai, E.; Plymate, S.R.; et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: A pilot clinical trial. Arch. Neurol. 2012, 69, 29–38. [Google Scholar] [CrossRef]
- Djupesland, P.G.; Skretting, A.; Winderen, M.; Holand, T. Breath actuated device improves delivery to target sites beyond the nasal valve. Laryngoscope 2006, 116, 466–472. [Google Scholar] [CrossRef]
- Djupesland, P.G. Nasal drug delivery devices: Characteristics and performance in a clinical perspective―A review. Drug Deliv.Transl. Res. 2013, 3, 42–62. [Google Scholar] [CrossRef]
- Hardy, J.G.; Lee, S.W.; Wilson, C.G. Intranasal drug delivery by spray and drops. J. Pharm. Pharmacol. 1985, 37, 294–297. [Google Scholar] [CrossRef]
- Van den Berg, M.P.; Romeijn, S.G.; Verhoef, J.C.; Merkus, F.W. Serial cerebrospinal fluid sampling in a rat model to study drug uptake from the nasal cavity. J. Neurosci. Methods 2002, 116, 99–107. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Van Woensel, M.; Wauthoz, N.; Rosière, R.; Amighi, K.; Mathieu, V.; Lefranc, F.; Van Gool, S.W.; De Vleeschouwer, S. Formulations for Intranasal Delivery of Pharmacological Agents to Combat Brain Disease: A New Opportunity to Tackle GBM? Cancers 2013, 5, 1020-1048. https://doi.org/10.3390/cancers5031020
Van Woensel M, Wauthoz N, Rosière R, Amighi K, Mathieu V, Lefranc F, Van Gool SW, De Vleeschouwer S. Formulations for Intranasal Delivery of Pharmacological Agents to Combat Brain Disease: A New Opportunity to Tackle GBM? Cancers. 2013; 5(3):1020-1048. https://doi.org/10.3390/cancers5031020
Chicago/Turabian StyleVan Woensel, Matthias, Nathalie Wauthoz, Rémi Rosière, Karim Amighi, Véronique Mathieu, Florence Lefranc, Stefaan W. Van Gool, and Steven De Vleeschouwer. 2013. "Formulations for Intranasal Delivery of Pharmacological Agents to Combat Brain Disease: A New Opportunity to Tackle GBM?" Cancers 5, no. 3: 1020-1048. https://doi.org/10.3390/cancers5031020
APA StyleVan Woensel, M., Wauthoz, N., Rosière, R., Amighi, K., Mathieu, V., Lefranc, F., Van Gool, S. W., & De Vleeschouwer, S. (2013). Formulations for Intranasal Delivery of Pharmacological Agents to Combat Brain Disease: A New Opportunity to Tackle GBM? Cancers, 5(3), 1020-1048. https://doi.org/10.3390/cancers5031020






