The Role of Radiation Induced Injury on Lung Cancer
Abstract
:1. Introduction
2. Results
2.1. Half-Life and Dose per Cell Turnover
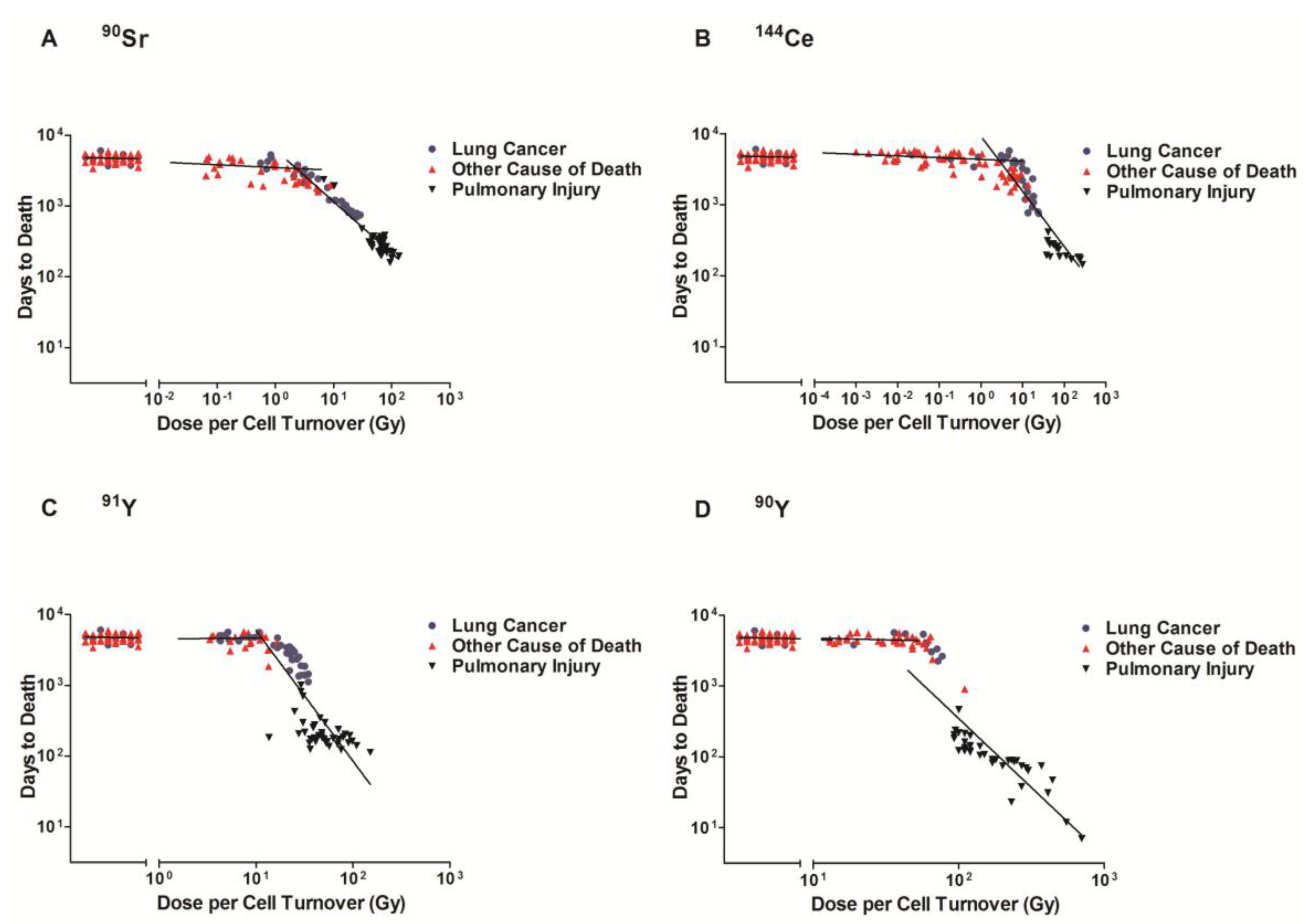
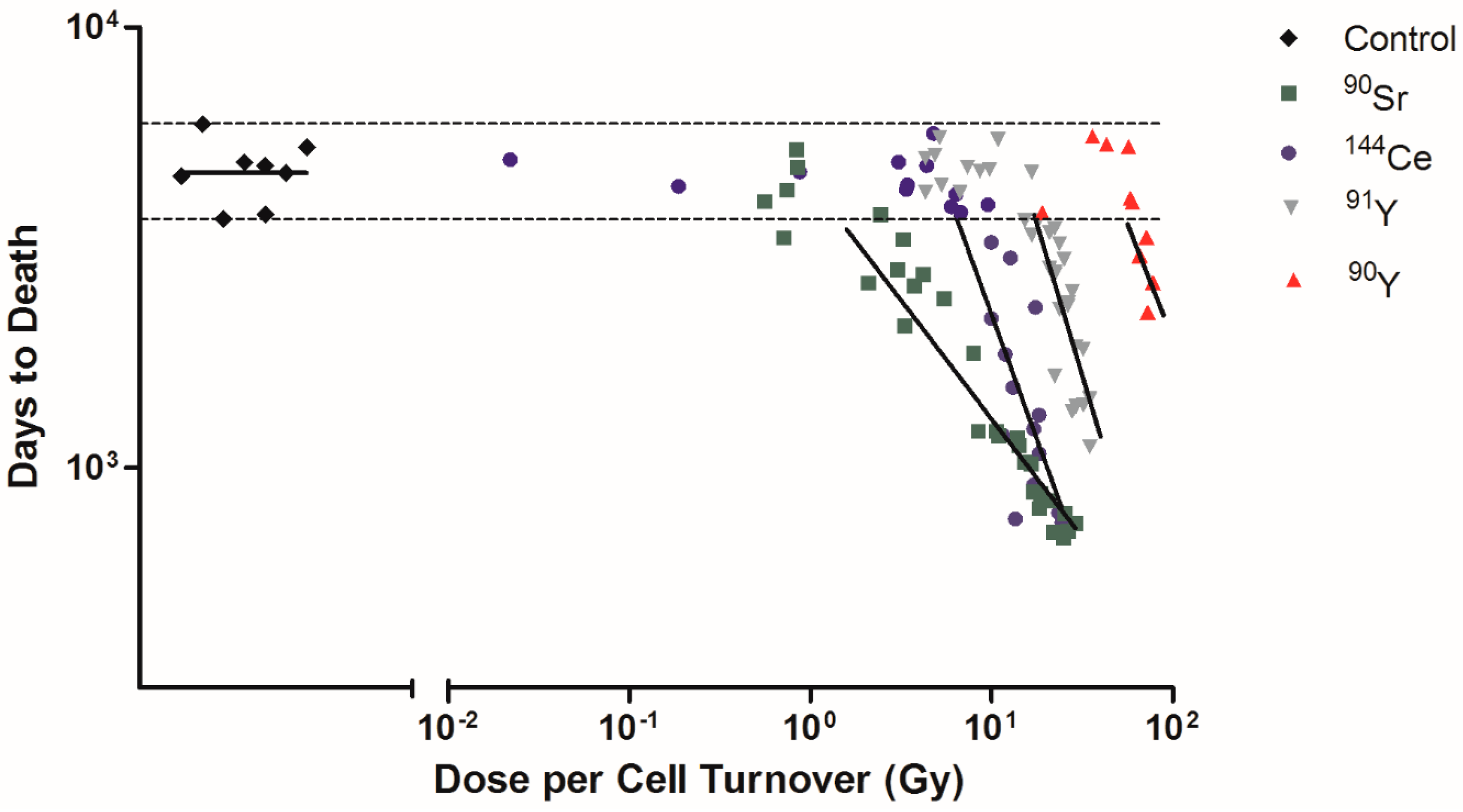
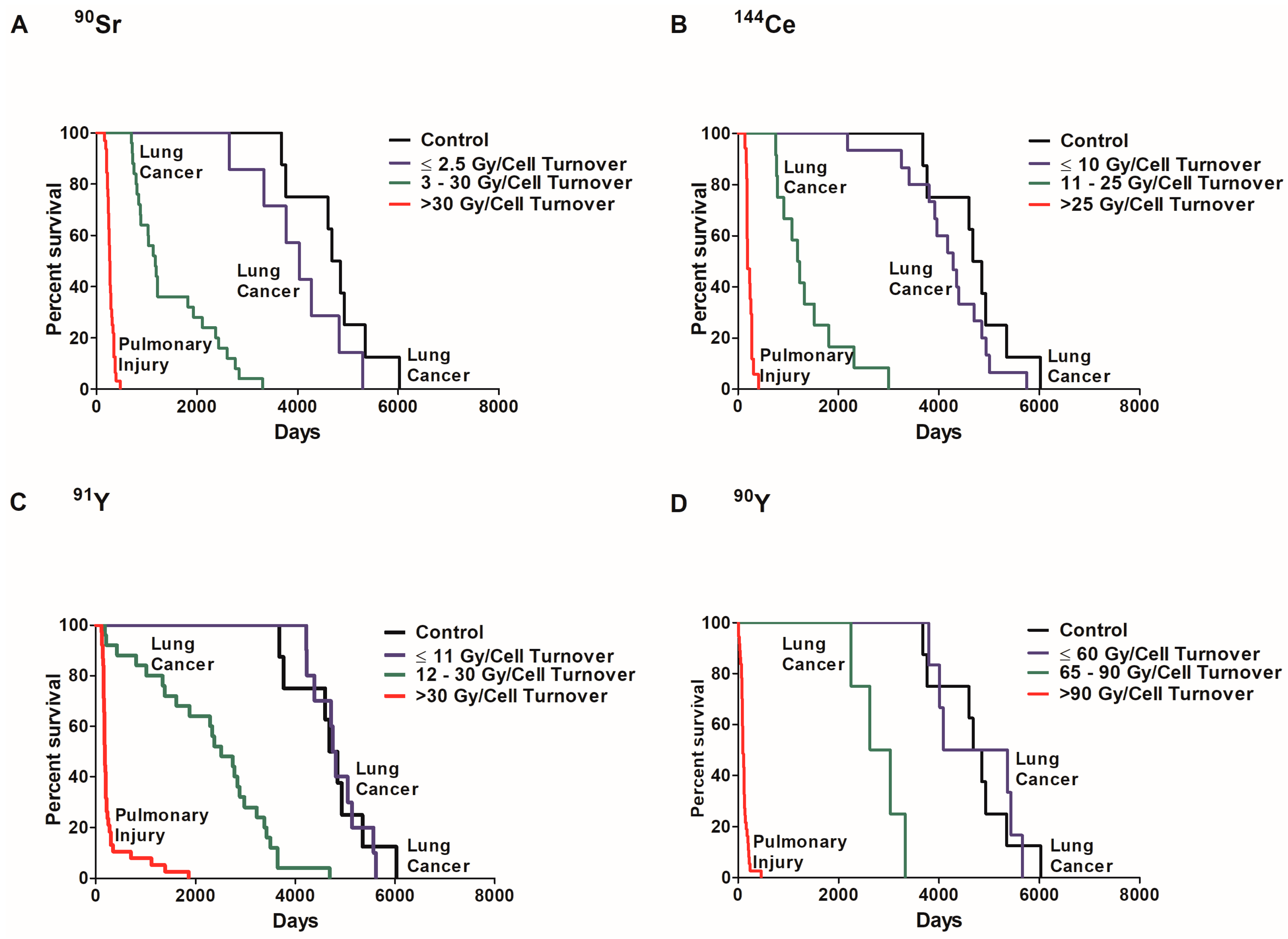
2.2. Dose Rate and Time to Death
2.3. Dose Rate and Dose Response Functions
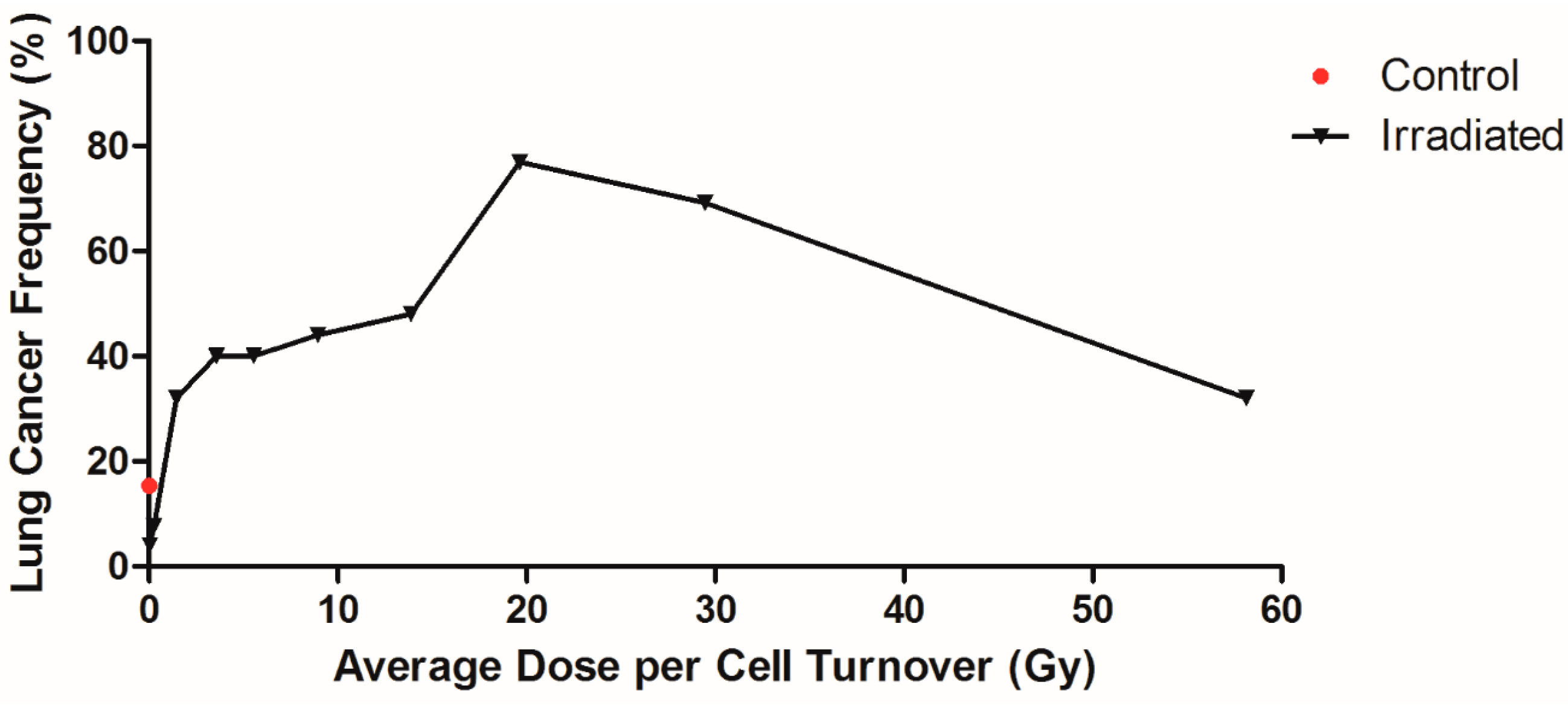
2.4. Influence of Dose per Cell Turnover
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Dose Rate Calculations
Dose per Cell Turnover
4.3. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- National Research Council. Health Risks from Exposure to Low Levels of Ionizing Radiation. In BEIR VII Phase 2; National Academies Press: Washington, DC, USA, 2006. [Google Scholar]
- International Commission on Radiological Protection (ICRP). The 2007 recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann. ICRP 2007, 37, 1–332. [Google Scholar]
- Tubiana, M.; Aurengo, A.; Averbeck, D.; Bonnin, A.; Le Guen, B.; Masse, R.; Monier, R.; Valleron, A.J.; de Vathaire, F. Dose-effect relationships and estimation of the carcinogenic effects of low doses of ionization radiation: The joint report of the Academie des Sciences (PARIS and of the Academie National de Medicine. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 317–319. [Google Scholar] [CrossRef]
- Brooks, A.L.; Eberlein, P.E.; Couch, L.A.; Boecker, B.B. The role of dose-rate on risk from internally-deposited radionuclides and the potential need to separate dose-rate effectiveness factor (DREF) from the dose and dose-rate effectiveness factor (DDREF). Health Phys. 2009, 97, 458–469. [Google Scholar] [CrossRef]
- Hoel, D.G. Comments on the DDREF estimate of the BEIR VII committee. Health Phys. 2015, 108, 351–356. [Google Scholar] [CrossRef]
- Brooks, A.L.; Hoel, D.G.; Preston, R.J. The role of dose rate in radiation cancer risk: Evaluating the effect of dose rate at the molecular, cellular and tissue levels using key events in critical pathways following exposure to low LET radiation. Int. J. Radiat. Biol. 2016, 92, 405–426. [Google Scholar] [CrossRef]
- Brooks, A.L.; McClellan, R.O. Chromosome aberrations and other effects produced by 90Sr-90Y in chinese hamsters. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1969, 16, 545–561. [Google Scholar] [CrossRef]
- Brooks, A.L. Chromosome damage in liver cells from low dose rate alpha, beta, and gamma irradiation: Derivation of RBE. Science 1975, 190, 1090–1092. [Google Scholar] [CrossRef]
- Raabe, O.G. Concerning the health effects of internally deposited radionuclides. Health Phys. 2010, 98, 515–536. [Google Scholar] [CrossRef] [PubMed]
- Raabe, O.G. Concerning ionizing radiation-induced cancer from internally deposited radionuclides. Int. J. Radiat. Biol. 2015, 91, 810–819. [Google Scholar] [CrossRef]
- Hahn, F.F.; Boecker, B.B.; Cuddihy, R.G.; Hobbs, C.H.; McClellan, R.O.; Snipes, M.B. Influence of radiation dose patterns on lung tumor incidence in dogs that inhaled beta emitters: A preliminary report. Radiat. Res. 1983, 96, 505–517. [Google Scholar] [CrossRef]
- Barcellos-Hoff, M.H. It takes a tissue to make a tumor: Epigenetics, cancer and the microenvironment. J. Mammary Gland Biol. Neoplasia 2001, 6, 213–221. [Google Scholar] [CrossRef]
- Barcellos-Hoff, M.H.; Brooks, A.L. Extracellular signaling through the microenvironment: A hypothesis relating carcinogenesis, bystander effects, and genomic instability. Radiat. Res. 2001, 156, 618–627. [Google Scholar] [CrossRef]
- Tomasetti, C.; Vogelstein, B. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science 2015, 347, 78–81. [Google Scholar] [CrossRef]
- Hall, E.J.; Giaccia, A.J. Radiobiology for the Radiologist, 6th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006; pp. 1–546. [Google Scholar]
- Marples, B.; Collis, S.J. Low-Dose Hyper-Radiosensitivity: Past, Present, and Future. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 1310–1318. [Google Scholar] [CrossRef]
- Hahn, F.; Muggenburg, B.A.; Snipes, M.B.; Boecker, B. The toxicity of insoluble cerium-144 inhaled by beagle dogs: Non-neoplastic effects. Radiat. Res. 2001, 155, 95–112. [Google Scholar] [CrossRef]
- Spitz, D.R.; Azzam, E.I.; Li, J.; Gius, D. Metabolic oxidation/reduction reactions and cellular responses to ionizing radiation: A unifying concept in stress response biology. Cancer Metastasis Rev. 2004, 23, 311–322. [Google Scholar] [CrossRef]
- Bogdándi, E.N.; Balogh, A.; Felgyinszki, N.; Szatmári, T.; Persa, E.; Hildebrandt, G.; Sáfrány, G.; Lumniczky, K. Effects of low-dose radiation on the immune system of mice after total-body irradiation. Radiat. Res. 2010, 174, 480–489. [Google Scholar] [CrossRef]
- Bauer, G. Low dose radiation and intercellular induction of apoptosis: Potential implications for the control of oncogenesis. Int. J. Radiat. Biol. 2007, 83, 873–888. [Google Scholar] [CrossRef]
- Brooks, A.L.; Benjamin, S.A.; Jones, R.K.; McClellan, R.O. Interaction of 144Ce and partial hepatectomy in the production of liver neoplasms in the Chinese hamster. Radiat. Res. 1982, 91, 573–588. [Google Scholar] [CrossRef]
- Bender, M.A.; Awa, A.A.; Brooks, A.L.; Evans, H.J.; Groer, P.G.; Littlefield, L.G.; Pereira, C.; Preston, R.J.; Wachholz, B.W. Current status of cytogenetic procedures to detect and quantify previous exposures to radiation. Mutat. Res. Genet. Toxicol. 1988, 196, 103–159. [Google Scholar] [CrossRef]
- Brooks, A.L. Whole animal and in vivo cell systems for detecting mutations. In Safety Evaluation of Drugs and Chemicals; Floyd, W., Ed.; Hemisphere Publishing Corporation: New York, NY, USA, 1986; pp. 167–185. [Google Scholar]
- Jostes, R.F.; Fleck, E.W.; Morgan, T.L.; Stiegler, G.L.; Cross, F.T. Southern blot and polymerase chain reaction exon analyses of HPRT- mutations induced by radon and radon progeny. Radiat. Res. 1994, 137, 371–379. [Google Scholar] [CrossRef]
- German Commission on Radiological Protection. Dose and Dose-Rate Effectiveness Factor (DDREF) Recommendation by German Commission on Radiological Protection with Scientific Grounds. Available online: http://www.ssk.de/SharedDocs/Beratungsergebnisse_E/2014/DDREF_e.html (accessed on 10 May 2017).
- Morgan, W.F.; Bair, W.J. Issues in low dose radiation biology: The controversy continues. A perspective. Radiat. Res. 2013, 179, 501–510. [Google Scholar] [CrossRef]
- Mauderly, J.L.; Daynes, R.A. Biennial Report on Long-Term Dose–response Studies of Inhaled or Injected Radionuclides, 1991–1993; Defense Technical Information Center: Fort Belvoir, VA, USA, 1994. [Google Scholar]
- Mauderly, J.L.; Pickrell, J.A.; Hobbs, C.H.; Benjamin, S.A.; Hahn, F.F.; Jones, R.K.; Barnes, J.E. The effects of inhaled 90Y fused clay aerosol on pulmonary function and related parameters of the Beagle dog. Radiat. Res. 1973, 56, 83–96. [Google Scholar] [CrossRef]
- Pickrell, J.A.; Schnizlein, C.T.; Hahn, F.F.; Snipes, M.B.; Jones, R.K. Radiation-induced pulmonary fibrosis: Study of changes in collagen constituents in different lung regions of beagle dogs after inhalation of beta-emitting radionuclides. Radiat. Res. 1978, 74, 363–377. [Google Scholar] [CrossRef]
- Kotton, D.N.; Morrisey, E.E. Lung regeneration: Mechanisms, applications and emerging stem cell populations. Nat. Med. 2014, 20, 822–832. [Google Scholar] [CrossRef]
- Barkauskas, C.E.; Cronce, M.J.; Rackley, C.R.; Bowie, E.J.; Keene, D.R.; Stripp, B.R.; Randell, S.H.; Noble, P.W.; Hogan, B.L.M. Type 2 alveolar cells are stem cells in adult lung. J. Clin. Investig. 2013, 123, 3025–3036. [Google Scholar] [CrossRef]
- Adamson, I.Y.R. Cellular Kinetics of the Lung. In Toxicology of Inhaled Materials; Witschi, H., Brian, J., Eds.; Springer: Berlin/Heidelberg, Germany, 1985; pp. 289–317. [Google Scholar]
- Johnson, N.F.; Hubbs, A.F.; Thomassen, D.G.; Thomassen, D.G.; Nettesheim, P. Epithelial progenitor cells in the rat respiratory tract. In Biology, Toxicology and Carcinogenesis of Respiratory Tract Epithelium; Thomassen, D.G., Nettesheim, P., Eds.; Hemisphere Publishing Corporation: Washington, DC, USA, 1990; pp. 88–89. [Google Scholar]
| Radionuclide Infused Aluminosilicate Particles | Physical Half-Life | Effective Half-Life in Lung (days) | Average Time to Deliver 95% of Total Dose (days) | Average Number of Cell Turnovers * | Average Dose/Cell Turnover (Gy) * |
|---|---|---|---|---|---|
| 90Sr | 29 year | 600 | 1473 | 49 | 26.65 |
| 144Ce | 285 days | 175 | 670 | 22 | 21.53 |
| 91Y | 59 days | 50 | 201 | 7 | 31.70 |
| 90Y | 2.6 days | 2.5 | 11 | 1 | 116.18 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puukila, S.; Thome, C.; Brooks, A.L.; Woloschak, G.; Boreham, D.R. The Role of Radiation Induced Injury on Lung Cancer. Cancers 2017, 9, 89. https://doi.org/10.3390/cancers9070089
Puukila S, Thome C, Brooks AL, Woloschak G, Boreham DR. The Role of Radiation Induced Injury on Lung Cancer. Cancers. 2017; 9(7):89. https://doi.org/10.3390/cancers9070089
Chicago/Turabian StylePuukila, Stephanie, Christopher Thome, Antone L. Brooks, Gayle Woloschak, and Douglas R. Boreham. 2017. "The Role of Radiation Induced Injury on Lung Cancer" Cancers 9, no. 7: 89. https://doi.org/10.3390/cancers9070089





