Supraphysiologic Testosterone Therapy in the Treatment of Prostate Cancer: Models, Mechanisms and Questions
Abstract
:1. Introduction
2. Physiologic Role of AR in Growth Repression
3. Oncogenic Role of AR in Prostate Cancer Progression
4. Historical Observations on Androgen Therapy of Prostate Cancer
5. Preclinical Observations on Androgen-Mediated Growth Repression of Prostate Cancer
6. Contemporary Clinical Studies of Testosterone Therapy for Prostate Cancer
6.1. Studies of Continuous Testosterone Treatment
6.2. Studies of Bipolar Androgen Therapy (BAT)
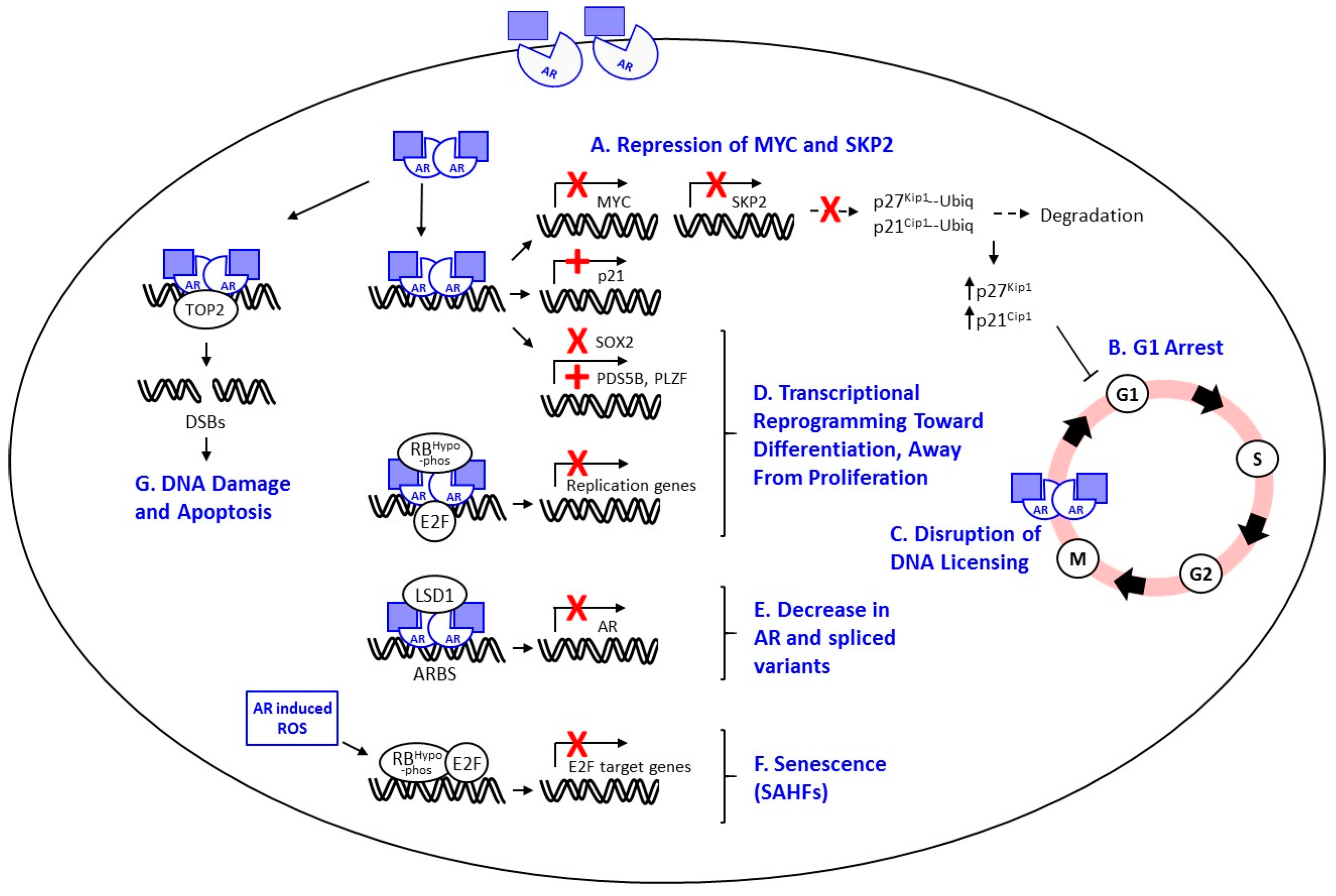
7. Proposed Mechanisms of Androgen-Mediated Growth Repression
7.1. Cell Cycle Arrest
7.2. Repression of SKP2 and MYC
7.3. Apoptosis
7.4. Disruption of AR-Mediated DNA Licensing
7.5. Transcriptional Repression of AR and AR Variants
7.6. Transcriptional Reprogramming and Differentiation
7.7. Induction of Cellular Senescence or Quiescence
7.8. Induction of DNA Damage
8. High Dose Estrogen Therapy for Breast Cancer–Clinical and Experimental Evidence
9. Potential Predictive Markers of Response to Androgen Therapy
9.1. Androgen Receptor
9.2. DNA Damage Response Genes
9.3. Steroid Metabolism and Transport Genes
10. Future Directions
11. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
Appendix A.1. Preclinical Observations on Androgen-Mediated Growth Repression of Prostate Cancer
Appendix A.1.1. LNCaPs
I. LNCaP 104-S, 104-R1, 104-R2, R1Ad, R2Ad
| Cell Line | Source | Derivation | In Vitro Growth Characteristics | In Vivo Growth Characteristics | Refs. |
|---|---|---|---|---|---|
| LNCaP | Lymph node metastasis in a 50-year-old Caucasian male with CRPC | Biphasic response in CSS (peak stimulation at 0.1 nM DHT, progressive growth suppression at 1 nM to 100 nM). Androgen repressed in 5% FBS | [78,139,140] | ||
| 104-S | LNCaP | Parental Line | Similar to original report Biphasic response in CSS (peak stimulation at 0.1 nM R1881, growth suppression at higher doses) | In vivo growth stimulated by androgens | [7,58] |
| 104-R1 | LNCaP 104-S | Passage in CSS × 10 mo | Proliferated more rapidly than 104-S cells in CSS Severely growth repressed by 0.1 nM or higher R1881 doses | In vivo growth inhibited by androgens | [7,57,58] |
| 104-R2 | LNCaP 104-S | Passage in CSS × 18 mo | |||
| R1Ad | LNCaP 104-R1 | Re-growth in castrate mice after T treatment in vivo | Lost androgen-repressed phenotype Androgen sensitive for growth | [141] | |
| R2Ad | LNCaP 104-R2 | Re-growth in castrate mice after T treatment in vivo | Lost androgen-repressed phenotype Androgen insensitive for growth-not affected by R1881 or bicalutamide | [80] | |
| MOP | LNCaP | Passage (of LNCaP passage 25 cells) in CSS × 10–12 mo | Androgen insensitive for growth Dose-dependent growth suppression in response to R1881 at 0.1 to 10 nM | In vivo growth inhibited by androgens | [75] |
| JAC | LNCaP | Passage (of LNCaP passage 55 cells) in CSS × 10–12 mo | [76] | ||
| ME | MOP | Regrowth in castrate mice after T treatment in vivo | Still showed androgen repressed growth in vitro | [76] | |
| LNCaP-abl | LNCaP | Long term passage in CSS | Biphasic response but with higher sensitivity than parental LNCaP (max proliferation at 0.001 nM R1881 vs. 0.01 nM) | [142] | |
| CWR22 | Primary PCa tumors initially injected subcutaneously into nude mice supplemented with T, then serially transplanted as cell suspension | Biphasic response to androgen, with optimal proliferation at 25 to 35 nM testosterone and growth repression at concentrations higher than 35 nM | [61,143,144] | ||
| CWR22R | CWR22 | Derived from a CWR22 tumor showing castration resistant re-growth in vivo | Not consistently stimulated by androgen Growth repressive effect left-shifted vs. parental CWR22 line, with repression induced at T levels of approximately 25 nM | [145] | |
| 22RV1 | CWR22R | Androgen-sensitive for growth without a biphasic response | [63] | ||
| ARCaP (MDA PCa 1) | Isolated from the ascites fluid of an 83-year-old Caucasian man with metastatic CRPC | Highly androgen-repressed growth (starting as low as 100 pM DHT) despite relatively low AR expression | Grew 3 times faster in castrated hosts than in intact male hosts; growth in castrated hosts was suppressed by exogenous T | [62] | |
| VCaP | From a vertebral metastatic lesion of patient with CRPC | 40% repression at 10 nM R1881. Detachment and disintegration of cells passaged in low androgen conditions (10% FBS) when treated with 1 nM T in vitro | Poor growth in intact (noncastrate) SCID mice [ 56] | [56,67,146] | |
| E006AA | From primary tumor of a 50-year-old African-American man with clinically localized PCa | Biphasic response, with proliferative response as low as 1 fM DHT and maximal proliferative at 0.1 pM DHT | [147] | ||
| MDA PCa 2b | From a bone metastasis of a patient with CRPC | Biphasic response, peak proliferation at 10 nM DHT with growth inhibitory effects at higher concentrations | Stopped growing or decreased in size after castration (response to high dose androgen not evaluated in vivo) | [59,60] | |
| MDA PCa 2b-hr | MDA PCa 2b | culture of MDA PCa 2b in CSS for 35 weeks | Biphasic response to T concentrations ranging from 0.1 ng/ml to 1000 ng/ml, with maximal proliferation 1 ng/mL T | [60] | |
| RC-77T | From primary tumor of a 63-year-old African American man with clinically localized PCa | Biphasic response, maximal growth at 0.1 nM R1881 and growth inhibition at higher doses | [148] | ||
| PC3-AR | From lumbar vertebral metastasis of a 62-year-old white man | PC3 with exogenous expression of AR | Androgen mediated growth repression at DHT 0.1 nM | In vivo growth inhibited by androgen levels presesnt in intact male mice | [129,149,150,151] |
II. MOP, JAC, ME
III. LNCaP-abl
IV. C4-2B
Appendix A.1.2. CWR22 and CWR22R
Appendix A.1.3. ARCaP
Appendix A.1.4. VCaP
Appendix A.1.5. E006AA
Appendix A.1.6. MDA PCa 2b and MDA PCa 2b-hr
Appendix A.1.7. RC-77N/E & RC-77T/E
Appendix A.1.8. PC-3
References
- Huggins, C.; Hodges, C.V. Studies on prostate cancer 1: The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941, 1, 293–297. [Google Scholar] [CrossRef]
- Fowler, J.E., Jr.; Whitmore, W.F., Jr. Considerations for the use of testosterone with systemic chemotherapy in prostatic cancer. Cancer 1982, 49, 1373–1377. [Google Scholar] [CrossRef]
- Prout, G.R., Jr.; Brewer, W.R. Response of men with advanced prostatic carcinoma to exogenous administration of testosterone. Cancer 1967, 20, 1871–1878. [Google Scholar] [CrossRef]
- Gardiner, R.A.; Sweeney, C.; Tilley, W.D. Testosterone therapy in castrate-resistant prostate cancer: A possible new approach. Eur. Urol. 2009, 56, 245–246. [Google Scholar] [CrossRef] [PubMed]
- Geck, P.; Maffini, M.V.; Szelei, J.; Sonnenschein, C.; Soto, A.M. Androgen-induced proliferative quiescence in prostate cancer cells: The role of AS3 as its mediator. Proc. Natl. Acad. Sci. USA 2000, 97, 10185–10190. [Google Scholar] [CrossRef] [PubMed]
- Soto, A.M.; Sonnenschein, C. The two faces of janus: Sex steroids as mediators of both cell proliferation and cell death. J. Natl. Cancer Inst. 2001, 93, 1673–1675. [Google Scholar] [CrossRef] [PubMed]
- Umekita, Y.; Hiipakka, R.A.; Kokontis, J.M.; Liao, S. Human prostate tumor growth in athymic mice: Inhibition by androgens and stimulation by finasteride. Proc. Natl. Acad. Sci. USA 1996, 93, 11802–11807. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Umekita, Y.; Guo, J.; Kokontis, J.M.; Hiipakka, R.A. Growth inhibition and regression of human prostate and breast tumors in athymic mice by tea epigallocatechin gallate. Cancer Lett. 1995, 96, 239–243. [Google Scholar] [CrossRef]
- Schweizer, M.T.; Antonarakis, E.S.; Wang, H.; Ajiboye, A.S.; Spitz, A.; Cao, H.; Luo, J.; Haffner, M.C.; Yegnasubramanian, S.; Carducci, M.A.; et al. Effect of bipolar androgen therapy for asymptomatic men with castration-resistant prostate cancer: Results from a pilot clinical study. Sci. Transl. Med. 2015, 7, 269ra2. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, M.T.; Wang, H.; Luber, B.; Nadal, R.; Spitz, A.; Rosen, D.M.; Cao, H.; Antonarakis, E.S.; Eisenberger, M.A.; Carducci, M.A.; et al. Bipolar Androgen Therapy for Men with Androgen Ablation Naive Prostate Cancer: Results from the Phase II BATMAN Study. Prostate 2016, 76, 1218–1226. [Google Scholar] [CrossRef] [PubMed]
- Teply, B.A.; Wang, H.; Sullivan, R.; Rifkind, I.; Bruns, A.; Decarli, M.; Sinibaldi, V.J.; Pratz, C.F.; Luo, J.; Carducci, M.A.; et al. Phase II study of bipolar androgen therapy (BAT) in men with metastatic castration-resistant prostate cancer (mCRPC) and progression on enzalutamide (enza). In Proceedings of the ASCO Annual Meeting, Chicago, IL, USA, 1–5 June 2017. [Google Scholar]
- Song, R.X.; Santen, R.J. Apoptotic action of estrogen. Apoptosis 2003, 8, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Song, R.X.; Mor, G.; Naftolin, F.; McPherson, R.A.; Song, J.; Zhang, Z.; Yue, W.; Wang, J.; Santen, R.J. Effect of long-term estrogen deprivation on apoptotic responses of breast cancer cells to 17beta-estradiol. J. Natl. Cancer Inst. 2001, 93, 1714–1723. [Google Scholar] [CrossRef] [PubMed]
- Santen, R.J.; Song, R.X.; Zhang, Z.; Kumar, R.; Jeng, M.H.; Masamura, A.; Lawrence, J., Jr.; Berstein, L.; Yue, W. Long-term estradiol deprivation in breast cancer cells up-regulates growth factor signaling and enhances estrogen sensitivity. Endocr.-Relat. Cancer 2005, 12 (Suppl. 1), S61–S73. [Google Scholar] [CrossRef] [PubMed]
- Sugimura, Y.; Cunha, G.R.; Bigsby, R.M. Androgenic induction of DNA synthesis in prostatic glands induced in the urothelium of testicular feminized (Tfm/Y) mice. Prostate 1986, 9, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Vander Griend, D.J.; Litvinov, I.V.; Isaacs, J.T. Conversion of androgen receptor signaling from a growth suppressor in normal prostate epithelial cells to an oncogene in prostate cancer cells involves a gain of function in c-Myc regulation. Int. J. Biol. Sci. 2014, 10, 627–642. [Google Scholar] [CrossRef] [PubMed]
- Antony, L.; van der Schoor, F.; Dalrymple, S.L.; Isaacs, J.T. Androgen receptor (AR) suppresses normal human prostate epithelial cell proliferation via AR/beta-catenin/TCF-4 complex inhibition of c-MYC transcription. Prostate 2014, 74, 1118–1131. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Altuwaijri, S.; Lai, K.P.; Wu, C.T.; Ricke, W.A.; Messing, E.M.; Yao, J.; Yeh, S.; Chang, C. Androgen receptor is a tumor suppressor and proliferator in prostate cancer. Proc. Natl. Acad. Sci. USA 2008, 105, 12182–12187. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.T.; Altuwaijri, S.; Ricke, W.A.; Huang, S.P.; Yeh, S.; Zhang, C.; Niu, Y.; Tsai, M.Y.; Chang, C. Increased prostate cell proliferation and loss of cell differentiation in mice lacking prostate epithelial androgen receptor. Proc. Natl. Acad. Sci. USA 2007, 104, 12679–12684. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.O.; Tian, J.; Huang, C.K.; Ma, Z.; Lai, K.P.; Hsiao, H.; Jiang, M.; Yeh, S.; Chang, C. Suppressor role of androgen receptor in proliferation of prostate basal epithelial and progenitor cells. J. Endocrinol. 2012, 213, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Bubendorf, L.; Kononen, J.; Koivisto, P.; Schraml, P.; Moch, H.; Gasser, T.C.; Willi, N.; Mihatsch, M.J.; Sauter, G.; Kallioniemi, O.P. Survey of gene amplifications during prostate cancer progression by high-throughout fluorescence in situ hybridization on tissue microarrays. Cancer Res. 1999, 59, 803–806. [Google Scholar] [PubMed]
- Ford, O.H., 3rd; Gregory, C.W.; Kim, D.; Smitherman, A.B.; Mohler, J.L. Androgen receptor gene amplification and protein expression in recurrent prostate cancer. J. Urol. 2003, 170, 1817–1821. [Google Scholar] [CrossRef] [PubMed]
- Linja, M.J.; Savinainen, K.J.; Saramaki, O.R.; Tammela, T.L.J.; Vessella, R.L.; Visakorpi, T. Amplification and Overexpression of Androgen Receptor Gene in Hormone-Refractory Prostate Cancer. Cancer Res. 2001, 61, 3550–3555. [Google Scholar] [PubMed]
- Visakorpi, T.; Hyytinen, E.; Koivisto, P.; Tanner, M.; Keinanen, R.; Palmberg, C.; Palotie, A.; Tammela, T.; Isola, J.; Kallioniemi, O.P. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat. Genet. 1995, 9, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Van der Kwast, T.H.; Schalken, J.; Ruizeveld de Winter, J.A.; van Vroonhoven, C.C.; Mulder, E.; Boersma, W.; Trapman, J. Androgen receptors in endocrine-therapy-resistant human prostate cancer. Int. J. Cancer 1991, 48, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Ruizeveld de Winter, J.A.; Janssen, P.J.; Sleddens, H.M.; Verleun-Mooijman, M.C.; Trapman, J.; Brinkmann, A.O.; Santerse, A.B.; Schroder, F.H.; van der Kwast, T.H. Androgen receptor status in localized and locally progressive hormone refractory human prostate cancer. Am. J. Pathol. 1994, 144, 735–746. [Google Scholar] [PubMed]
- Mohler, J.L.; Gregory, C.W.; Ford, O.H., 3rd; Kim, D.; Weaver, C.M.; Petrusz, P.; Wilson, E.M.; French, F.S. The androgen axis in recurrent prostate cancer. Clin. Cancer Res. 2004, 10, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Taplin, M.E.; Bubley, G.J.; Shuster, T.D.; Frantz, M.E.; Spooner, A.E.; Ogata, G.K.; Keer, H.N.; Balk, S.P. Mutation of the androgen-receptor gene in metastatic androgen-independent prostate cancer. N. Engl. J. Med. 1995, 332, 1393–1398. [Google Scholar] [CrossRef] [PubMed]
- Holzbeierlein, J.; Lal, P.; LaTulippe, E.; Smith, A.; Satagopan, J.; Zhang, L.; Ryan, C.; Smith, S.; Scher, H.; Scardino, P.; et al. Gene expression analysis of human prostate carcinoma during hormonal therapy identifies androgen-responsive genes and mechanisms of therapy resistance. Am. J. Pathol. 2004, 164, 217–227. [Google Scholar] [CrossRef]
- Stanbrough, M.; Bubley, G.J.; Ross, K.; Golub, T.R.; Rubin, M.A.; Penning, T.M.; Febbo, P.G.; Balk, S.P. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006, 66, 2815–2825. [Google Scholar] [CrossRef] [PubMed]
- Latil, A.; Bieche, I.; Vidaud, D.; Lidereau, R.; Berthon, P.; Cussenot, O.; Vidaud, M. Evaluation of androgen, estrogen (ERα and ERβ), and progesterone receptor expression in human prostate cancer by real-time quantitative reverse transcription-polymerase chain reaction assays. Cancer Res. 2001, 61, 1919–1926. [Google Scholar] [PubMed]
- Miyamoto, H.; Rahman, M.M.; Chang, C. Molecular basis for the antiandrogen withdrawal syndrome. J. Cell. Biochem. 2004, 91, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.D.; Welsbie, D.S.; Tran, C.; Baek, S.H.; Chen, R.; Vessella, R.; Rosenfeld, M.G.; Sawyers, C.L. Molecular determinants of resistance to antiandrogen therapy. Nat. Med. 2004, 10, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Brooke, G.N.; Bevan, C.L. The role of androgen receptor mutations in prostate cancer progression. Curr. Genom. 2009, 10, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Culig, Z.; Hobisch, A.; Cronauer, M.V.; Cato, A.C.; Hittmair, A.; Radmayr, C.; Eberle, J.; Bartsch, G.; Klocker, H. Mutant androgen receptor detected in an advanced-stage prostatic carcinoma is activated by adrenal androgens and progesterone. Mol. Endocrinol. 1993, 7, 1541–1550. [Google Scholar] [PubMed]
- Gingrich, J.R.; Barrios, R.J.; Kattan, M.W.; Nahm, H.S.; Finegold, M.J.; Greenberg, N.M. Androgen-independent prostate cancer progression in the TRAMP model. Cancer Res. 1997, 57, 4687–4691. [Google Scholar] [PubMed]
- Taplin, M.E. Drug insight: Role of the androgen receptor in the development and progression of prostate cancer. Nat. Clin. Pract. Oncol. 2007, 4, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Balk, S.P. Mechanisms mediating androgen receptor reactivation after castration. Urol. Oncol. 2009, 27, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Y.; Malloy, P.J.; Krishnan, A.V.; Swami, S.; Navone, N.M.; Peehl, D.M.; Feldman, D. Glucocorticoids can promote androgen-independent growth of prostate cancer cells through a mutated androgen receptor. Nat. Med. 2000, 6, 703–706. [Google Scholar] [CrossRef] [PubMed]
- Steinkamp, M.P.; O’Mahony, O.A.; Brogley, M.; Rehman, H.; Lapensee, E.W.; Dhanasekaran, S.; Hofer, M.D.; Kuefer, R.; Chinnaiyan, A.; Rubin, M.A.; et al. Treatment-dependent androgen receptor mutations in prostate cancer exploit multiple mechanisms to evade therapy. Cancer Res. 2009, 69, 4434–4442. [Google Scholar] [CrossRef] [PubMed]
- Korpal, M.; Korn, J.M.; Gao, X.; Rakiec, D.P.; Ruddy, D.A.; Doshi, S.; Yuan, J.; Kovats, S.G.; Kim, S.; Cooke, V.G.; et al. An F876L Mutation in Androgen Receptor Confers Genetic and Phenotypic Resistance to MDV3100 (Enzalutamide). Cancer Discov. 2013, 3, 1030–1043. [Google Scholar] [CrossRef] [PubMed]
- Balbas, M.D.; Evans, M.J.; Hosfield, D.J.; Wongvipat, J.; Arora, V.K.; Watson, P.A.; Chen, Y.; Greene, G.L.; Shen, Y.; Sawyers, C.L. Overcoming mutation-based resistance to antiandrogens with rational drug design. eLife 2013, 2, e00499. [Google Scholar] [CrossRef] [PubMed]
- Haile, S.; Sadar, M.D. Androgen receptor and its splice variants in prostate cancer. Cell. Mol. Life Sci. 2011, 68, 3971–3981. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, E. Endocrine therapy in the management of prostatic cancer. Clin. Endocrinol. Metab. 1980, 9, 369–381. [Google Scholar] [CrossRef]
- Robinson, M.R.; Shearer, R.J.; Fergusson, J.D. Adrenal suppression in the treatment of carcinoma of the prostate. Br. J. Urol. 1974, 46, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Samson, D.J.; Shearer, R.J.; Fergusson, J.D. Systematic review and meta-analysis of monotherapy compared with combined androgen blockade for patients with advanced prostate carcinoma. Cancer 2002, 95, 361–376. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, B.; Bennett, C.; Seidenfeld, J.; Samson, D.; Wilt, T. Maximal androgen blockade for advanced prostate cancer. Cochrane Database Syst. Rev. 2000, CD001526. [Google Scholar] [CrossRef]
- Caubet, J.F.; Tosteson, T.D.; Dong, E.W.; Naylon, E.M.; Whiting, G.W.; Ernstoff, M.S.; Ross, S.D. Maximum androgen blockade in advanced prostate cancer: A meta-analysis of published randomized controlled trials using nonsteroidal antiandrogens. Urology 1997, 49, 71–78. [Google Scholar] [CrossRef]
- De Bono, J.S. Abiraterone acetate improves survival in metastatic castration-resistant prostate cancer: Phase III results. In Proceedings of the 2010 European Society for Medical Oncology, Milan, Italy, 8–12 October 2010. [Google Scholar]
- Scher, H.I.; Beer, T.M.; Higano, C.S.; Anand, A.; Taplin, M.E.; Efstathiou, E.; Rathkopf, D.; Shelkey, J.; Yu, E.Y.; Alumkal, J.; et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: A phase 1–2 study. Lancet 2010, 375, 1437–1446. [Google Scholar] [CrossRef]
- Huggins, C. Two principles in endocrine therapy of cancers: Hormone deprival and hormone interference. Cancer Res. 1965, 25, 1163–1167. [Google Scholar] [PubMed]
- Brendler, H.; Chase, W.E.; Scott, W.W. Prostatic cancer; further investigation of hormonal relationships. Arch. Surg. 1950, 61, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Mathew, P. Prolonged control of progressive castration-resistant metastatic prostate cancer with testosterone replacement therapy: The case for a prospective trial. Ann. Oncol. 2008, 19, 395–396. [Google Scholar] [CrossRef] [PubMed]
- Pearson, O.H. Discussion of Dr. Huggins’ paper: Control of cancers of man by endocrinological methods. Cancer Res. 1957, 17, 473–479. [Google Scholar] [PubMed]
- Hussain, M.; Tangen, C.M.; Berry, D.L.; Higano, C.S.; Crawford, E.D.; Liu, G.; Wilding, G.; Prescott, S.; Kanaga Sundaram, S.; Small, E.J.; et al. Intermittent versus continuous androgen deprivation in prostate cancer. N. Engl. J. Med. 2013, 368, 1314–1325. [Google Scholar] [CrossRef] [PubMed]
- Thelen, P.; Heinrich, E.; Bremmer, F.; Trojan, L.; Strauss, A. Testosterone boosts for treatment of castration resistant prostate cancer: An experimental implementation of intermittent androgen deprivation. Prostate 2013, 73, 1699–1709. [Google Scholar] [CrossRef] [PubMed]
- Kokontis, J.M.; Hay, N.; Liao, S. Progression of LNCaP prostate tumor cells during androgen deprivation: Hormone-independent growth, repression of proliferation by androgen, and role for p27Kip1 in androgen-induced cell cycle arrest. Mol. Endocrinol. 1998, 12, 941–953. [Google Scholar] [CrossRef] [PubMed]
- Kokontis, J.; Takakura, K.; Hay, N.; Liao, S. Increased androgen receptor activity and altered c-myc expression in prostate cancer cells after long-term androgen deprivation. Cancer Res. 1994, 54, 1566–1573. [Google Scholar] [PubMed]
- Navone, N.M.; Olive, M.; Ozen, M.; Davis, R.; Troncoso, P.; Tu, S.M.; Johnston, D.; Pollack, A.; Pathak, S.; von Eschenbach, A.C.; et al. Establishment of two human prostate cancer cell lines derived from a single bone metastasis. Clin. Cancer Res. 1997, 3, 2493–2500. [Google Scholar] [PubMed]
- Hara, T.; Nakamura, K.; Araki, H.; Kusaka, M.; Yamaoka, M. Enhanced androgen receptor signaling correlates with the androgen-refractory growth in a newly established MDA PCa 2b-hr human prostate cancer cell subline. Cancer Res. 2003, 63, 5622–5628. [Google Scholar] [PubMed]
- Nagabhushan, M.; Miller, C.M.; Pretlow, T.P.; Giaconia, J.M.; Edgehouse, N.L.; Schwartz, S.; Kung, H.J.; de Vere White, R.W.; Gumerlock, P.H.; Resnick, M.I.; et al. CWR22: The first human prostate cancer xenograft with strongly androgen-dependent and relapsed strains both in vivo and in soft agar. Cancer Res. 1996, 56, 3042–3046. [Google Scholar] [PubMed]
- Zhau, H.Y.; Chang, S.M.; Chen, B.Q.; Wang, Y.; Zhang, H.; Kao, C.; Sang, Q.A.; Pathak, S.J.; Chung, L.W. Androgen-repressed phenotype in human prostate cancer. Proc. Natl. Acad. Sci. USA 1996, 93, 15152–15157. [Google Scholar] [CrossRef] [PubMed]
- Attardi, B.J.; Burgenson, J.; Hild, S.A.; Reel, J.R. Steroid hormonal regulation of growth, prostate specific antigen secretion, and transcription mediated by the mutated androgen receptor in CWR22Rv1 human prostate carcinoma cells. Mol. Cell. Endocrinol. 2004, 222, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Fowler, J.E., Jr.; Whitmore, W.F., Jr. The response of metastatic adenocarcinoma of the prostate to exogenous testosterone. J. Urol. 1981, 126, 372–375. [Google Scholar] [CrossRef]
- Morris, M.J.; Huang, D.; Kelly, W.K.; Slovin, S.F.; Stephenson, R.D.; Eicher, C.; Delacruz, A.; Curley, T.; Schwartz, L.H.; Scher, H.I. Phase 1 trial of high-dose exogenous testosterone in patients with castration-resistant metastatic prostate cancer. Eur. Urol. 2009, 56, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Szmulewitz, R.; Mohile, S.; Posadas, E.; Kunnavakkam, R.; Karrison, T.; Manchen, E.; Stadler, W.M. A randomized phase 1 study of testosterone replacement for patients with low-risk castration-resistant prostate cancer. Eur. Urol. 2009, 56, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Denmeade, S.R.; Isaacs, J.T. Bipolar androgen therapy: The rationale for rapid cycling of supraphysiologic androgen/ablation in men with castration resistant prostate cancer. Prostate 2010, 70, 1600–1607. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.; van Allen, E.M.; Wu, Y.M.; Schultz, N.; Lonigro, R.J.; Mosquera, J.M.; Montgomery, B.; Taplin, M.E.; Pritchard, C.C.; Attard, G.; et al. Integrative clinical genomics of advanced prostate cancer. Cell 2015, 161, 1215–1228. [Google Scholar] [CrossRef] [PubMed]
- Litvinov, I.V.; Vander Griend, D.J.; Antony, L.; Dalrymple, S.; de Marzo, A.M.; Drake, C.G.; Isaacs, J.T. Androgen receptor as a licensing factor for DNA replication in androgen-sensitive prostate cancer cells. Proc. Natl. Acad. Sci. USA 2006, 103, 15085–15090. [Google Scholar] [CrossRef] [PubMed]
- Vander Griend, D.J.; Litvinov, I.V.; Isaacs, J.T. Stabilizing androgen receptor in mitosis inhibits prostate cancer proliferation. Cell Cycle 2007, 6, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, J.T.; D’Antonio, J.M.; Chen, S.; Antony, L.; Dalrymple, S.P.; Ndikuyeze, G.H.; Luo, J.; Denmeade, S.R. Adaptive auto-regulation of androgen receptor provides a paradigm shifting rationale for bipolar androgen therapy (BAT) for castrate resistant human prostate cancer. Prostate 2012, 72, 1491–1505. [Google Scholar] [CrossRef] [PubMed]
- Cinar, B.; Koeneman, K.S.; Edlund, M.; Prins, G.S.; Zhau, H.E.; Chung, L.W. Androgen receptor mediates the reduced tumor growth, enhanced androgen responsiveness, and selected target gene transactivation in a human prostate cancer cell line. Cancer Res. 2001, 61, 7310–7317. [Google Scholar] [PubMed]
- Heisler, L.E.; Evangelou, A.; Lew, A.M.; Trachtenberg, J.; Elsholtz, H.P.; Brown, T.J. Androgen-dependent cell cycle arrest and apoptotic death in PC-3 prostatic cell cultures expressing a full-length human androgen receptor. Mol. Cell. Endocrinol. 1997, 126, 59–73. [Google Scholar] [CrossRef]
- Joly-Pharaboz, M.O.; Soave, M.C.; Nicolas, B.; Mebarki, F.; Renaud, M.; Foury, O.; Morel, Y.; Andre, J.G. Androgens inhibit the proliferation of a variant of the human prostate cancer cell line LNCaP. J. Steroid Biochem. Mol. Biol. 1995, 55, 67–76. [Google Scholar] [CrossRef]
- Joly-Pharaboz, M.O.; Ruffion, A.; Roch, A.; Michel-Calemard, L.; Andre, J.; Chantepie, J.; Nicolas, B.; Panaye, G. Inhibition of growth and induction of apoptosis by androgens of a variant of LNCaP cell line. J. Steroid Biochem. Mol. Biol. 2000, 73, 237–249. [Google Scholar] [CrossRef]
- Joly-Pharaboz, M.O.; Kalach, J.J.; Pharaboz, J.; Chantepie, J.; Nicolas, B.; Baille, M.L.; Ruffion, A.; Benahmed, M.; Andre, J. Androgen inhibits the growth of carcinoma cell lines established from prostate cancer xenografts that escape androgen treatment. J. Steroid Biochem. Mol. Biol. 2008, 111, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Kokontis, J.; Tang, F.; Godfrey, B.; Liao, S.; Lin, A.; Chen, Y.; Xiang, J. Androgen and its receptor promote Bax-mediated apoptosis. Mol. Cell. Biol. 2006, 26, 1908–1916. [Google Scholar] [CrossRef] [PubMed]
- De Launoit, Y.; Veilleux, R.; Dufour, M.; Simard, J.; Labrie, F. Characteristics of the biphasic action of androgens and of the potent antiproliferative effects of the new pure antiestrogen EM-139 on cell cycle kinetic parameters in LNCaP human prostatic cancer cells. Cancer Res. 1991, 51, 5165–5170. [Google Scholar] [PubMed]
- Tsihlias, J.; Zhang, W.; Bhattacharya, N.; Flanagan, M.; Klotz, L.; Slingerland, J. Involvement of p27Kip1 in G1 arrest by high dose 5α-dihydrotestosterone in LNCaP human prostate cancer cells. Oncogene 2000, 19, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Chuu, C.P.; Kokontis, J.M.; Hiipakka, R.A.; Fukuchi, J.; Lin, H.P.; Lin, C.Y.; Huo, C.; Su, L.C.; Liao, S. Androgen suppresses proliferation of castration-resistant LNCaP 104-R2 prostate cancer cells through androgen receptor, Skp2, and c-Myc. Cancer Sci. 2011, 102, 2022–2028. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Jenster, G.; Epner, D.E. Androgen induction of cyclin-dependent kinase inhibitor p21 gene: Role of androgen receptor and transcription factor Sp1 complex. Mol. Endocrinol. 2000, 14, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Schulz, H.; Wolf, D.A. The F-box protein SKP2 mediates androgen control of p27 stability in LNCaP human prostate cancer cells. BMC Cell Biol. 2002, 3, 22. [Google Scholar] [CrossRef]
- Kokontis, J.M.; Lin, H.P.; Jiang, S.S.; Lin, C.Y.; Fukuchi, J.; Hiipakka, R.A.; Chung, C.J.; Chan, T.M.; Liao, S.; Chang, C.H.; et al. Androgen Suppresses the Proliferation of Androgen Receptor-Positive Castration-Resistant Prostate Cancer Cells via Inhibition of Cdk2, CyclinA, and Skp2. PLoS ONE 2014, 9, e109170. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Pan, Y.; Regan, K.M.; Wu, C.; Zhang, X.; Tindall, D.J.; Huang, H. Androgens repress expression of the F-box protein Skp2 via p107 dependent and independent mechanisms in LNCaP prostate cancer cells. Prostate 2012, 72, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Polyak, K.; Lee, M.H.; Erdjument-Bromage, H.; Koff, A.; Roberts, J.M.; Tempst, P.; Massague, J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell 1994, 78, 59–66. [Google Scholar] [CrossRef]
- Wolf, D.A.; Kohlhuber, F.; Schulz, P.; Fittler, F.; Eick, D. Transcriptional down-regulation of c-myc in human prostate carcinoma cells by the synthetic androgen mibolerone. Br. J. Cancer 1992, 65, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Bretones, G.; Acosta, J.C.; Caraballo, J.M.; Ferrandiz, N.; Gomez-Casares, M.T.; Albajar, M.; Blanco, R.; Ruiz, P.; Hung, W.C.; Albero, M.P.; et al. SKP2 oncogene is a direct MYC target gene and MYC down-regulates p27(KIP1) through SKP2 in human leukemia cells. J. Biol. Chem. 2011, 286, 9815–9825. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Chen, L.; Milazzo, G.; Gherardi, S.; Perini, G.; Willmore, E.; Newell, D.R.; Tweddle, D.A. SKP2 is a direct transcriptional target of MYCN and a potential therapeutic target in neuroblastoma. Cancer Lett. 2015, 363, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Herbst, A.; Tworkowski, K.A.; Salghetti, S.E.; Tansey, W.P. Skp2 regulates Myc protein stability and activity. Mol. Cell 2003, 11, 1177–1188. [Google Scholar] [CrossRef]
- Chuu, C.P.; Kokontis, J.M.; Hiipakka, R.A.; Fukuchi, J.; Lin, H.P.; Lin, C.Y.; Huo, C.; Su, L.C. Androgens as therapy for androgen receptor-positive castration-resistant prostate cancer. J. Biomed. Sci. 2011, 18, 63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, C. F-box protein Skp2: A novel transcriptional target of E2F. Oncogene 2006, 25, 2615–2627. [Google Scholar] [CrossRef] [PubMed]
- Berchem, G.J.; Bosseler, M.; Sugars, L.Y.; Voeller, H.J.; Zeitlin, S.; Gelmann, E.P. Androgens induce resistance to bcl-2-mediated apoptosis in LNCaP prostate cancer cells. Cancer Res. 1995, 55, 735–738. [Google Scholar] [PubMed]
- Korsmeyer, S.J. BCL-2 gene family and the regulation of programmed cell death. Cancer Res. 1999, 59 (Suppl. 7), 1693s–1700s. [Google Scholar] [CrossRef]
- Lin, Y.; Lu, Z.; Kokontis, J.; Xiang, J. Androgen receptor primes prostate cancer cells to apoptosis through down-regulation of basal p21 expression. Biochem. Biophys. Res. Commun. 2013, 430, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Takeda, D.Y.; Dutta, A. DNA replication and progression through S phase. Oncogene 2005, 24, 2827–2843. [Google Scholar] [CrossRef] [PubMed]
- Murthy, S.; Wu, M.; Bai, V.U.; Hou, Z.; Menon, M.; Barrack, E.R.; Kim, S.H.; Reddy, G.P. Role of androgen receptor in progression of LNCaP prostate cancer cells from G1 to S phase. PLoS ONE 2013, 8, e56692. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; He, H.H.; Chen, S.; Coleman, I.; Wang, H.; Fang, Z.; Chen, S.; Nelson, P.S.; Liu, X.S.; Brown, M.; et al. Androgen receptor gene expression in prostate cancer is directly suppressed by the androgen receptor through recruitment of lysine-specific demethylase 1. Cancer Cell 2011, 20, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Kiyota, H.; Nakata, D.; Masaki, T.; Kusaka, M.; Egawa, S. A novel androgen-dependent prostate cancer xenograft model derived from skin metastasis of a Japanese patient. Prostate 2009, 69, 1660–1667. [Google Scholar] [CrossRef] [PubMed]
- Nakata, D.; Nakayama, K.; Masaki, T.; Tanaka, A.; Kusaka, M.; Watanabe, T. Growth Inhibition by Testosterone in an Androgen Receptor Splice Variant-Driven Prostate Cancer Model. Prostate 2016, 76, 1536–1545. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, W.; Zhang, Y.; Yuan, X.; Xu, K.; Yu, J.; Chen, Z.; Beroukhim, R.; Wang, H.; Lupien, M.; et al. Androgen receptor regulates a distinct transcription program in androgen-independent prostate cancer. Cell 2009, 138, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Gao, Y.; He, H.H.; Han, D.; Han, W.; Avery, A.; Macoska, J.A.; Liu, X.; Chen, S.; Ma, F.; et al. Androgen Receptor Tumor Suppressor Function Is Mediated by Recruitment of Retinoblastoma Protein. Cell Rep. 2016, 17, 966–976. [Google Scholar] [CrossRef] [PubMed]
- Ruan, D.; He, J.; Li, C.F.; Lee, H.J.; Liu, J.; Lin, H.K.; Chan, C.H. Skp2 deficiency restricts the progression and stem cell features of castration-resistant prostate cancer by destabilizing Twist. Oncogene 2017, 36, 4299–4310. [Google Scholar] [CrossRef] [PubMed]
- Kregel, S.; Kiriluk, K.J.; Rosen, A.M.; Cai, Y.; Reyes, E.E.; Otto, K.B.; Tom, W.; Paner, G.P.; Szmulewitz, R.Z.; Vander Griend, D.J.; et al. Sox2 is an androgen receptor-repressed gene that promotes castration-resistant prostate cancer. PLoS ONE 2013, 8, e53701. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, Y.; Chen, Y.; Chen, S.; Jia, X.; Sun, T.; Liu, Y.; Li, X.; Xiang, R.; Li, N.; et al. SOX2 promotes tumor metastasis by stimulating epithelial-to-mesenchymal transition via regulation of WNT/beta-catenin signal network. Cancer Lett. 2013, 336, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Mu, P.; Zhang, Z.; Benelli, M.; Karthaus, W.R.; Hoover, E.; Chen, C.C.; Wongvipat, J.; Ku, S.Y.; Gao, D.; Cao, Z.; et al. SOX2 promotes lineage plasticity and antiandrogen resistance in TP53- and RB1-deficient prostate cancer. Science 2017, 355, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Maffini, M.V.; Geck, P.; Powell, C.E.; Sonnenschein, C.; Soto, A.M. Mechanism of androgen action on cell proliferation: AS3 protein as a mediator of proliferative arrest in the rat prostate. Endocrinology 2002, 143, 2708–2714. [Google Scholar] [CrossRef] [PubMed]
- Maffini, M.; Denes, V.; Sonnenschein, C.; Soto, A.; Geck, P. APRIN is a unique Pds5 paralog with features of a chromatin regulator in hormonal differentiation. J. Steroid Biochem. Mol. Biol. 2008, 108, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Denes, V.; Pilichowska, M.; Makarovskiy, A.; Carpinito, G.; Geck, P. Loss of a cohesin-linked suppressor APRIN (Pds5b) disrupts stem cell programs in embryonal carcinoma: An emerging cohesin role in tumor suppression. Oncogene 2010, 29, 3446–3452. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Nenseth, H.Z.; Saatcioglu, F. Role of PLZF as a tumor suppressor in prostate cancer. Oncotarget 2017, 8, 71317–71324. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.L.; Botta, G.; Gao, S.; Li, T.; van Allen, E.M.; Treacy, D.J.; Cai, C.; He, H.H.; Sweeney, C.J.; Brown, M.; et al. PLZF, a tumor suppressor genetically lost in metastatic castration-resistant prostate cancer, is a mediator of resistance to androgen deprivation therapy. Cancer Res. 2015, 75, 1944–1948. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Wang, Z. Identification and characterization of PLZF as a prostatic androgen-responsive gene. Prostate 2004, 59, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Coleman, I.; Morrissey, C.; Zhang, X.; True, L.D.; Gulati, R.; Etzioni, R.; Bolouri, H.; Montgomery, B.; White, T.; et al. Substantial interindividual and limited intraindividual genomic diversity among tumors from men with metastatic prostate cancer. Nat. Med. 2016, 22, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.Q.; Unger, P.; Yang, Q.; Kinoshita, Y.; Singh, K.; McMahon, L.; Nastiuk, K.; Sha, K.; Krolewski, J.; Burstein, D. Loss of PLZF expression in prostate cancer by immunohistochemistry correlates with tumor aggressiveness and metastasis. PLoS ONE 2015, 10, e0121318. [Google Scholar] [CrossRef] [PubMed]
- Storer, M.; Mas, A.; Robert-Moreno, A.; Pecoraro, M.; Ortells, M.C.; Di Giacomo, V.; Yosef, R.; Pilpel, N.; Krizhanovsky, V.; Sharpe, J.; et al. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell 2013, 155, 1119–1130. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J. Cellular senescence as a tumor-suppressor mechanism. Trends Cell Biol. 2001, 11, S27–S31. [Google Scholar] [CrossRef]
- Roediger, J.; Hessenkemper, W.; Bartsch, S.; Manvelyan, M.; Huettner, S.S.; Liehr, T.; Esmaeili, M.; Foller, S.; Petersen, I.; Grimm, M.O.; et al. Supraphysiological androgen levels induce cellular senescence in human prostate cancer cells through the Src-Akt pathway. Mol. Cancer 2014, 13, 214. [Google Scholar] [CrossRef] [PubMed]
- Narita, M.; Nunez, S.; Heard, E.; Narita, M.; Lin, A.W.; Hearn, S.A.; Spector, D.L.; Hannon, G.J.; Lowe, S.W. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell 2003, 113, 703–716. [Google Scholar] [CrossRef]
- Mirochnik, Y.; Veliceasa, D.; Williams, L.; Maxwell, K.; Yemelyanov, A.; Budunova, I.; Volpert, O.V. Androgen receptor drives cellular senescence. PLoS ONE 2012, 7, e31052. [Google Scholar] [CrossRef] [PubMed]
- Bui, A.T.; Huang, M.E.; Havard, M.; Laurent-Tchenio, F.; Dautry, F.; Tchenio, T. Transient exposure to androgens induces a remarkable self-sustained quiescent state in dispersed prostate cancer cells. Cell Cycle 2017, 16, 879–893. [Google Scholar] [CrossRef] [PubMed]
- Haffner, M.C.; Aryee, M.J.; Toubaji, A.; Esopi, D.M.; Albadine, R.; Gurel, B.; Isaacs, W.B.; Bova, G.S.; Liu, W.; Xu, J.; et al. Androgen-induced TOP2B-mediated double-strand breaks and prostate cancer gene rearrangements. Nat. Genet. 2010, 42, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Teply, B.A.; Kachhap, S.; Eisenberger, M.A.; Denmeade, S.R. Extreme Response to High-dose Testosterone in BRCA2- and ATM-mutated Prostate Cancer. Eur. Urol. 2016, 71, 499. [Google Scholar] [CrossRef] [PubMed]
- Jordan, V.C. The new biology of estrogen-induced apoptosis applied to treat and prevent breast cancer. Endocr. Relat. Cancer 2015, 22, R1–R31. [Google Scholar] [CrossRef] [PubMed]
- Coelingh Bennink, H.J.; Verhoeven, C.; Dutman, A.E.; Thijssen, J. The use of high-dose estrogens for the treatment of breast cancer. Maturitas 2017, 95, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, S.; Farahmand, L.; Hosseinzade, A.; Eslami, S.Z.; Majidzadeh, A.K. Estrogen can restore Tamoxifen sensitivity in breast cancer cells amidst the complex network of resistance. Biomed. Pharmacother. 2017, 93, 1320–1325. [Google Scholar] [CrossRef] [PubMed]
- Ariazi, E.A.; Cunliffe, H.E.; Lewis-Wambi, J.S.; Slifker, M.J.; Willis, A.L.; Ramos, P.; Tapia, C.; Kim, H.R.; Yerrum, S.; Sharma, C.G.; et al. Estrogen induces apoptosis in estrogen deprivation-resistant breast cancer through stress responses as identified by global gene expression across time. Proc. Natl. Acad. Sci. USA 2011, 108, 18879–18886. [Google Scholar] [CrossRef] [PubMed]
- Lewis-Wambi, J.S.; Jordan, V.C. Estrogen regulation of apoptosis: How can one hormone stimulate and inhibit? Breast Cancer Res. 2009, 11, 206. [Google Scholar] [CrossRef] [PubMed]
- Maximov, P.; Sengupta, S.; Lewis-Wambi, J.S.; Kim, H.R.; Curpan, R.F.; Jordan, V.C. The Conformation of the Estrogen Receptor Directs Estrogen-Induced Apoptosis in Breast Cancer: A Hypothesis. Horm. Mol. Biol. Clin. Investig. 2011, 5, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, J.P.; Santen, R.J.; Yue, W. Adenosine monophosphate activated protein kinase (AMPK), a mediator of estradiol-induced apoptosis in long-term estrogen deprived breast cancer cells. Apoptosis 2015, 20, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Litvinov, I.V.; Antony, L.; Dalrymple, S.L.; Becker, R.; Cheng, L.; Isaacs, J.T. PC3, but not DU145, human prostate cancer cells retain the coregulators required for tumor suppressor ability of androgen receptor. Prostate 2006, 66, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Hamada, A.; Sissung, T.; Price, D.K.; Danesi, R.; Chau, C.H.; Sharifi, N.; Venzon, D.; Maeda, K.; Nagao, K.; Sparreboom, A.; et al. Effect of SLCO1B3 haplotype on testosterone transport and clinical outcome in Caucasian patients with androgen-independent prostatic cancer. Clin. Cancer Res. 2008, 14, 3312–3318. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, N.; Hamada, A.; Sissung, T.; Danesi, R.; Venzon, D.; Baum, C.; Gulley, J.L.; Price, D.K.; Dahut, W.L.; Figg, W.D. A polymorphism in a transporter of testosterone is a determinant of androgen independence in prostate cancer. BJU Int. 2008, 102, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ramirez, J.; Gamazon, E.R.; Mirkov, S.; Chen, P.; Wu, K.; Sun, C.; Cox, N.J.; Cook, E., Jr.; Das, S.; et al. Genetic factors affecting gene transcription and catalytic activity of UDP-glucuronosyltransferases in human liver. Hum. Mol. Genet. 2014, 23, 5558–5569. [Google Scholar] [CrossRef] [PubMed]
- Paquet, S.; Fazli, L.; Grosse, L.; Verreault, M.; Tetu, B.; Rennie, P.S.; Belanger, A.; Barbier, O. Differential expression of the androgen-conjugating UGT2B15 and UGT2B17 enzymes in prostate tumor cells during cancer progression. J. Clin. Endocrinol. Metab. 2012, 97, E428–E432. [Google Scholar] [CrossRef] [PubMed]
- Gauthier-Landry, L.; Belanger, A.; Barbier, O. Multiple roles for UDP-glucuronosyltransferase (UGT)2B15 and UGT2B17 enzymes in androgen metabolism and prostate cancer evolution. J. Steroid Biochem. Mol. Biol. 2015, 145, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Nieto, C.M.; Rider, L.C.; Cramer, S.D. Influence of stromal-epithelial interactions on androgen action. Endocr. Relat. Cancer 2014, 21, T147–T160. [Google Scholar] [CrossRef] [PubMed]
- Leach, D.A.; Buchanan, G. Stromal Androgen Receptor in Prostate Cancer Development and Progression. Cancers (Basel) 2017, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Leach, D.A.; Need, E.F.; Toivanen, R.; Trotta, A.P.; Palethorpe, H.M.; Tamblyn, D.J.; Kopsaftis, T.; England, G.M.; Smith, E.; Drew, P.A.; et al. Stromal androgen receptor regulates the composition of the microenvironment to influence prostate cancer outcome. Oncotarget 2015, 6, 16135–16150. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.P.; Chen, L.Y.; Luethy, A.; Kim, Y.; Kani, K.; MacLeod, A.R.; Gross, M.E. Androgen receptor in cancer-associated fibroblasts influences stemness in cancer cells. Endocr. Relat. Cancer 2017, 24, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Horoszewicz, J.S.; Leong, S.S.; Chu, T.M.; Wajsman, Z.L.; Friedman, M.; Papsidero, L.; Kim, U.; Chai, L.S.; Kakati, S.; Arya, S.K.; et al. The LNCaP cell line—A new model for studies on human prostatic carcinoma. Prog. Clin. Biol. Res. 1980, 37, 115–132. [Google Scholar] [PubMed]
- Horoszewicz, J.S.; Leong, S.S.; Kawinski, E.; Karr, J.P.; Rosenthal, H.; Chu, T.M.; Mirand, E.A.; Murphy, G.P. LNCaP model of human prostatic carcinoma. Cancer Res. 1983, 43, 1809–1818. [Google Scholar] [PubMed]
- Chuu, C.P.; Hiipakka, R.A.; Fukuchi, J.; Kokontis, J.M.; Liao, S. Androgen causes growth suppression and reversion of androgen-independent prostate cancer xenografts to an androgen-stimulated phenotype in athymic mice. Cancer Res. 2005, 65, 2082–2084. [Google Scholar] [CrossRef] [PubMed]
- Culig, Z.; Hoffmann, J.; Erdel, M.; Eder, I.E.; Hobisch, A.; Hittmair, A.; Bartsch, G.; Utermann, G.; Schneider, M.R.; Parczyk, K.; et al. Switch from antagonist to agonist of the androgen receptor blocker bicalutamide is associated with prostate tumour progression in a new model system. Br. J. Cancer 1999, 81, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Pretlow, T.G.; Wolman, S.R.; Micale, M.A.; Pelley, R.J.; Kursh, E.D.; Resnick, M.I.; Bodner, D.R.; Jacobberger, J.W.; Delmoro, C.M.; Giaconia, J.M.; et al. Xenografts of primary human prostatic carcinoma. J. Natl Cancer Inst. 1993, 85, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Wainstein, M.A.; He, F.; Robinson, D.; Kung, H.J.; Schwartz, S.; Giaconia, J.M.; Edgehouse, N.L.; Pretlow, T.P.; Bodner, D.R.; Kursh, E.D.; et al. CWR22: Androgen-dependent xenograft model derived from a primary human prostatic carcinoma. Cancer Res. 1994, 54, 6049–6052. [Google Scholar] [PubMed]
- Gregory, C.W.; Hamil, K.G.; Kim, D.; Hall, S.H.; Pretlow, T.G.; Mohler, J.L.; French, F.S. Androgen receptor expression in androgen-independent prostate cancer is associated with increased expression of androgen-regulated genes. Cancer Res. 1998, 58, 5718–5724. [Google Scholar] [PubMed]
- Loberg, R.D.; St. John, L.N.; Day, L.S.L.; Neeley, C.K.; Pienta, K.J. Development of the VCaP Androgen Independent Model of Prostate Cancer. Urol. Oncol. 2006, 24, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Koochekpour, S.; Maresh, G.A.; Katner, A.; Parker-Johnson, K.; Lee, T.J.; Hebert, F.E.; Kao, Y.S.; Skinner, J.; Rayford, W. Establishment and characterization of a primary androgen-responsive African-American prostate cancer cell line, E006AA. Prostate 2004, 60, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Kaighn, M.E.; Narayan, K.S.; Ohnuki, Y.; Lechner, J.F.; Jones, L.W. Establishment and characterization of a human prostatic carcinoma cell line (PC-3). Investig. Urol. 1979, 17, 16–23. [Google Scholar]
- Litvinov, I.V.; Antony, L.; Isaacs, J.T. Molecular characterization of an improved vector for evaluation of the tumor suppressor versus oncogene abilities of the androgen receptor. Prostate 2004, 61, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Altuwaijri, S.; Wu, C.C.; Niu, Y.J.; Mizokami, A.; Chang, H.C.; Chang, C. Expression of human AR cDNA driven by its own promoter results in mild promotion, but not suppression, of growth in human prostate cancer PC-3 cells. Asian J. Androl. 2007, 9, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.C.; Hsieh, J.T.; Gleave, M.E.; Brown, N.M.; Pathak, S.; Chung, L.W. Derivation of androgen-independent human LNCaP prostatic cancer cell sublines: role of bone stromal cells. Int. J. Cancer 1994, 57, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Thalmann, G.N.; Anezinis, P.E.; Chang, S.M.; Zhau, H.E.; Kim, E.E.; Hopwood, V.L.; Pathak, S.; von Eschenbach, A.C.; Chung, L.W. Androgen-independent cancer progression and bone metastasis in the LNCaP model of human prostate cancer. Cancer Res. 1994, 54, 2577–2581. [Google Scholar] [PubMed]
- Pfitzenmaier, J.; Quinn, J.E.; Odman, A.M.; Zhang, J.; Keller, E.T.; Vessella, R.L.; Corey, E. Characterization of C4-2 prostate cancer bone metastases and their response to castration. J. Bone Miner Res. 2003, 18, 1882–1888. [Google Scholar] [CrossRef] [PubMed]
- D’Antonio, J.M.; Vander Griend, D.J.; Antony, L.; Ndikuyeze, G.; Dalrymple, S.L.; Koochekpour, S.; Isaacs, J.T. Loss of Androgen Receptor-Dependent Growth Suppression by Prostate Cancer Cells Can Occur Independently from Acquiring Oncogenic Addiction to Androgen Receptor Signaling. PLoS ONE 2010, 5, e11475. [Google Scholar] [CrossRef] [PubMed]
- Theodore, S.; Sharp, S.; Zhou, J.; Turner, T.; Li, H.; Miki, J.; Ji, Y.; Patel, V.; Yates, C.; Rhim, J.S. Establishment and characterization of a pair of non-malignant and malignant tumor derived cell lines from an African American prostate cancer patient. Int. J. Oncol. 2010, 37, 1477–1482. [Google Scholar] [PubMed]
- Yuan, S.; Trachtenberg, J.; Mills, G.B.; Brown, T.J.; Xu, F.; Keating, A. Androgen-induced inhibition of cell proliferation in an androgen-insensitive prostate cancer cell line (PC-3) transfected with a human androgen receptor complementary DNA. Cancer Res. 1993, 53, 1304–1311. [Google Scholar] [PubMed]
- Garcia-Arenas, R.; Lin, F.F.; Lin, D.; Jin, L.P.; Shih, C.C.; Chang, C.; Lin, M.F. The expression of prostatic acid phosphatase is transcriptionally regulated in human prostate carcinoma cells. Mol. Cell. Endocrinol. 1995, 111, 29–37. [Google Scholar] [CrossRef]
| Patient Population | No. of Patients | Treatment Regimen | Serum T Level | PSA Response | Objective Response | Median Time to Progression | Caner Related Adverse Effects | Ref. |
|---|---|---|---|---|---|---|---|---|
| CRPC (disease burden or symptoms not designated) | 12 | T via 5 mg transdermal patch or 1% gel for 1 week, 1 month, or until disease progression | physiologic (342–876 ng/dL) | 1 patient had PSA decline >50% from baseline | none | 84 days (23–247 days) | [65] | |
| CRPC with minimal metastatic disease | 15 | transdermal T at 25, 5.0 or 75 mg/day | physiologic (94–824 ng/dL) | 3/15 (20%) had PSA declines from baseline (largest decline 43%) | none | 63 days (14–672 days) | one patient with symptomatic progression | [66] |
| Asymptomatic CRPC with low to moderate metastatic burden | 16 | T (400 mg IM day 1 of 28) and etoposide (100 mg oral daily; days 1 to 14 of 28) | T > 1500 ng/dL (~50 nM) at 2 days after T injection (range 920 to >3200 ng/dL), above 600 ng/dL at 2 weeks, and 150 ng/dL by 28 days | 7/14 (50%) had PSA declines from baseline (≥50%) | radiographic responses in 5/10 (50%), and 4 continued on treatment for ≥1 year | 11 months (3 to not reached) | 2 patients were not evaluable because they came off study after only one cycle of therapy due to toxicity | [9] |
| CRPC post progression on enzalutamide | 30 | alternating 3 month cycles of BAT (T 400 mg IM on days 1, 29 or 57), followed by 3 months of ADT alone | not reported | 9/30 (30%) men achieved a ≥50% decline in PSA from baseline | 50% of patients achieving an objective radiographic response | 8.6 months (4.7 to not reached) | 3 patients progressed per RECIST criteria and 3 had unconfirmed progression on bone scan | [11] |
| Asymptomatic hormone naïve with low metastatic burden or biochemically recurrent disease, who achieved PSA <4 ng/dL after 6 months of ADT | 29 | T 400 mg IM on days 1, 29, and 57 | not reported | 17/29 (59%) achieved primary endpoint of PSA < 4 ng/dL after 18 months | 4 of 10 evaluable patients had complete and 4 had partial responses (80%) | not given | 3 patients taken off study prior to completing 2 cycles due to concerns for early progression | [10] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammad, O.S.; Nyquist, M.D.; Schweizer, M.T.; Balk, S.P.; Corey, E.; Plymate, S.; Nelson, P.S.; Mostaghel, E.A. Supraphysiologic Testosterone Therapy in the Treatment of Prostate Cancer: Models, Mechanisms and Questions. Cancers 2017, 9, 166. https://doi.org/10.3390/cancers9120166
Mohammad OS, Nyquist MD, Schweizer MT, Balk SP, Corey E, Plymate S, Nelson PS, Mostaghel EA. Supraphysiologic Testosterone Therapy in the Treatment of Prostate Cancer: Models, Mechanisms and Questions. Cancers. 2017; 9(12):166. https://doi.org/10.3390/cancers9120166
Chicago/Turabian StyleMohammad, Osama S., Michael D. Nyquist, Michael T. Schweizer, Stephen P. Balk, Eva Corey, Stephen Plymate, Peter S. Nelson, and Elahe A. Mostaghel. 2017. "Supraphysiologic Testosterone Therapy in the Treatment of Prostate Cancer: Models, Mechanisms and Questions" Cancers 9, no. 12: 166. https://doi.org/10.3390/cancers9120166
APA StyleMohammad, O. S., Nyquist, M. D., Schweizer, M. T., Balk, S. P., Corey, E., Plymate, S., Nelson, P. S., & Mostaghel, E. A. (2017). Supraphysiologic Testosterone Therapy in the Treatment of Prostate Cancer: Models, Mechanisms and Questions. Cancers, 9(12), 166. https://doi.org/10.3390/cancers9120166




