Reduced Contractility and Motility of Prostatic Cancer-Associated Fibroblasts after Inhibition of Heat Shock Protein 90
Abstract
:1. Introduction
2. Results
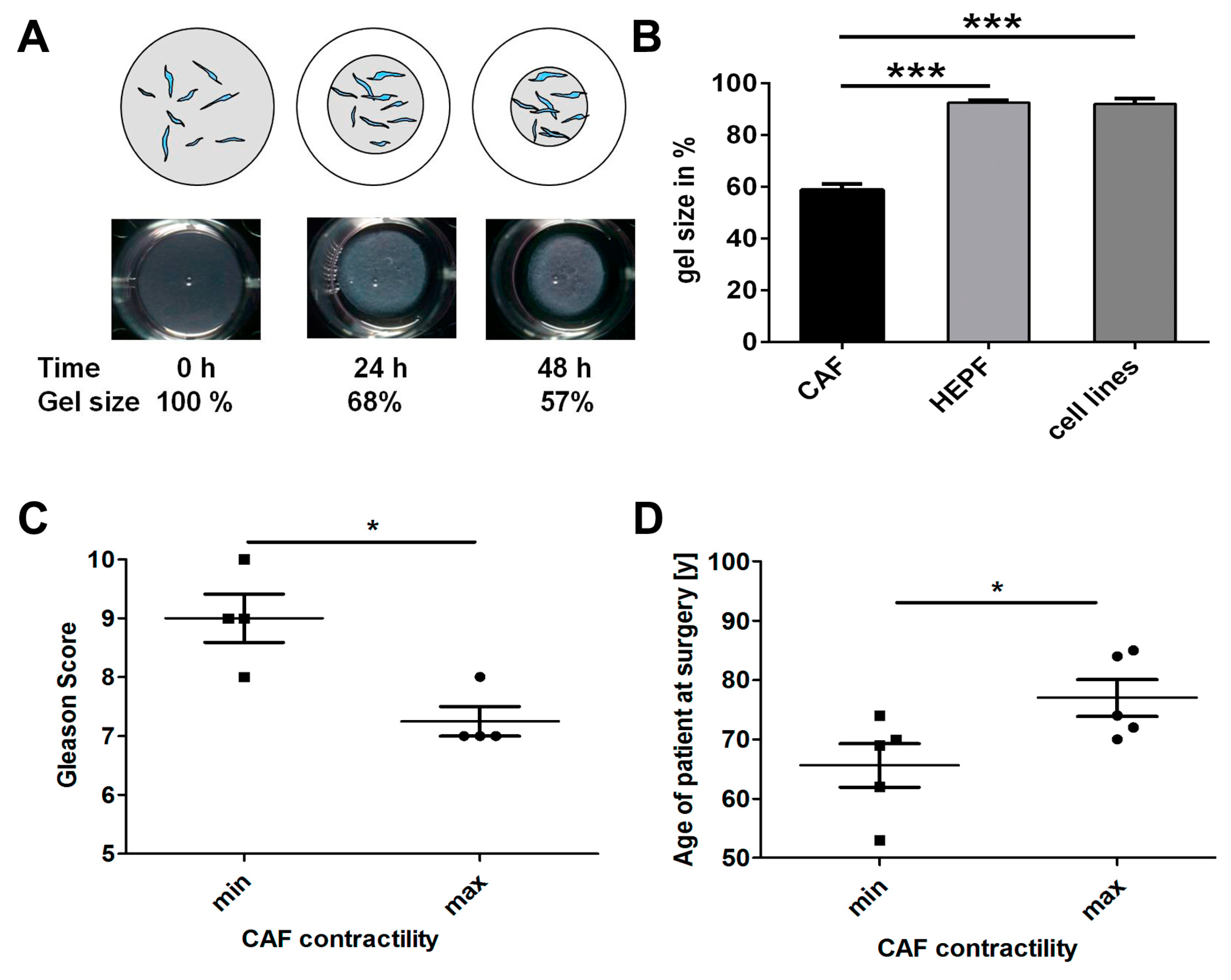
2.1. CAF Isolation
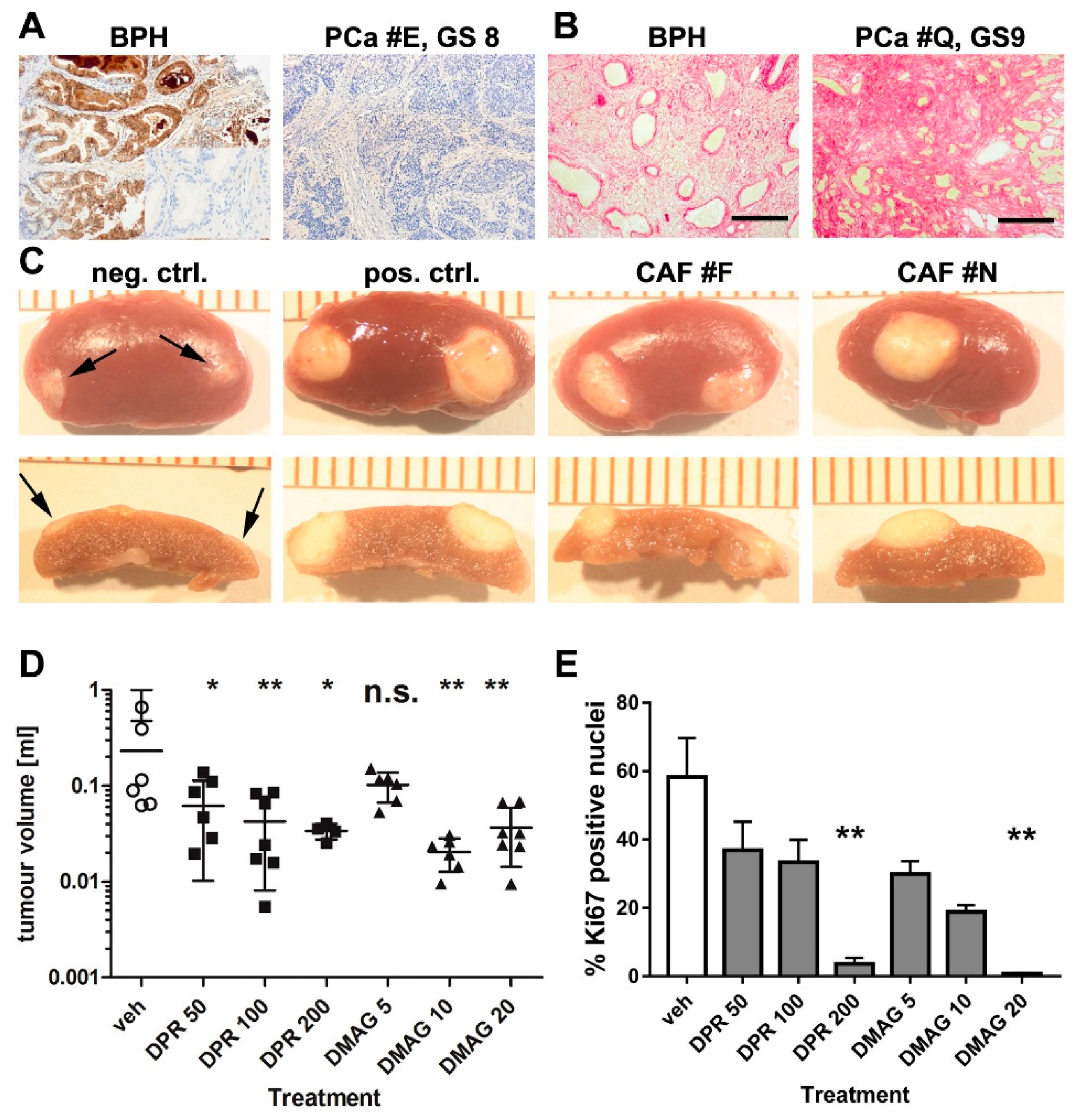
2.2. The Effects of HSP90 Inhibitors upon CAF-Induced Tumourigenesis in Vivo
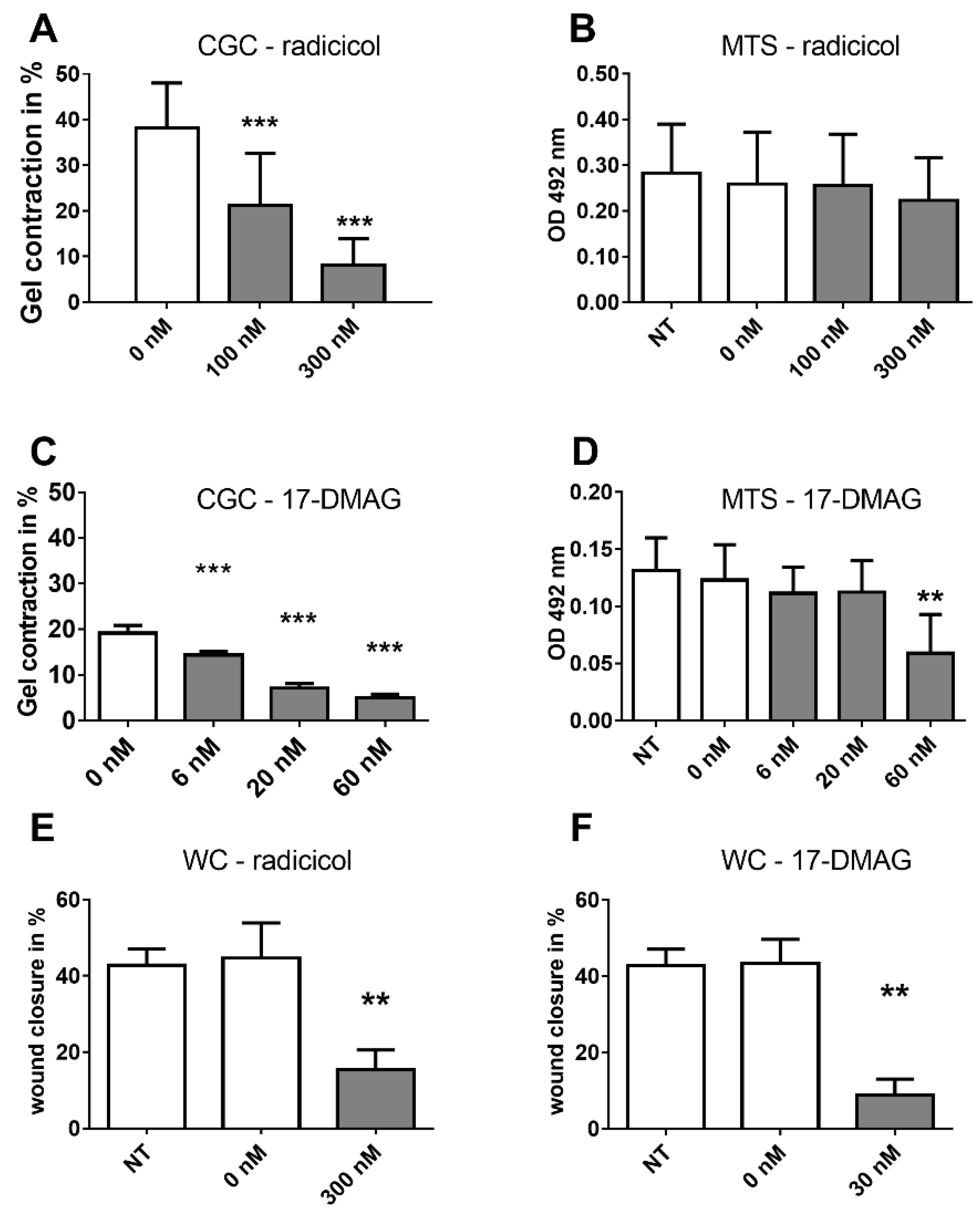
2.3. Effects of HSP90 Inhibitors Upon CAF Contractility in Vitro
2.4. CAF Contractility and Patient Parameters
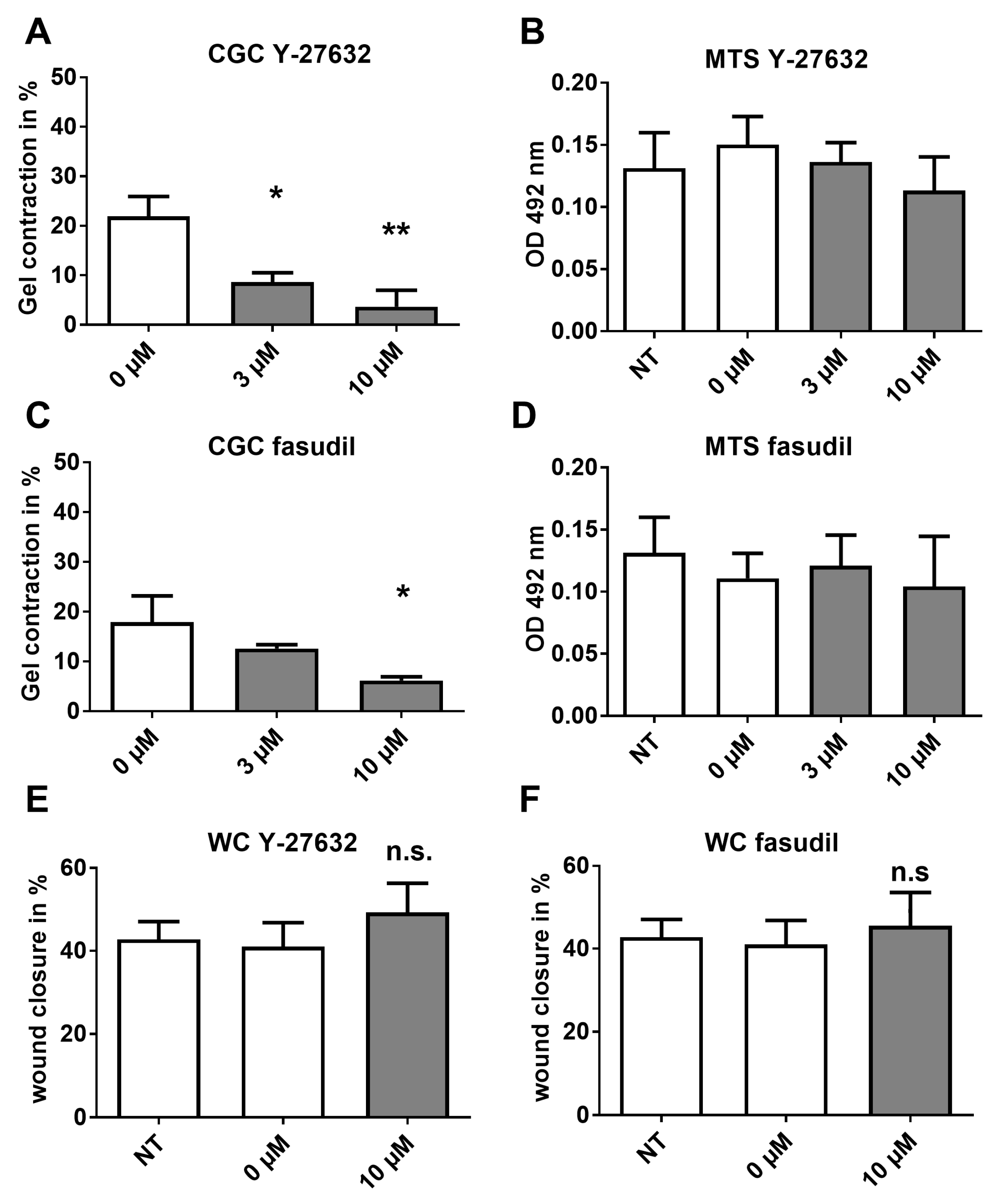
2.5. Analysis of HSP Inhibitors upon Contracility and Potential Cytotoxicity
2.6. Effects of HSP90 Inhibitors upon Motility
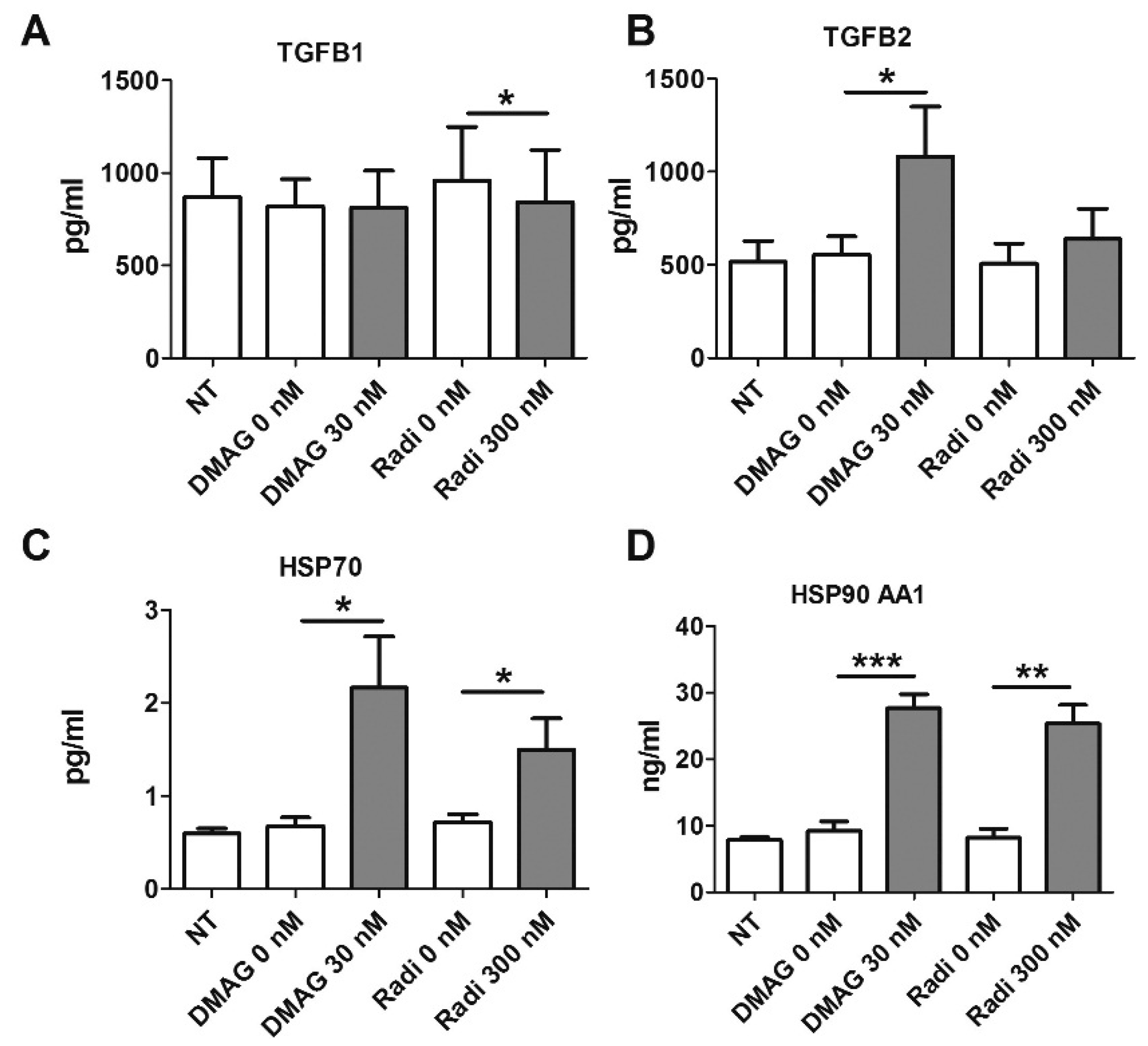
2.7. HSP90 Inhibitors and Secretion of TGFβ
2.8. HSP Levels Following Treatment with HSP90 Inhibitors
2.9. Effects of HSP90 Inhibitors upon ROCK in Vitro
3. Discussion
4. Materials and Methods
4.1. Tissue and Cells
4.2. Chemicals
4.3. Cell Culture Assays
4.4. Multiplex Assays
4.5. In Vivo Studies
4.6. Immunohistochemistry and Staining of Tissue Sections
4.7. Statistics
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CAF | Cancer associated fibroblastCGC: Collagen gel contraction |
| GS | Gleason Score |
| HSP | Heat shock protein |
| PCa | Prostate cancer |
| TME | Tumour microenvironment |
| WC | Wound closure |
References
- Olumi, A.F.; Grossfeld, G.D.; Hayward, S.W.; Carroll, P.R.; Tlsty, T.D.; Cunha, G.R. Carcinoma-associated fibroblasts direct tumour progression of initiated human prostatic epithelium. Cancer Res. 1999, 59, 5002–5011. [Google Scholar] [PubMed]
- Orimo, A.; Gupta, P.B.; Sgroi, D.C.; Arenzana-Seisdedos, F.; Delaunay, T.; Naeem, R.; Carey, V.J.; Richardson, A.L.; Weinberg, R.A. Stromal fibroblasts present in invasive human breast carcinomas promote tumour growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 2005, 121, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Ao, M.; Franco, O.E.; Park, D.; Raman, D.; Williams, K.; Hayward, S.W. Cross-talk between Paracrine-acting cytokine and chemokine pathways promotes malignancy in benign human prostatic epithelium. Cancer Res. 2007, 67, 4244–4253. [Google Scholar] [CrossRef] [PubMed]
- Franco, O.E.; Jiang, M.; Strand, D.W.; Peacock, J.; Fernandez, S.; Jackson, R.S., 2nd; Revelo, M.P.; Bhowmick, N.A.; Hayward, S.W. Altered TGF-β signaling in a subpopulation of human stromal cells promotes prostatic carcinogenesis. Cancer Res. 2011, 71, 1272–1281. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Zeisberg, M. Fibroblasts in cancer. Nat. Rev. Cancer 2006, 6, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Levental, K.R.; Yu, H.; Kass, L.; Lakins, J.N.; Egeblad, M.; Erler, J.T.; Fong, S.F.; Csiszar, K.; Giaccia, A.; Weninger, W.; et al. Matrix crosslinking forces tumour progression by enhancing integrin signaling. Cell 2009, 139, 891–906. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.W.; Hooper, S.; Crighton, D.; Li, A.; Konig, I.; Munro, J.; Trivier, E.; Wickman, G.; Morin, P.; Croft, D.R.; et al. LIM kinases are required for invasive path generation by tumour and tumour-associated stromal cells. J. Cell Biol. 2010, 191, 169–185. [Google Scholar] [CrossRef] [PubMed]
- Gaggioli, C.; Hooper, S.; Hidalgo-Carcedo, C.; Grosse, R.; Marshall, J.F.; Harrington, K.; Sahai, E. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat. Cell Biol. 2007, 9, 1392–1400. [Google Scholar] [CrossRef] [PubMed]
- Samuel, M.S.; Lopez, J.I.; McGhee, E.J.; Croft, D.R.; Strachan, D.; Timpson, P.; Munro, J.; Schroder, E.; Zhou, J.; Brunton, V.G.; et al. Actomyosin-mediated cellular tension drives increased tissue stiffness and β-catenin activation to induce epidermal hyperplasia and tumour growth. Cancer Cell 2011, 19, 776–791. [Google Scholar] [CrossRef] [PubMed]
- Sluka, P.; Davis, I.D. Cell mates: Paracrine and stromal targets for prostate cancer therapy. Nat. Rev. Urol. 2013, 10, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Campbell, I.; Polyak, K.; Haviv, I. Clonal mutations in the cancer-associated fibroblasts: The case against genetic coevolution. Cancer Res. 2009, 69, 6765–6768. [Google Scholar] [CrossRef] [PubMed]
- Franco, O.E.; Hayward, S.W. Targeting the tumour stroma as a novel therapeutic approach for prostate cancer. Adv. Pharmacol. 2012, 65, 267–313. [Google Scholar] [PubMed]
- Barrott, J.J.; Haystead, T.A. HSP90, an unlikely ally in the war on cancer. FEBS J. 2013, 280, 1381–1396. [Google Scholar] [CrossRef] [PubMed]
- Tatokoro, M.; Koga, F.; Yoshida, S.; Kihara, K. Heat Shock Protein 90 targeting therapy: State of the art and future perspective. EXCLI J. 2015, 14, 48–58. [Google Scholar] [PubMed]
- Taiyab, A.; Rao, C. HSP90 modulates actin dynamics: Inhibition of HSP90 leads to decreased cell motility and impairs invasion. Biochim. Biophys. Acta 2011, 1813, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.A.; Klein, D.; Moser, C.; Gaumann, A.; Glockzin, G.; Dahlke, M.H.; Dietmaier, W.; Bolder, U.; Schlitt, H.J.; Geissler, E.K.; et al. Inhibition of Heat Shock Protein 90 impairs epidermal growth factor-mediated signaling in gastric cancer cells and reduces tumour growth and vascularization in vivo. Mol. Cancer. Ther. 2007, 6, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Belotti, D.; Burger, A.M.; Fisher-Nielson, K.; Borsotti, P.; Riccardi, E.; Thillainathan, J.; Hollingshead, M.; Sausville, E.A.; Giavazzi, R. Antiangiogenic properties of 17-(Dimethylaminoethylamino)-17-Demethoxygeldanamycin: An orally bioavailable Heat Shock Protein 90 modulator. Clin. Cancer Res. 2004, 10, 4813–4821. [Google Scholar] [CrossRef] [PubMed]
- Radovanac, K.; Morgner, J.; Schulz, J.N.; Blumbach, K.; Patterson, C.; Geiger, T.; Mann, M.; Krieg, T.; Eckes, B.; Fassler, R.; et al. Stabilization of integrin-linked kinase by the Hsp90-CHIP axis impacts cellular force generation, migration and the fibrotic response. EMBO J. 2013, 32, 1409–1424. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Lee, J.H.; Ahn, H.M.; Song, S.Y.; Kim, Y.O.; Lew, D.H.; Yun, C.O. Heat Shock Protein 90 inhibitor decreases collagen synthesis of keloid fibroblasts and attenuates the extracellular matrix on the keloid spheroid model. Plast. Reconstr. Surg. 2015, 136, 328e–337e. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.W.; Kim, J.; Che, Z.M.; Oh, E.S.; Kim, G.; Eom, S.H.; Im, S.H.; Ha, H.H.; Chang, Y.T.; Williams, D.R.; et al. A triazine compound S06 inhibits proinvasive crosstalk between carcinoma cells and stromal fibroblasts via binding to Heat Shock Protein 90. Chem. Biol. 2011, 18, 1581–1590. [Google Scholar] [CrossRef] [PubMed]
- Hance, M.W.; Dole, K.; Gopal, U.; Bohonowych, J.E.; Jezierska-Drutel, A.; Neumann, C.A.; Liu, H.; Garraway, I.P.; Isaacs, J.S. Secreted Hsp90 is a novel regulator of the epithelial to mesenchymal transition (EMT) in prostate cancer. J. Biol. Chem. 2012, 287, 37732–37744. [Google Scholar] [CrossRef] [PubMed]
- Bohonowych, J.E.; Hance, M.W.; Nolan, K.D.; Defee, M.; Parsons, C.H.; Isaacs, J.S. Extracellular Hsp90 mediates an NF-κB dependent inflammatory stromal program: Implications for the prostate tumour microenvironment. Prostate 2014, 74, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, H.C.; Kote-Jarai, Z.; Ross-Adams, H.; Warren, A.Y.; Burge, J.; George, A.; Bancroft, E.; Jhavar, S.; Leongamornlert, D.; Tymrakiewicz, M.; et al. The rs10993994 risk allele for prostate cancer results in clinically relevant changes in microseminoprotein-β expression in tissue and urine. PLoS ONE 2010, 5, e13363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orr, B.; Grace, O.C.; Brown, P.; Riddick, A.C.; Stewart, G.D.; Franco, O.E.; Hayward, S.W.; Thomson, A.A. Reduction of pro-tumourigenic activity of human prostate cancer-associated fibroblasts using Dlk1 or SCUBE1. Dis. Model. Mech. 2013, 6, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Kiskowski, M.A.; Jackson, R.S., 2nd; Banerjee, J.; Li, X.; Kang, M.; Iturregui, J.M.; Franco, O.E.; Hayward, S.W.; Bhowmick, N.A. Role for stromal heterogeneity in prostate tumourigenesis. Cancer Res. 2011, 71, 3459–3470. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, H.; Mundel, T.M.; Kieran, M.W.; Kalluri, R. Identification of fibroblast heterogeneity in the tumour microenvironment. Cancer Biol. Ther. 2006, 5, 1640–1646. [Google Scholar] [CrossRef] [PubMed]
- Kassen, A.; Sutkowski, D.M.; Ahn, H.; Sensibar, J.A.; Kozlowski, J.M.; Lee, C. Stromal cells of the human prostate: Initial isolation and characterization. Prostate 1996, 28, 89–97. [Google Scholar] [CrossRef]
- Junqueira, L.C.; Bignolas, G.; Brentani, R.R. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem. J. 1979, 11, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Tlsty, T.D.; Coussens, L.M. Tumour stroma and regulation of cancer development. Annu. Rev. Pathol. 2006, 1, 119–150. [Google Scholar] [CrossRef] [PubMed]
- Ayala, G.; Tuxhorn, J.A.; Wheeler, T.M.; Frolov, A.; Scardino, P.T.; Ohori, M.; Wheeler, M.; Spitler, J.; Rowley, D.R. Reactive stroma as a predictor of biochemical-free recurrence in prostate cancer. Clin. Cancer Res. 2003, 9, 4792–4801. [Google Scholar] [PubMed]
- Oikawa, T.; Onozawa, C.; Kuranuki, S.; Igarashi, Y.; Sato, M.; Ashino, H.; Shimamura, M.; Toi, M.; Kurakata, S. Dipalmitoylation of radicicol results in improved efficacy against tumour growth and angiogenesis in vivo. Cancer Sci. 2007, 98, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Floris, G.; Debiec-Rychter, M.; Wozniak, A.; Stefan, C.; Normant, E.; Faa, G.; Machiels, K.; Vanleeuw, U.; Sciot, R.; Schoffski, P. The Heat Shock Protein 90 inhibitor IPI-504 induces KIT degradation, tumour shrinkage, and cell proliferation arrest in xenograft models of gastrointestinal stromal tumours. Mol. Cancer Ther. 2011, 10, 1897–1908. [Google Scholar] [CrossRef] [PubMed]
- Oh, W.K.; Galsky, M.D.; Stadler, W.M.; Srinivas, S.; Chu, F.; Bubley, G.; Goddard, J.; Dunbar, J.; Ross, R.W. Multicenter phase II trial of the Heat Shock Protein 90 inhibitor, Retaspimycin Hydrochloride (IPI-504), in patients with castration-resistant prostate cancer. Urology 2011, 78, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Vogt, J.; Traynor, R.; Sapkota, G.P. The specificities of small molecule inhibitors of the TGFss and BMP pathways. Cell Signal. 2011, 23, 1831–1842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casey, T.M.; Eneman, J.; Crocker, A.; White, J.; Tessitore, J.; Stanley, M.; Harlow, S.; Bunn, J.Y.; Weaver, D.; Muss, H.; et al. Cancer associated fibroblasts stimulated by transforming growth factor β1 (TGF-β1) increase invasion rate of tumour cells: A population study. Breast Cancer Res. Treat. 2008, 110, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Peehl, D.M.; Sellers, R.G. Induction of smooth muscle cell phenotype in cultured human prostatic stromal cells. Exp. Cell Res. 1997, 232, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Gerdes, M.J.; Larsen, M.; Dang, T.D.; Ressler, S.J.; Tuxhorn, J.A.; Rowley, D.R. Regulation of rat prostate stromal cell myodifferentiation by androgen and TGF-β1. Prostate 2004, 58, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.M.; Mukerji, R.; Samadi, A.K.; Zhang, X.; Zhao, H.; Blagg, B.S.; Cohen, M.S. Novel C-terminal Hsp90 inhibitor for Head and Neck Squamous Cell Cancer (HNSCC) with in vivo efficacy and improved toxicity profiles compared with standard agents. Ann. Surg. Oncol. 2012, 19, S483–S490. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, K.J.; Langmann, G.; Ai, J.; Ramos-Garcia, R.; Vessella, R.L.; Wang, Z. Hsp90 Inhibitor 17-AAG inhibits progression of LuCaP35 xenograft prostate tumours to castration resistance. Prostate 2012, 72, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Franco, O.E.; Shaw, A.K.; Strand, D.W.; Hayward, S.W. Cancer associated fibroblasts in cancer pathogenesis. Semin. Cell Dev. Biol. 2010, 21, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Wegiel, B.; Evans, S.; Hellsten, R.; Otterbein, L.E.; Bjartell, A.; Persson, J.L. Molecular pathways in the progression of hormone-independent and metastatic prostate cancer. Curr. Cancer Drug Targets 2010, 10, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, D.; Toh, B.H.; Liu, J.P. TGF-β and cancer: Is Smad3 a repressor of hTERT gene? Cell Res. 2006, 16, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Pruitt, F.L.; He, Y.; Franco, O.E.; Jiang, M.; Cates, J.M.; Hayward, S.W. Cathepsin D acts as an essential mediator to promote malignancy of benign prostatic epithelium. Prostate 2013, 73, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, J.; Loo, A.; Jaeger, S.; Bagdasarian, L.; Yu, J.; Chung, F.; Korn, J.; Ruddy, D.; Guo, R.; et al. Targeting HSF1 sensitizes cancer cells to HSP90 inhibition. Oncotarget 2013, 4, 816–829. [Google Scholar] [CrossRef] [PubMed]
- Taipale, M.; Krykbaeva, I.; Koeva, M.; Kayatekin, C.; Westover, K.D.; Karras, G.I.; Lindquist, S. Quantitative analysis of HSP90-client interactions reveals principles of substrate recognition. Cell 2012, 150, 987–1001. [Google Scholar] [CrossRef] [PubMed]
- Eskew, J.D.; Sadikot, T.; Morales, P.; Duren, A.; Dunwiddie, I.; Swink, M.; Zhang, X.; Hembruff, S.; Donnelly, A.; Rajewski, R.A.; et al. Development and characterization of a novel C-terminal inhibitor of Hsp90 in androgen dependent and independent prostate cancer cells. BMC Cancer 2011. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Zhang, C.; Shafi, A.A.; Sequeira, M.; Acquaviva, J.; Friedland, J.C.; Sang, J.; Smith, D.L.; Weigel, N.L.; Wada, Y.; et al. Potent activity of the Hsp90 inhibitor ganetespib in prostate cancer cells irrespective of androgen receptor status or variant receptor expression. Int. J. Oncol. 2013, 42, 35–43. [Google Scholar] [PubMed]
- Lin, Z.; Peng, R.; Li, Z.; Wang, Y.; Lu, C.; Shen, Y.; Wang, J.; Shi, G. 17-ABAG, a Novel geldanamycin derivative, inhibits LNCaP-cell proliferation through Heat Shock Protein 90 inhibition. Int. J. Mol. Med. 2015, 36, 424–432. [Google Scholar] [PubMed]
- Wang, J.; Li, Z.; Lin, Z.; Zhao, B.; Wang, Y.; Peng, R.; Wang, M.; Lu, C.; Shi, G.; Shen, Y. 17-DMCHAG, a new geldanamycin derivative, inhibits prostate cancer cells through Hsp90 inhibition and survivin downregulation. Cancer Lett. 2015, 362, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Moses, M.A.; Henry, E.C.; Ricke, W.A.; Gasiewicz, T.A. The Heat Shock Protein 90 inhibitor, (−)-epigallocatechin gallate, has anticancer activity in a novel human prostate cancer Progression model. Cancer Prev. Res. 2015, 8, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Pacey, S.; Wilson, R.H.; Walton, M.; Eatock, M.M.; Hardcastle, A.; Zetterlund, A.; Arkenau, H.T.; Moreno-Farre, J.; Banerji, U.; Roels, B.; et al. A phase I study of the Heat Shock Protein 90 inhibitor alvespimycin (17-DMAG) given intravenously to patients with advanced solid tumours. Clin. Cancer Res. 2011, 17, 1561–1570. [Google Scholar] [CrossRef] [PubMed]
- Sidera, K.; Patsavoudi, E. HSP90 inhibitors: Current development and potential in cancer therapy. Recent. Pat. Anticancer Drug Discov. 2014, 9, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, L.; Bolaender, A.; Patel, H.J.; Taldone, T. Heat Shock Protein (HSP) drug discovery and development: Targeting heat shock proteins in disease. Curr. Top. Med. Chem. 2016. [Google Scholar] [CrossRef]
- Gleason, D.F.; Mellinger, G.T. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J. Urol. 1974, 111, 58–64. [Google Scholar] [CrossRef]
- Hayward, S.W.; Dahiya, R.; Cunha, G.R.; Bartek, J.; Deshpande, N.; Narayan, P. Establishment and characterization of an immortalized but non-transformed human prostate epithelial cell line: BPH-1. In Vitro Cell Dev. Biol. Anim. 1995, 31, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Webber, M.M.; Trakul, N.; Thraves, P.S.; Bello-DeOcampo, D.; Chu, W.W.; Storto, P.D.; Huard, T.K.; Rhim, J.S.; Williams, D.E. A human prostatic stromal myofibroblast cell line WPMY-1: A model for stromal-epithelial interactions in prostatic neoplasia. Carcinogenesis 1999, 20, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Hallowes, R.C.; Bone, E.J.; Jones, W. A new dimension in the culture of human breast. In Tissue Culture in Medical Research; Richards, R.J., Rajan, K.T., Eds.; Pergamon Press: Oxford, UK, 1980; pp. 213–220. [Google Scholar]
- Abramoff, M.D.; Magalhaes, P.J.; Ram, S.J. Image processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Tuominen, V.J.; Ruotoistenmaki, S.; Viitanen, A.; Jumppanen, M.; Isola, J. ImmunoRatio: A publicly available web application for quantitative image analysis of Estrogen Receptor (ER), Progesterone Receptor (PR), and Ki-67. Breast Cancer Res. 2010. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henke, A.; Franco, O.E.; Stewart, G.D.; Riddick, A.C.P.; Katz, E.; Hayward, S.W.; Thomson, A.A. Reduced Contractility and Motility of Prostatic Cancer-Associated Fibroblasts after Inhibition of Heat Shock Protein 90. Cancers 2016, 8, 77. https://doi.org/10.3390/cancers8090077
Henke A, Franco OE, Stewart GD, Riddick ACP, Katz E, Hayward SW, Thomson AA. Reduced Contractility and Motility of Prostatic Cancer-Associated Fibroblasts after Inhibition of Heat Shock Protein 90. Cancers. 2016; 8(9):77. https://doi.org/10.3390/cancers8090077
Chicago/Turabian StyleHenke, Alex, Omar E. Franco, Grant D. Stewart, Antony C.P. Riddick, Elad Katz, Simon W. Hayward, and Axel A. Thomson. 2016. "Reduced Contractility and Motility of Prostatic Cancer-Associated Fibroblasts after Inhibition of Heat Shock Protein 90" Cancers 8, no. 9: 77. https://doi.org/10.3390/cancers8090077




