Recent Breakthroughs in the Conversion of Ethanol to Butadiene
Abstract
:1. Introduction
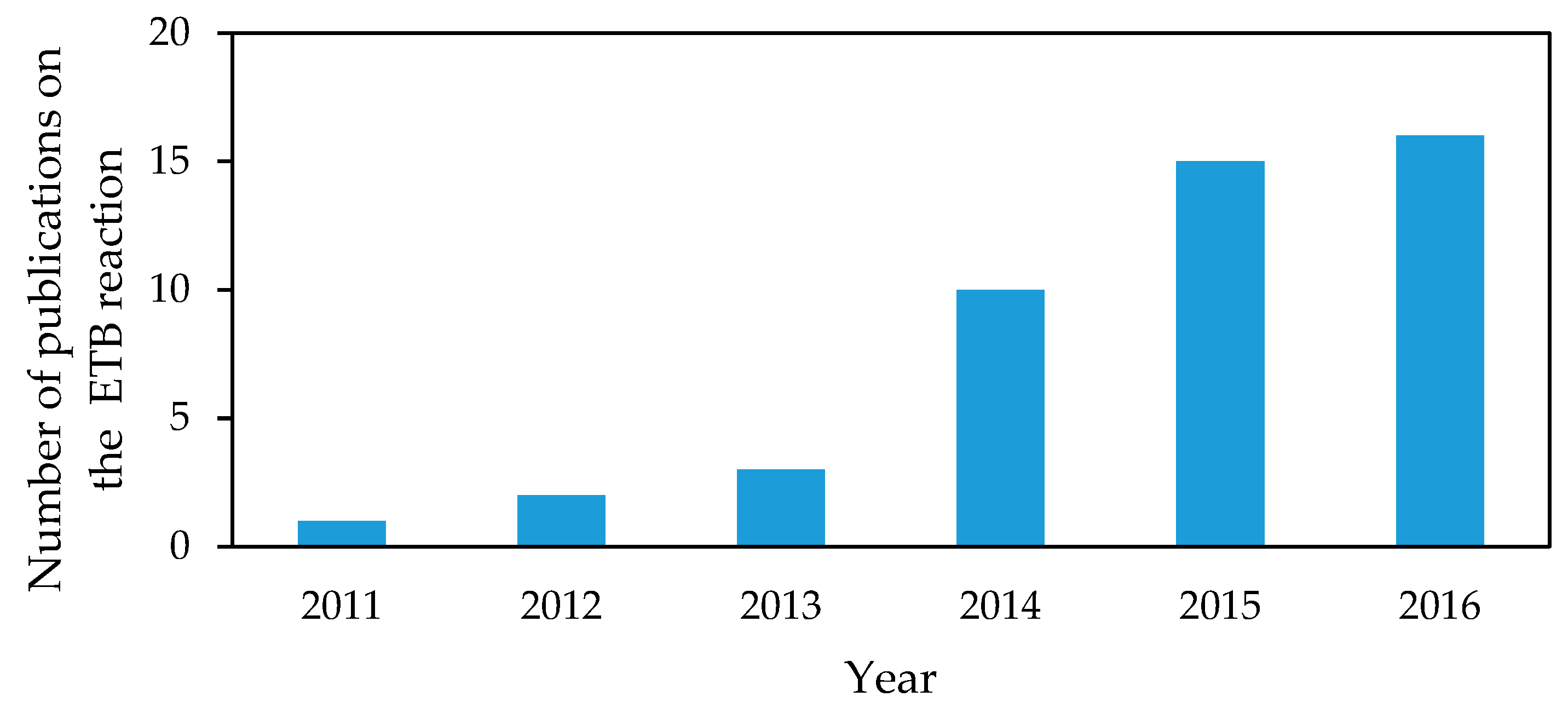
1.1. Scope of the Review
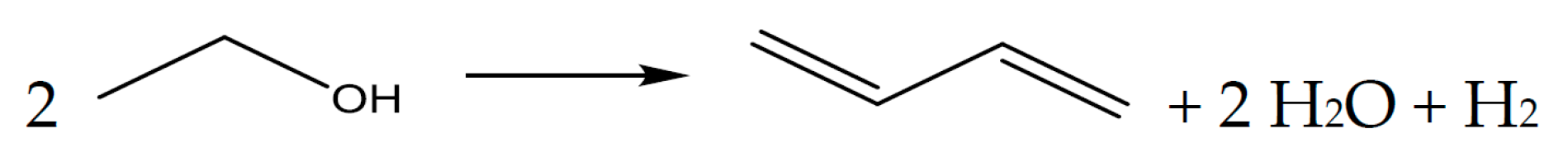
1.2. Reaction Details Reaction of Ethanol to Butadiene
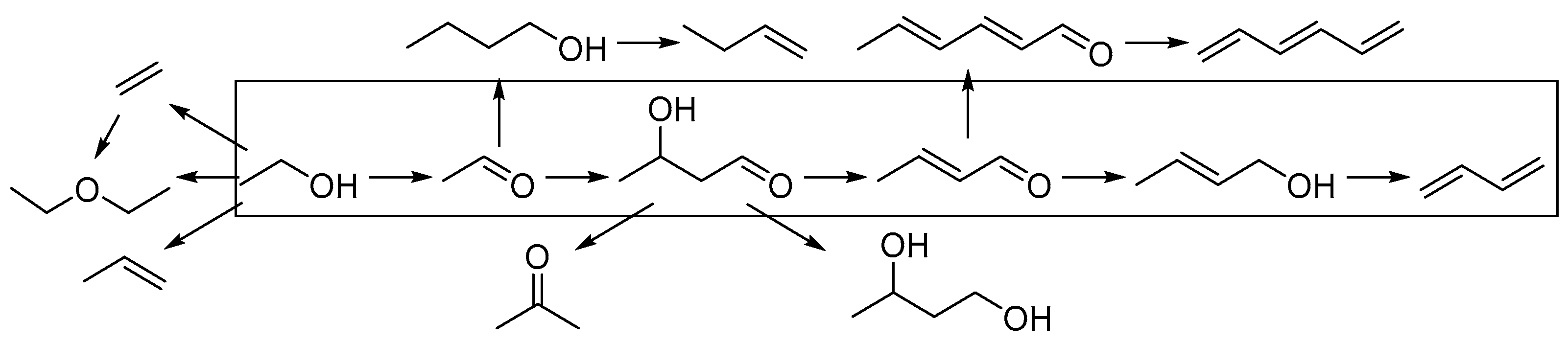
1.2.1. Generalities Mechanism
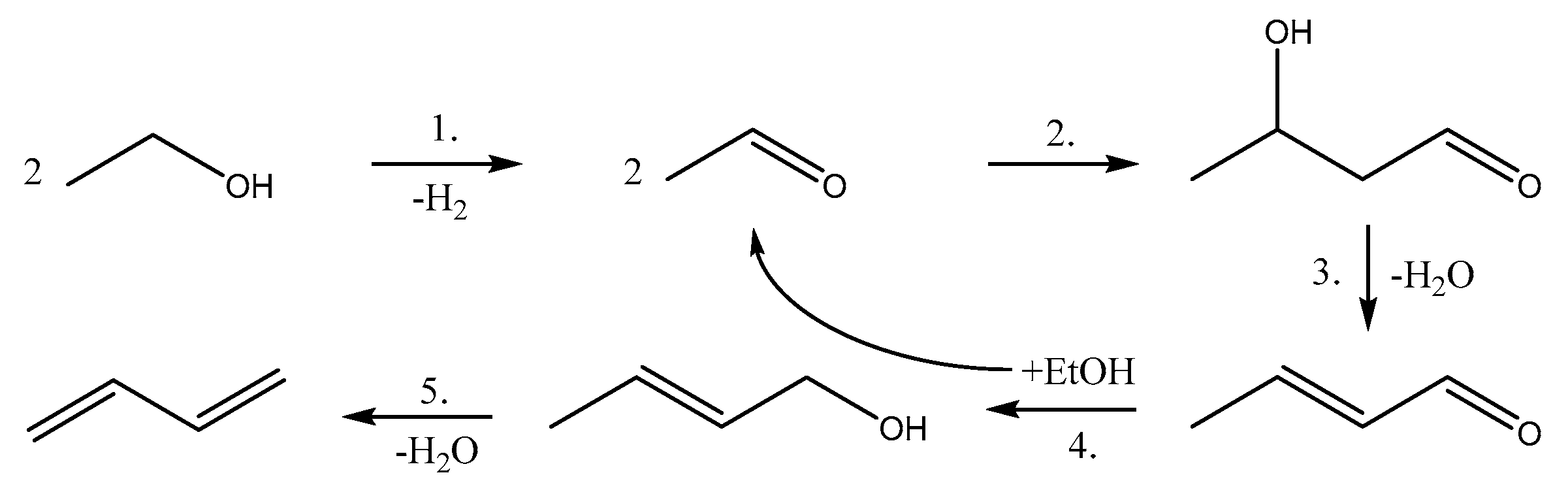
1.2.2. The Kagan Mechanism
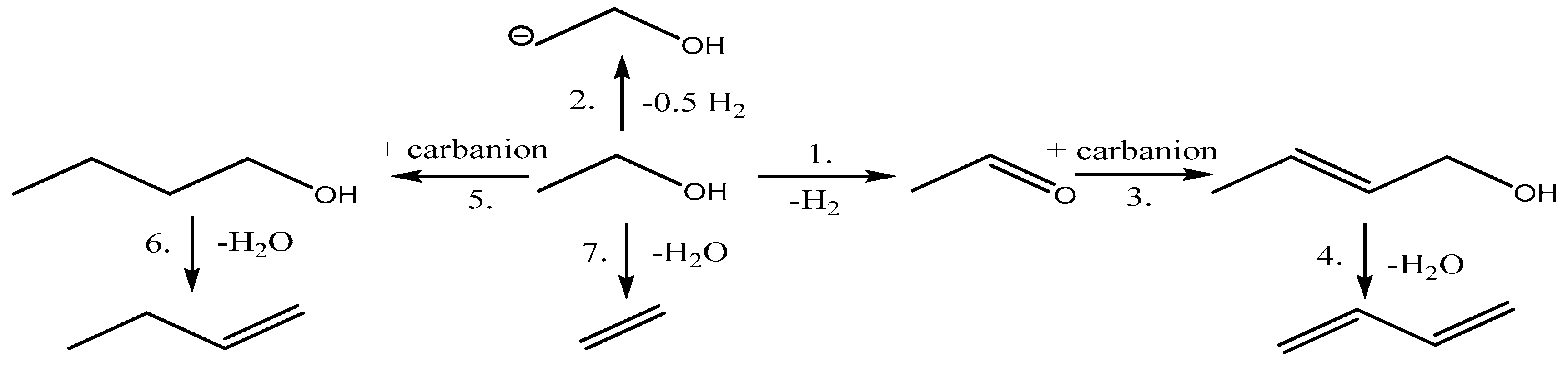
1.2.3. The Cavani Mechanism
1.2.4. Byproducts
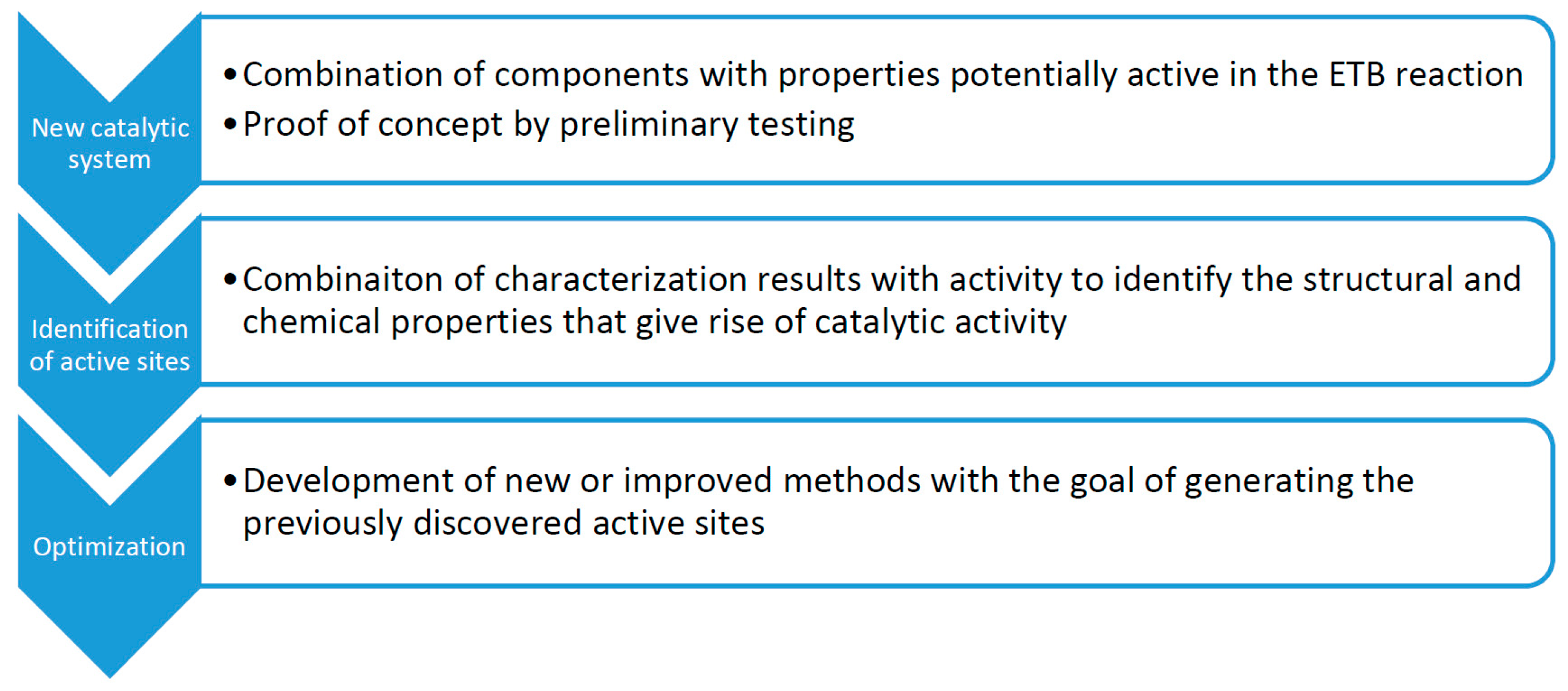
1.3. Catalyst Design
1.4. Performances and Reaction Conditions
2. Catalytic Systems
2.1. Magnesium-Silica System
2.1.1. Introduction
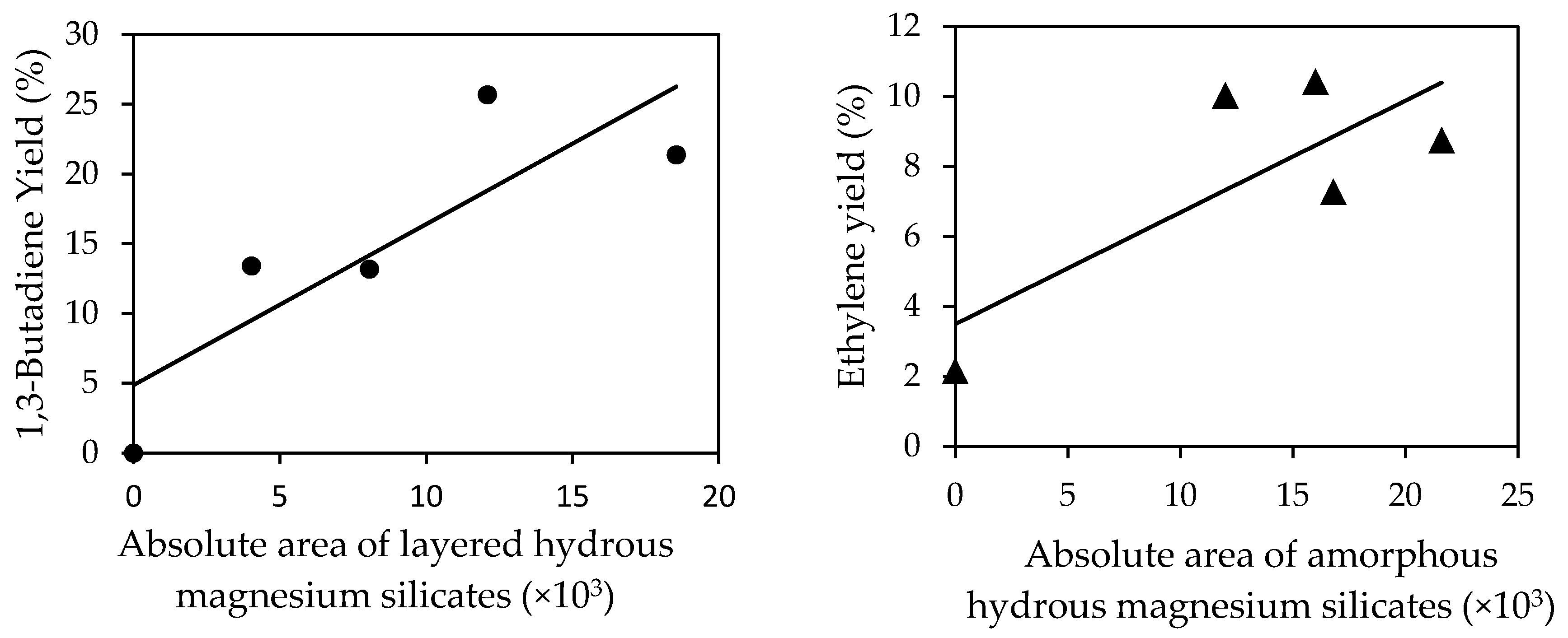
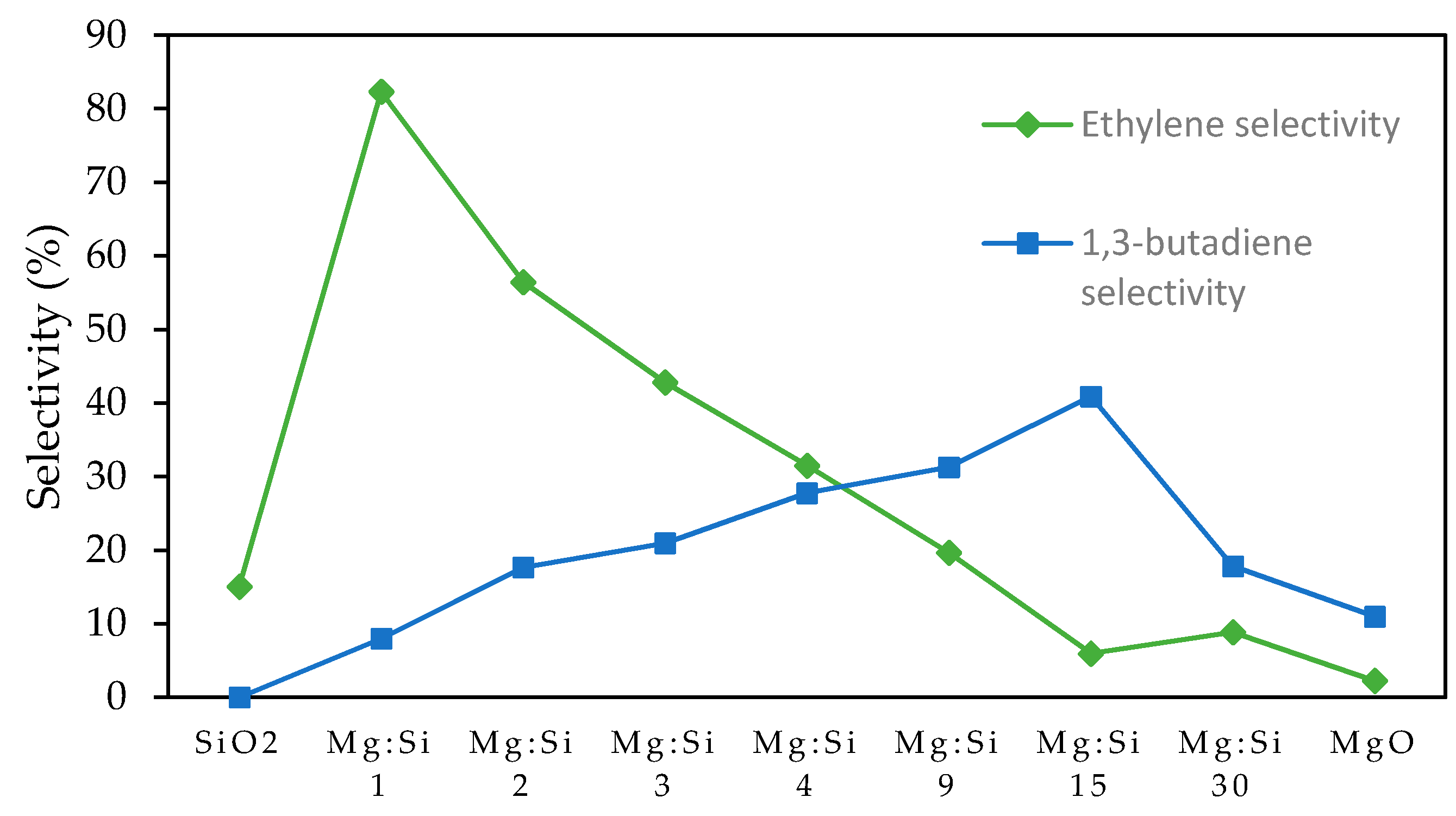
2.1.2. Unpromoted MgO-SiO2
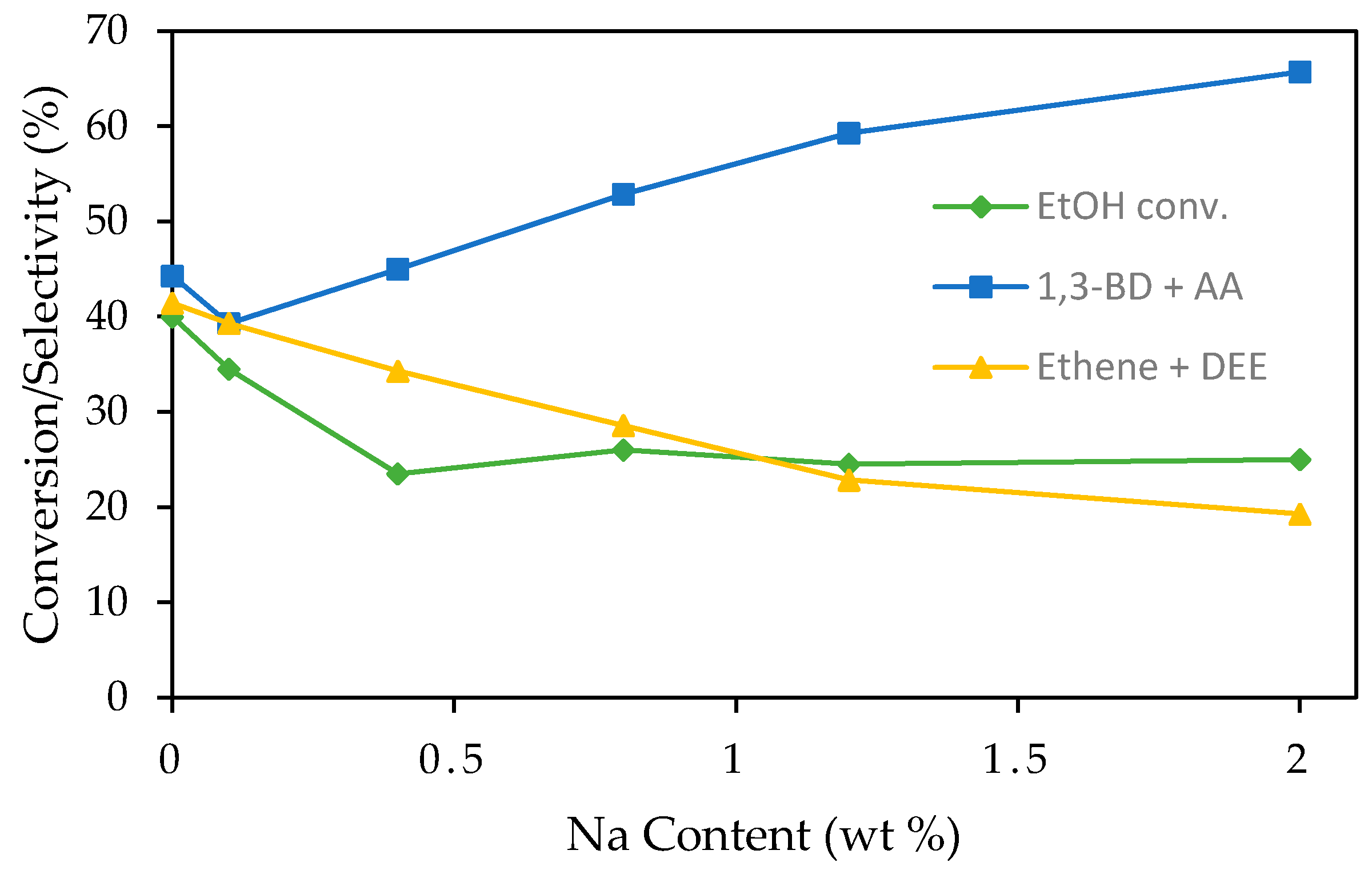
2.1.3. Metal-Promoted MgO-SiO2
2.1.4. Lewis Acid Promoted MgO-SiO2
2.1.5. Promoted Magnesium Silicate Minerals
2.2. Zirconium Catalytic System
2.2.1. Introduction
2.2.2. Catalysts for the One-Step Process
2.2.3. Catalysts for the Two-Step Process
2.3. Other Catalytic Systems
Other Acid Catalysts
3. Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ouhadi, T.; Abdou-Sabet, S.; Wussow, H.-G.; Ryan, L.M.; Plummer, L.; Baumann, F.E.; Lohmar, J.; Vermeire, H.F.; Malet, F.L.G. Thermoplastic elastomers. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2014; pp. 1–41. [Google Scholar]
- Dahlmann, M.; Grub, J.; Löser, E. Butadiene. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011; Vol. 100 C, pp. 1–24. [Google Scholar]
- Shylesh, S.; Gokhale, A.A.; Scown, C.D.; Kim, D.; Ho, C.R.; Bell, A.T. From sugars to wheels: The conversion of ethanol to 1,3-butadiene over metal-promoted magnesia-silicate catalysts. ChemSusChem 2016, 9, 1462–1472. [Google Scholar] [CrossRef] [PubMed]
- Ezinkwo, G.O.; Tretyakov, V.P.; Aliyu, A.; Ilolov, A.M. Fundamental issues of catalytic conversion of bio-ethanol into butadiene. ChemBioEng Rev. 2014, 1, 194–203. [Google Scholar] [CrossRef]
- Sun, J.; Wang, Y. Recent advances in catalytic conversion of ethanol to chemicals. ACS Catal. 2014, 4, 1078–1090. [Google Scholar] [CrossRef]
- Burla, J.; Fehnel, R.; Louie, P.; Terpeluk, P. Two-Step Production of 1,3-Butadiene from Ethanol. Available online: http://repository.upenn.edu/cgi/viewcontent.cgi?article=1033&context=cbe_sdr (accessed on 12 December 2016).
- Makshina, E.V.; Janssens, W.; Sels, B.F.; Jacobs, P.A. Catalytic study of the conversion of ethanol into 1,3-butadiene. Catal. Today 2012, 198, 338–344. [Google Scholar] [CrossRef]
- Makshina, E.V.; Dusselier, M.; Janssens, W.; Degrève, J.; Jacobs, P.A.; Sels, B.F. Review of old chemistry and new catalytic advances in the on-purpose synthesis of butadiene. Chem. Soc. Rev. 2014, 43, 7917–7953. [Google Scholar] [CrossRef] [PubMed]
- Angelici, C.; Weckhuysen, B.M.; Bruijnincx, P.C.A. Chemocatalytic conversion of ethanol into butadiene and other bulk chemicals. ChemSusChem 2013, 6, 1595–1614. [Google Scholar] [CrossRef] [PubMed]
- Jones, M. Catalytic transformation of ethanol into 1,3-butadiene. Chem. Cent. J. 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, M.; Keir, C.; Iulio, C.; Robertson, R.; Williams, C.; Apperley, D. Investigations into the conversion of ethanol into 1,3-butadiene. Catal. Sci. Technol. 2011, 1, 267–272. [Google Scholar] [CrossRef]
- Chieregato, A.; Velasquez Ochoa, J.; Bandinelli, C.; Fornasari, G.; Cavani, F.; Mella, M. On the chemistry of ethanol on basic oxides: Revising mechanisms and intermediates in the Lebedev and Guerbet reactions. ChemSusChem 2015, 8, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, J.V.; Bandinelli, C.; Vozniuk, O.; Chieregato, A.; Malmusi, A.; Recchi, C.; Cavani, F. An analysis of the chemical, physical and reactivity features of MgO–SiO2 catalysts for butadiene synthesis with the Lebedev process. Green Chem. 2016, 18, 1653–1663. [Google Scholar] [CrossRef]
- Chieregato, A.; Ochoa, J.V.; Cavani, F. Olefins from biomass. In Chemicals and Fuels from Bio-Based Building Blocks; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2016; pp. 1–32. [Google Scholar]
- Jones, H.E.; Stahly, E.E.; Corson, B.B. Butadiene from ethanol. reaction mechanism. J. Am. Chem. Soc. 1949, 71, 1822–1828. [Google Scholar] [CrossRef]
- Sushkevich, V.L.; Ivanova, I.I.; Ordomsky, V.V.; Taarning, E. Design of a metal-promoted oxide catalyst for the selective synthesis of butadiene from ethanol. ChemSusChem 2014, 7, 2527–2536. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Liu, Z.; Zhang, M.; Tong, L. Study on the mechanism of butadiene formation from ethanol. Catal. Letters 2014, 144, 2071–2079. [Google Scholar] [CrossRef]
- Müller, P.; Burt, S.P.; Love, A.M.; McDermott, W.P.; Wolf, P.; Hermans, I. Mechanistic study on the Lewis acid catalyzed synthesis of 1,3-butadiene over Ta-BEA using modulated operando DRIFTS-MS. ACS Catal. 2016, 6, 6823–6832. [Google Scholar] [CrossRef]
- Hayashi, Y.; Akiyama, S.; Miyaji, A.; Sekiguchi, Y.; Sakamoto, Y.; Shiga, A.; Koyama, T.; Motokura, K.; Baba, T. Experimental and computational studies of the roles of MgO and Zn in talc for the selective formation of 1,3-butadiene in the conversion of ethanol. Phys. Chem. Chem. Phys. 2016, 18, 25191–25209. [Google Scholar] [CrossRef] [PubMed]
- Veibel, S.; Nielsen, J.I. On the mechanism of the Guerbet reaction. Tetrahedron 1967, 23, 1723–1733. [Google Scholar] [CrossRef]
- Kozlowski, J.T.; Davis, R.J. Heterogeneous catalysts for the Guerbet coupling of alcohols. ACS Catal. 2013, 3, 1588–1600. [Google Scholar] [CrossRef]
- Ho, C.R.; Shylesh, S.; Bell, A.T. Mechanism and kinetics of ethanol coupling to butanol over hydroxyapatite. ACS Catal. 2016, 6, 939–948. [Google Scholar] [CrossRef]
- Scalbert, J.; Thibault-Starzyk, F.; Jacquot, R.; Morvan, D.; Meunier, F. Ethanol condensation to butanol at high temperatures over a basic heterogeneous catalyst: How relevant is acetaldehyde self-aldolization? J. Catal. 2014, 311, 28–32. [Google Scholar] [CrossRef]
- Da Ros, S.; Jones, M.D.; Mattia, D.; Schwaab, M.; Barbosa-Coutinho, E.; Rabelo-Neto, R.C.; Bellot Noronha, F.; Carlos Pinto, J. Microkinetic analysis of ethanol to 1,3-butadiene reactions over MgO–SiO2 catalysts based on characterization of experimental fluctuations. Chem. Eng. J. 2016, 308, 988–1000. [Google Scholar] [CrossRef]
- Angelici, C.; Velthoen, M.E. Z.; Weckhuysen, B.M.; Bruijnincx, P.C. A. Effect of preparation method and CuO promotion in the conversion of ethanol into 1,3-butadiene over SiO2–MgO catalysts. ChemSusChem 2014, 7, 2505–2515. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.K.; Ganguly, N.D. One-step catalytic conversion of ethanol to butadiene in the fixed bed. II Binary- and ternary-oxide catalysts. J. Appl. Chem. 1962, 12, 105–110. [Google Scholar] [CrossRef]
- Chung, S.-H.; Angelici, C.; Hinterding, S.O.M.; Weingarth, M.; Baldus, M.; Houben, K.; Weckhuysen, B.M.; Bruijnincx, P.C.A. On the role of magnesium silicates in wet-kneaded silica-magnesia catalysts for the Lebedev ethanol-to-butadiene process. ACS Catal. 2016, 6, 4034–4045. [Google Scholar] [CrossRef]
- Larina, O.V.; Kyriienko, P.I.; Trachevskii, V.V.; Vlasenko, N.V.; Soloviev, S.O. Effect of mechanochemical treatment on acidic and catalytic properties of MgO–SiO2 composition in the conversion of ethanol to 1,3-butadiene. Theor. Exp. Chem. 2016, 51, 387–393. [Google Scholar] [CrossRef]
- Janssens, W.; Makshina, E.V.; Vanelderen, P.; De Clippel, F.; Houthoofd, K.; Kerkhofs, S.; Martens, J.A.; Jacobs, P.A.; Sels, B.F. Ternary Ag/MgO–SiO2 catalysts for the conversion of ethanol into butadiene. ChemSusChem 2015, 8, 994–1008. [Google Scholar] [CrossRef] [PubMed]
- Da Ros, S.; Jones, M.D.; Mattia, D.; Pinto, J.C.; Schwaab, M.; Noronha, F.B.; Kondrat, S.A.; Clarke, T.C.; Taylor, S.H. Ethanol to 1,3-butadiene conversion by using ZrZn-containing MgO/SiO2 systems prepared by Co-precipitation and effect of catalyst acidity modification. ChemCatChem 2016, 8, 2376–2386. [Google Scholar] [CrossRef] [Green Version]
- Larina, O.V.; Kyriienko, P.I.; Soloviev, S.O. Ethanol conversion to 1,3-butadiene on ZnO/MgO–SiO2 catalysts: effect of ZnO content and MgO:SiO2 ratio. Catal. Lett. 2015, 145, 1162–1168. [Google Scholar] [CrossRef]
- Sushkevich, V.L.; Ivanova, I.I. Ag-promoted ZrBEA zeolites obtained by post-synthetic modification for conversion of ethanol to butadiene. ChemSusChem 2016, 9, 2216–2225. [Google Scholar] [CrossRef] [PubMed]
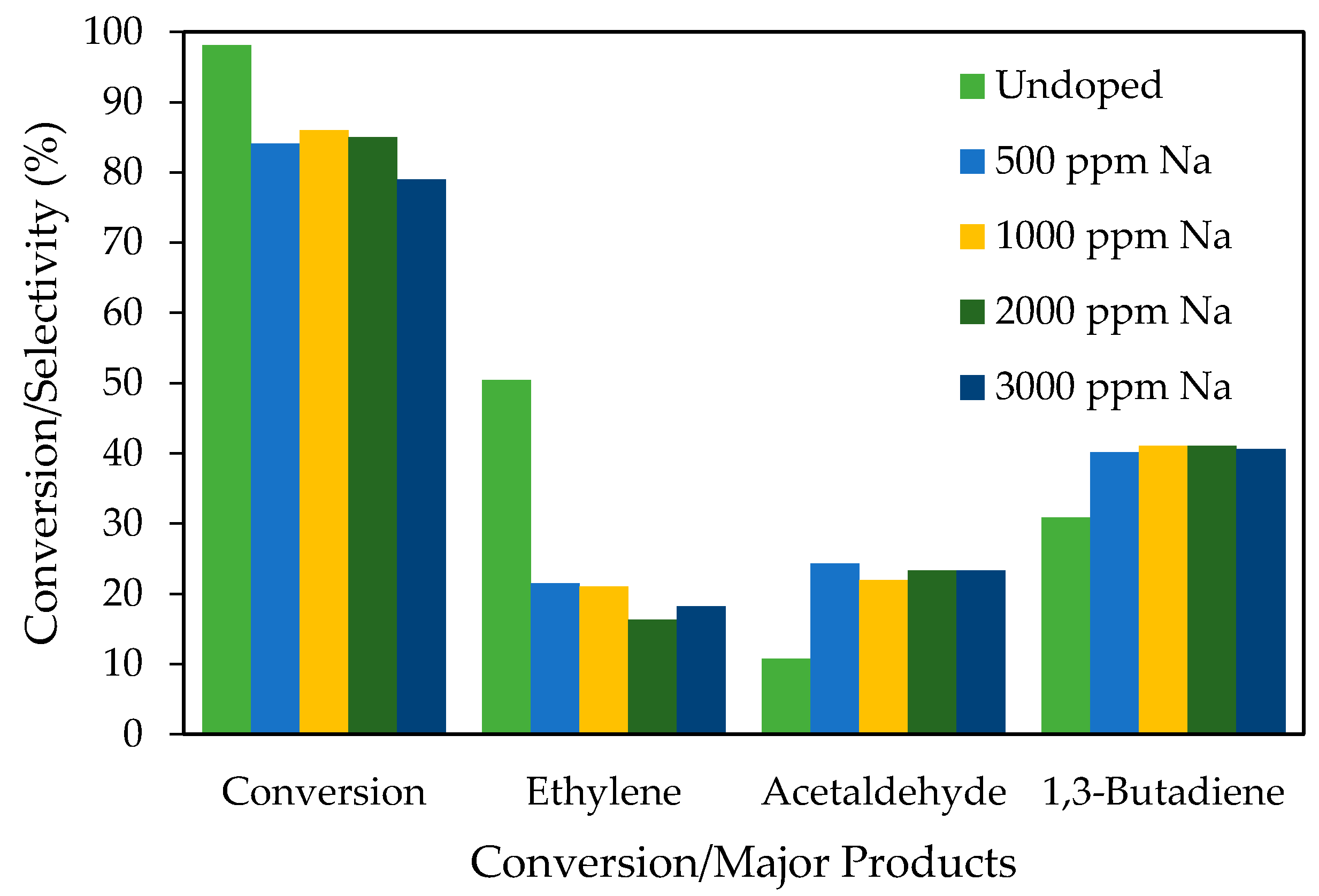
- Baylon, R.A.L.; Sun, J.; Wang, Y. Conversion of ethanol to 1,3-butadiene over Na doped ZnxZryOz mixed metal oxides. Catal. Today 2014, 259, 446–452. [Google Scholar] [CrossRef]
- Larina, O.V.; Kyriienko, P.I.; Soloviev, S.O. Effect of lanthanum in Zn-La(-Zr)-Si oxide compositions on their activity in the conversion of ethanol into 1,3-butadiene. Theor. Exp. Chem. 2016, 52, 51–56. [Google Scholar] [CrossRef]
- Han, Z.; Li, X.; Zhang, M.; Liu, Z.; Gao, M. Sol–gel synthesis of ZrO2–SiO2 catalysts for the transformation of bioethanol and acetaldehyde into 1,3-butadiene. RSC Adv. 2015, 5, 103982–103988. [Google Scholar] [CrossRef]
- Cheong, J.L.; Shao, Y.; Tan, S.J. R.; Li, X.; Zhang, Y.; Lee, S.S. Highly active and selective Zr/MCF catalyst for production of 1,3-butadiene from ethanol in a dual fixed bed reactor system. ACS Sustain. Chem. Eng. 2016, 4, 4887–4894. [Google Scholar] [CrossRef]
- De Baerdemaeker, T.; Feyen, M.; Müller, U.; Yilmaz, B.; Xiao, F.S.; Zhang, W.; Yokoi, T.; Bao, X.; Gies, H.; De Vos, D.E. Bimetallic Zn and Hf on silica catalysts for the conversion of ethanol to 1,3-butadiene. ACS Catal. 2015, 5, 3393–3397. [Google Scholar] [CrossRef]
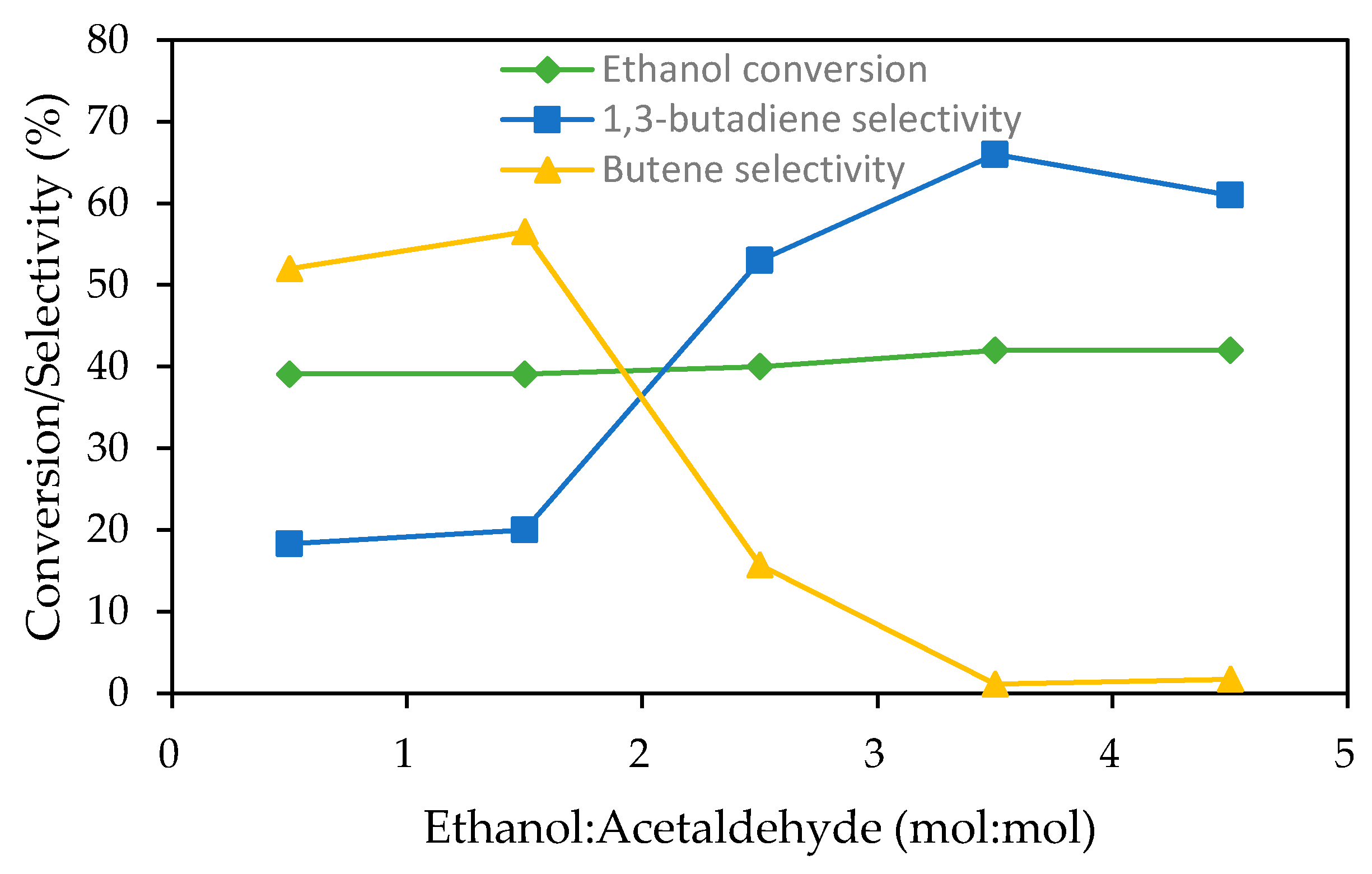
- Kyriienko, P.I.; Larina, O.V.; Soloviev, S.O.; Orlyk, S.M.; Dzwigaj, S. High selectivity of TaSiBEA zeolite catalysts in 1,3-butadiene production from ethanol and acetaldehyde mixture. Catal. Commun. 2016, 77, 123–126. [Google Scholar] [CrossRef]
- Kyriienko, P.I.; Larina, O.V.; Popovych, N.O.; Soloviev, S.O.; Millot, Y.; Dzwigaj, S. Effect of the niobium state on the properties of NbSiBEA as bifunctional catalysts for gas- and liquid-phase tandem processes. J. Mol. Catal. A 2016, 424, 27–36. [Google Scholar] [CrossRef]
- La-Salvia, N.; Lovón-Quintana, J.J.; Valença, G.P. Vapor-phase catalytic conversion of ethanol into 1,3-butadiene on Cr-Ba/MCM-41 catalysts. Brazilian J. Chem. Eng. 2015, 32, 489–500. [Google Scholar] [CrossRef]
- Corson, B.; Jones, H.; Welling, C.; Hinckley, J.; Stahly, E. Butadiene from ethyl alcohol. Catalysis in the one-and two-stop processes. Ind. Eng. Chem. 1950, 42, 359–373. [Google Scholar] [CrossRef]
- Ohnishi, R.; Akimoto, T.; Tanabe, K. Pronounced catalytic activity and selectivity of MgO–SiO2–Na2O for synthesis of buta-1,3-diene from ethanol. J. Chem. Soc., Chem. Commun. 1985, 70, 1613–1614. [Google Scholar] [CrossRef]
- Patel, A.D.; Meesters, K.; den Uil, H.; de Jong, E.; Blok, K.; Patel, M.K. Sustainability assessment of novel chemical processes at early stage: Application to biobased processes. Energy Environ. Sci. 2012, 5, 8430. [Google Scholar] [CrossRef]
- Lewandowski, M.; Babu, G.S.; Vezzoli, M.; Jones, M.D.; Owen, R.E.; Mattia, D.; Plucinski, P.; Mikolajska, E.; Ochenduszko, A.; Apperley, D.C. Investigations into the conversion of ethanol to 1,3-butadiene using MgO:SiO2 supported catalysts. Catal. Commun. 2014, 49, 25–28. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Hensen, E.J. M. Highly efficient and robust Au/MgCuCr2O4 catalyst for gas-phase oxidation of ethanol to acetaldehyde. J. Am. Chem. Soc. 2013, 135, 14032–14035. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.K.; Avasthi, B.N. One-step catalytic conversion of ethanol to butadiene in a fluidized bed. Ind. Eng. Chem. Process Des. Dev. 1963, 2, 45–51. [Google Scholar] [CrossRef]
- Kim, T.W.; Kim, J.W.; Kim, S.Y.; Chae, H.J.; Kim, J.R.; Jeong, S.Y.; Kim, C.U. Butadiene production from bioethanol and acetaldehyde over tantalum oxide-supported spherical silica catalysts for circulating fluidized bed. Chem. Eng. J. 2014, 278, 217–223. [Google Scholar] [CrossRef]
- Quattlebaum, W.M.; Toussaint, W.J.; Dunn, J.T. Deoxygenation of certain aldehydes and ketones: preparation of butadiene and styrene. J. Am. Chem. Soc. 1947, 1491, 593–599. [Google Scholar] [CrossRef]
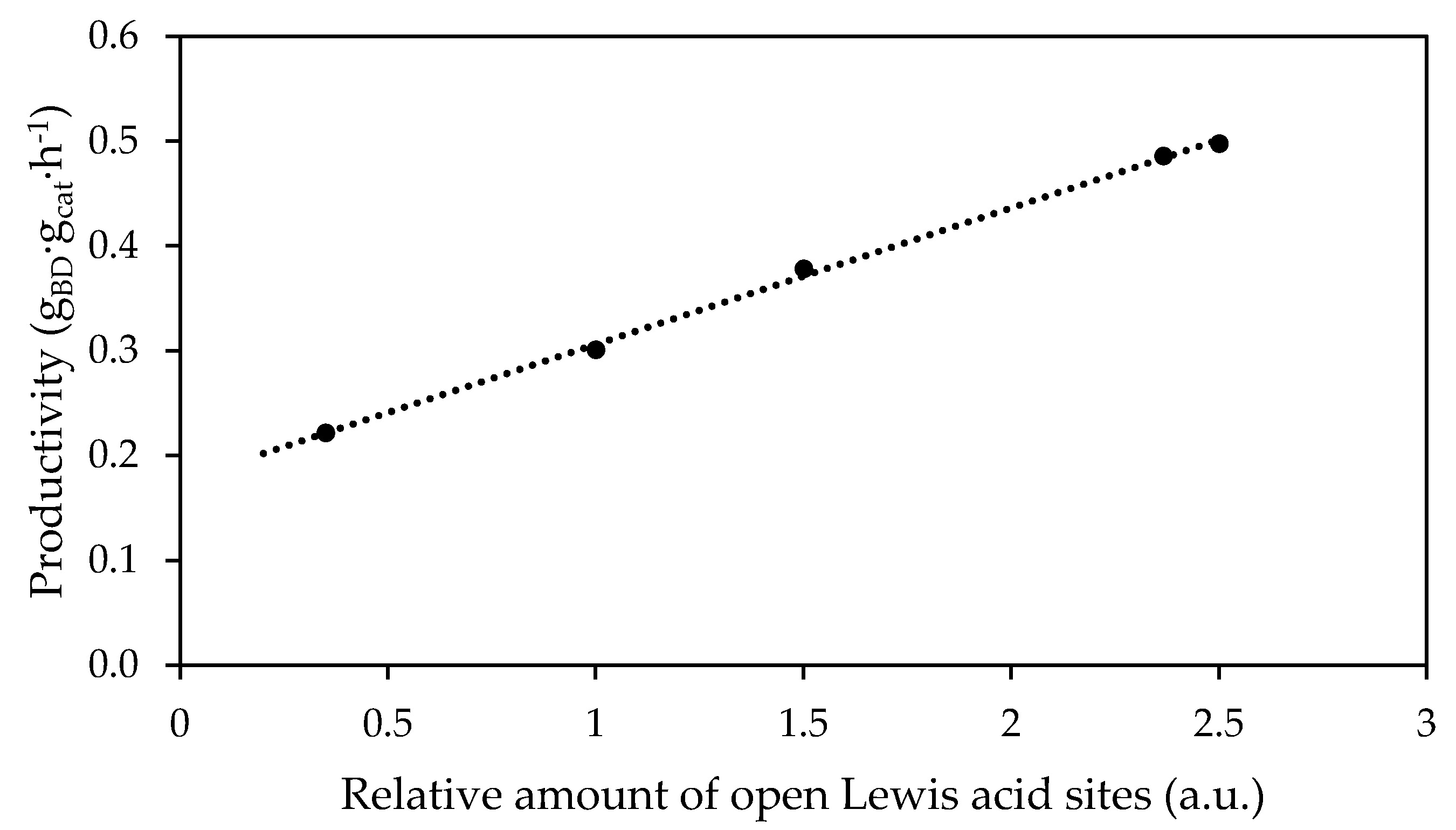
- Sushkevich, V.L.; Palagin, D.; Ivanova, I.I. With open arms: Open sites of ZrBEA zeolite facilitate selective synthesis of butadiene from ethanol. ACS Catal. 2015, 5, 4833–4836. [Google Scholar] [CrossRef]
- Angelici, C.; Meirer, F.; Van Der Eerden, A.M.J.; Schaink, H.L.; Goryachev, A.; Hofmann, J.P.; Hensen, E.J. M.; Weckhuysen, B.M.; Bruijnincx, P.C.A. Ex situ and operando studies on the role of copper in Cu-promoted SiO2–MgO catalysts for the Lebedev ethanol-to-butadiene process. ACS Catal. 2015, 5, 6005–6015. [Google Scholar] [CrossRef]
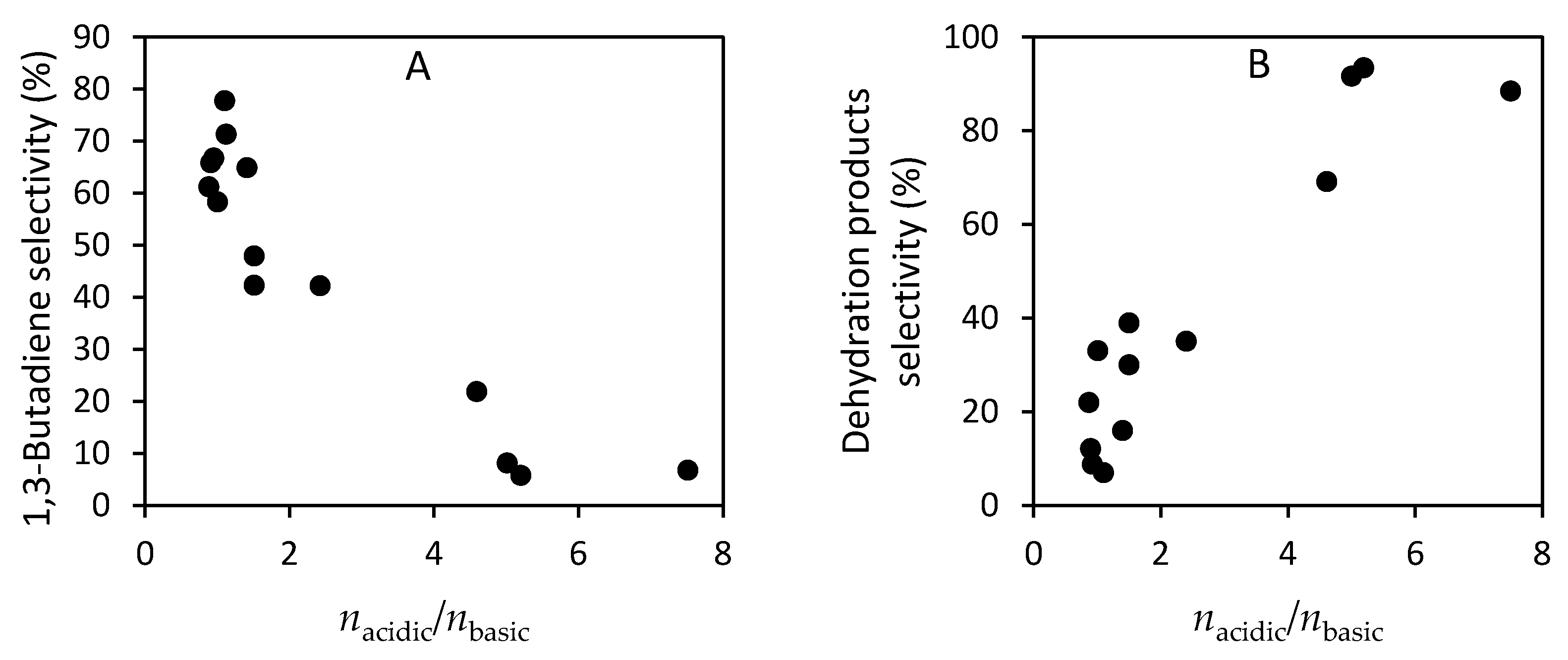
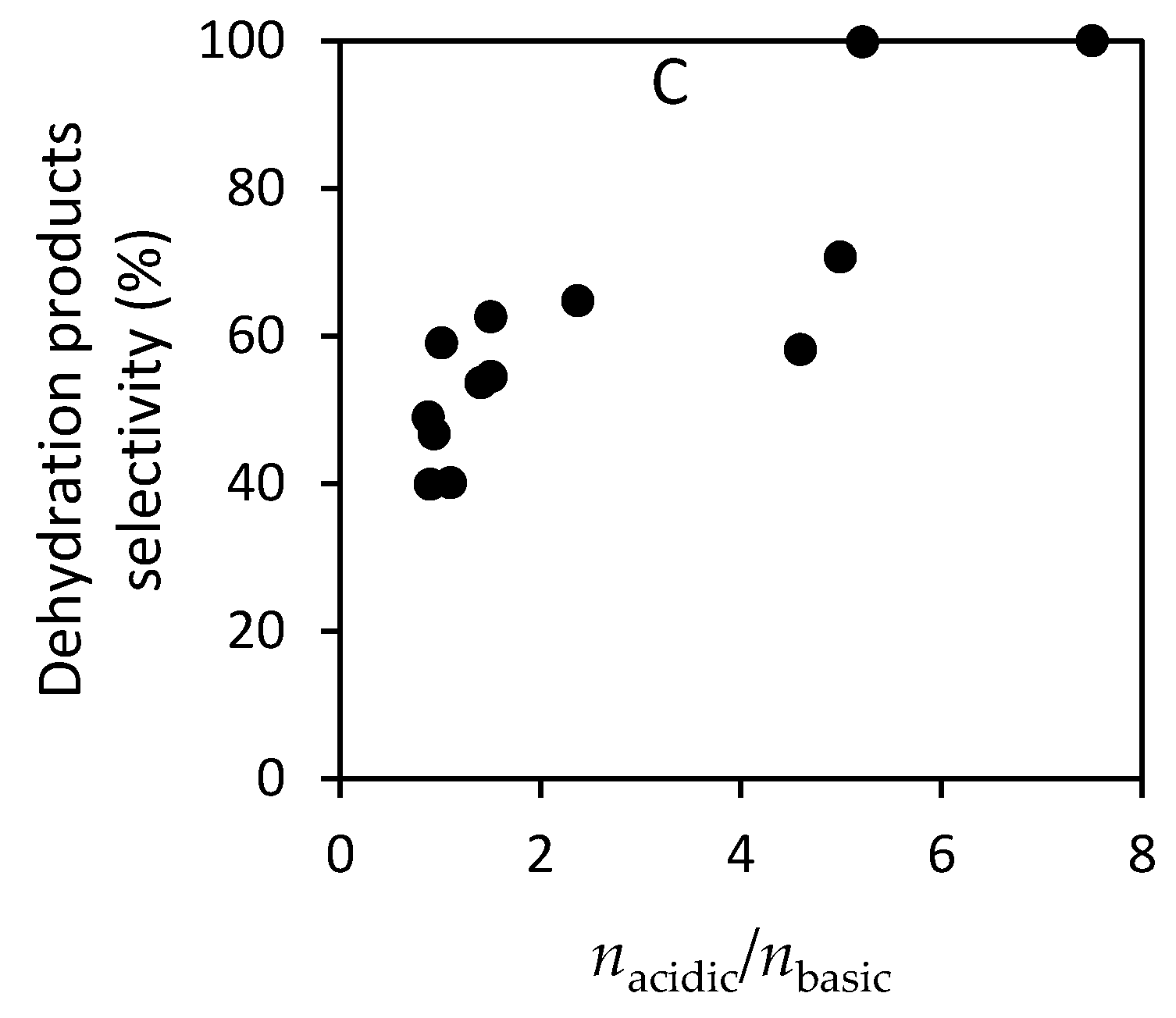
- Angelici, C.; Velthoen, M.E.Z.; Weckhuysen, B.M.; Bruijnincx, P.C.A. Influence of acid–base properties on the Lebedev ethanol-to-butadiene process catalyzed by SiO2–MgO materials. Catal. Sci. Technol. 2015, 5, 2869–2879. [Google Scholar] [CrossRef]
- Kvisle, S.; Aguero, A.; Sneeden, R.P.A. Transformation of ethanol into 1,3-butadiene over magnesium oxide/silica catalysts. Appl. Catal. 1988, 43, 117–131. [Google Scholar] [CrossRef]
- Ordomskiy, V.V.; Sushkevich, V.L.; Ivanova, I.I. One-Step Method for Butadiene Production. U.S. Patent 8,921,635, 30 December 2014. [Google Scholar]
- Ordomsky, V.V.; Sushkevich, V.L.; Ivanova, I.I. Study of acetaldehyde condensation chemistry over magnesia and zirconia supported on silica. J. Mol. Catal. A 2010, 333, 85–93. [Google Scholar] [CrossRef]
- Sekiguchi, Y.; Akiyama, S.; Urakawa, W.; Koyama, T.R.; Miyaji, A.; Motokura, K.; Baba, T. One-step catalytic conversion of ethanol into 1,3-butadiene using zinc-containing talc. Catal. Commun. 2015, 68, 20–24. [Google Scholar] [CrossRef]
- Simakova, O. A.; Davis, R.J.; Murzin, D.Y. Biomass Processing over Gold Catalysts; SpringerBriefs in Molecular Science; Springer International Publishing: Heidelberg, Germany, 2013. [Google Scholar]
- Guan, Y.; Hensen, E.J.M. Ethanol dehydrogenation by gold catalysts: The effect of the gold particle size and the presence of oxygen. Appl. Catal. A 2009, 361, 49–56. [Google Scholar] [CrossRef]
- Shimizu, K.I.; Sugino, K.; Sawabe, K.; Satsuma, A. Oxidant-free dehydrogenation of alcohols heterogeneously catalyzed by cooperation of silver clusters and acid-base sites on alumina. Chem. -A Eur. J. 2009, 15, 2341–2351. [Google Scholar] [CrossRef] [PubMed]
- Sushkevich, V.L.; Ivanova, I.I.; Taarning, E. Mechanistic study of ethanol dehydrogenation over silica-supported silver. ChemCatChem 2013, 5, 2367–2373. [Google Scholar] [CrossRef]
- Klein, A.; Keisers, K.; Palkovits, R. Formation of 1,3-butadiene from ethanol in a two-step process using modified zeolite-β catalysts. Appl. Catal. A 2015, 1, 192–202. [Google Scholar] [CrossRef]
- Larina, O.V.; Kyriienko, P.I.; Soloviev, S.O. Effect of the addition of zirconium dioxide on the catalytic properties of ZnO/MgO-SiO2 compositions in the production of 1,3-butadiene from ethanol. Theor. Exp. Chem. 2015, 51, 244–249. [Google Scholar] [CrossRef]
- Kitayama, Y.; Michishita, A. Catalytic activity of fibrous clay mineral sepiolite for butadiene formation from ethanol. J. Chem. Soc. Chem. Commun. 1981, 9, 401–402. [Google Scholar] [CrossRef]
- Kitayama, Y.; Satoh, M.; Kodama, T. Preparation of large surface area nickel magnesium silicate and its catalytic activity for conversion of ethanol into buta-1,3-diene. Catal. Lett. 1996, 36, 95–97. [Google Scholar] [CrossRef]
- Kitayama, Y.; Shimizu, K.; Kodama, T.; Murai, S.; Mizusima, T.; Hayakawa, M.; Muraoka, M. Role of intracrystalline tunnels of sepiolite for catalytic activity. Stud. Surf. Sci. Catal. 2002, 142, 675–682. [Google Scholar]
- Parr, R.G.; Pearson, R.G. Absolute hardness: Companion parameter to absolute electronegativity. J. Am. Chem. Soc. 1983, 105, 7512–7516. [Google Scholar] [CrossRef]
- Yang, W.; Parr, R.G. Hardness, softness, and the fukui function in the electronic theory of metals and catalysis. Proc. Natl. Acad. Sci. 1985, 82, 6723–6726. [Google Scholar] [CrossRef] [PubMed]
- Toussaint, W.J.; Dunn, J.T.; Jackson, D.R. Production of butadiene from alcohol. Ind. Eng. Chem. 1947, 39, 120–125. [Google Scholar] [CrossRef]
- Sushkevich, V.L.; Ivanova, I.I.; Tolborg, S.; Taarning, E. Meerwein-Ponndorf-Verley-Oppenauer reaction of crotonaldehyde with ethanol over Zr-containing catalysts. J. Catal. 2014, 316, 121–129. [Google Scholar] [CrossRef]
- Sushkevich, V.L.; Ivanova, I.I.; Taarning, E. Ethanol conversion into butadiene over Zr-containing molecular sieves doped with silver. Green Chem. 2015, 17, 2552–2559. [Google Scholar] [CrossRef]
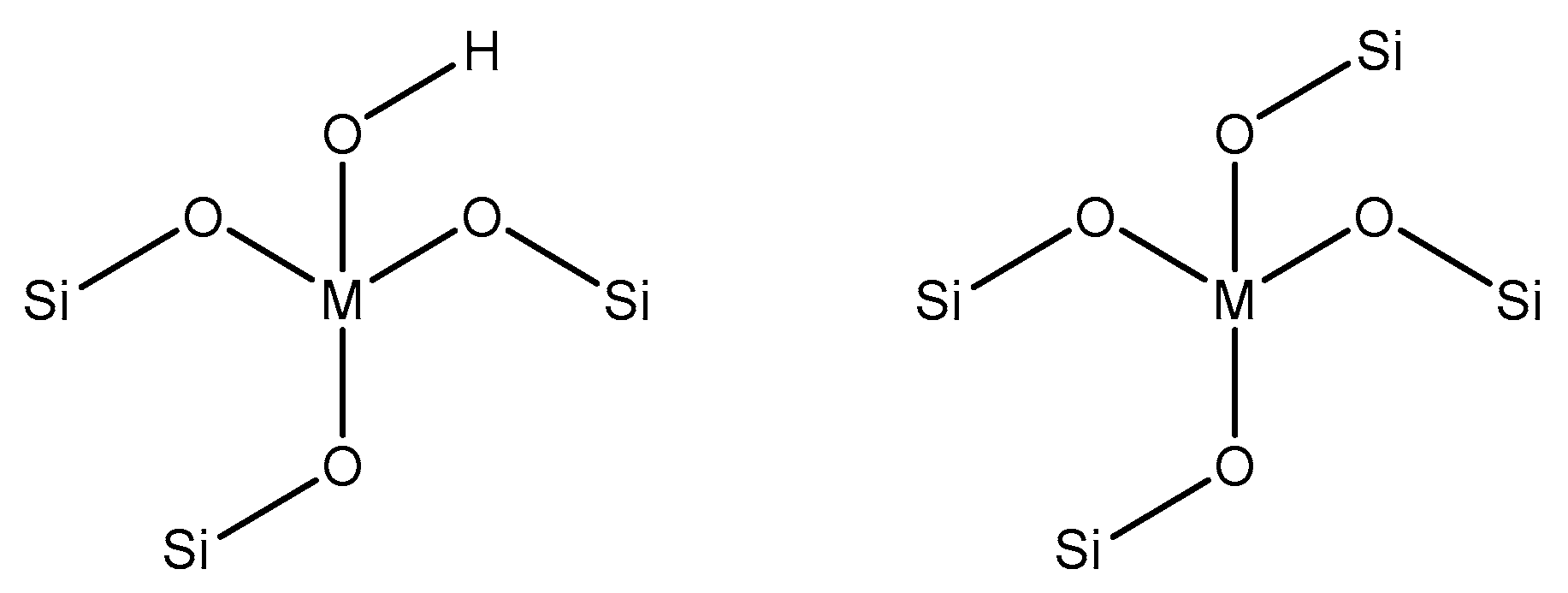
- Sushkevich, V.L.; Vimont, A.; Travert, A.; Ivanova, I.I. Spectroscopic evidence for open and closed Lewis acid sites in ZrBEA zeolites spectroscopic evidence for open and closed Lewis acid sites in ZrBEA zeolites. J. Phys. Chem. C 2015, 119, 17633–17639. [Google Scholar] [CrossRef]
- Courtney, T.D.; Chang, C.; Gorte, R.J.; Lobo, R.F.; Fan, W.; Nikolakis, V. Microporous and mesoporous materials effect of water treatment on Sn-BEA zeolite: Origin of 960 cm−1 FTIR peak. Microporous Mesoporous Mater. 2015, 210, 69–76. [Google Scholar] [CrossRef]
- Ratnasamy, P.; Srinivas, D.; Knözinger, H. Active sites and reactive intermediates in titanium silicate molecular sieves. Adv. Catal. 2004, 48, 1–169. [Google Scholar] [CrossRef]
- Boronat, M.; Concepción, P.; Corma, A.; Renz, M.; Valencia, S. Determination of the catalytically active oxidation Lewis acid sites in Sn-beta zeolites, and their optimisation by the combination of theoretical and experimental studies. J. Catal. 2005, 234, 111–118. [Google Scholar] [CrossRef]
- Boronat, M.; Concepción, P.; Corma, A.; Navarro, M.T.; Renz, M.; Valencia, S. Reactivity in the confined spaces of zeolites: The interplay between spectroscopy and theory to develop structure–activity relationships for catalysis. Phys. Chem. Chem. Phys. 2009, 11, 2876–2884. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.W.; Cordon, M.J.; Di Iorio, J.R.; Vega-vila, J.C.; Ribeiro, F.H.; Gounder, R. Titration and quantification of open and closed Lewis acid sites in Sn-Beta zeolites that catalyze glucose isomerization. J. Catal. 2016, 335, 141–154. [Google Scholar] [CrossRef]
- Zhu, Y.; Chuah, G.; Jaenicke, S. Chemo- and regioselective Meerwein-Ponndorf-Verley and Oppenauer reactions catalyzed by Al-free Zr-zeolite beta. J. Catal. 2004, 227, 1–10. [Google Scholar] [CrossRef]
- Sun, J.; Zhu, K.; Gao, F.; Wang, C.; Liu, J.; Peden, C.H.F.; Wang, Y. Direct conversion of bio-ethanol to isobutene on nanosized ZnxZryOz mixed oxides with balanced acid-base sites. J. Am. Chem. Soc. 2011, 133, 11096–11099. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Su, W.N.; Rick, J.; Yang, S.C.; Cheng, J.H.; Pan, C.J.; Lee, J.F.; Hwang, B.J. Hierarchical copper-decorated nickel nanocatalysts supported on La2O3 for low-temperature steam reforming of ethanol. ChemSusChem 2014, 7, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Frey, A.M.; Karmee, S.K.; de Jong, K.P.; Bitter, J.H.; Hanefeld, U. Supported La2O3 and MgO nanoparticles as solid base catalysts for aldol reactions while suppressing dehydration at room temperature. ChemCatChem 2013, 5, 594–600. [Google Scholar] [CrossRef]
- Boukha, Z.; Fitian, L.; López-Haro, M.; Mora, M.; Ruiz, J.R.; Jiménez-Sanchidrián, C.; Blanco, G.; Calvino, J.J.; Cifredo, G.A.; Trasobares, S.; et al. Influence of the calcination temperature on the nano-structural properties, surface basicity, and catalytic behavior of alumina-supported lanthana samples. J. Catal. 2010, 272, 121–130. [Google Scholar] [CrossRef]
- Kozlowski, J.T.; Davis, R.J. Sodium modification of zirconia catalysts for ethanol coupling to 1-butanol. J. Energy Chem. 2013, 22, 58–64. [Google Scholar] [CrossRef]
- Han, Y.; Lee, S.S.; Ying, J.Y. Spherical siliceous mesocellular foam particles for high-speed size exclusion chromatography. Chem. Mater. 2007, 19, 2292–2298. [Google Scholar] [CrossRef]
- Legendre, M.; Cornet, D. Catalytic oxidation of ethanol over tantalum oxide. J. Catal. 1972, 25, 194–203. [Google Scholar] [CrossRef]
- Tanabe, K. Catalytic application of niobium compounds. Catal. Today 2003, 78, 65–77. [Google Scholar] [CrossRef]
- Jehng, J.M.; Wachs, I.E. The molecular structures and reactivity of supported niobium oxide catalysts. Catal. Today 1990, 8, 37–55. [Google Scholar] [CrossRef]
- Jehng, J.-M.; Wachs, I.E. Molecular structures of supported niobium oxide catalysts under ambient conditions. J. Mol. Catal. 1991, 67, 369–387. [Google Scholar] [CrossRef]
- Kosslick, H.; Lischke, G.; Parlitz, B.; Storek, W.; Fricke, R. Acidity and active sites of Al-MCM-41. Appl. Catal. A 1999, 184, 49–60. [Google Scholar] [CrossRef]
- Aimon, D.; Panier, E. La mise en pratique de l’économie circulaire chez Michelin. Ann. des Mines-Responsab. Environ. 2014. [Google Scholar] [CrossRef]
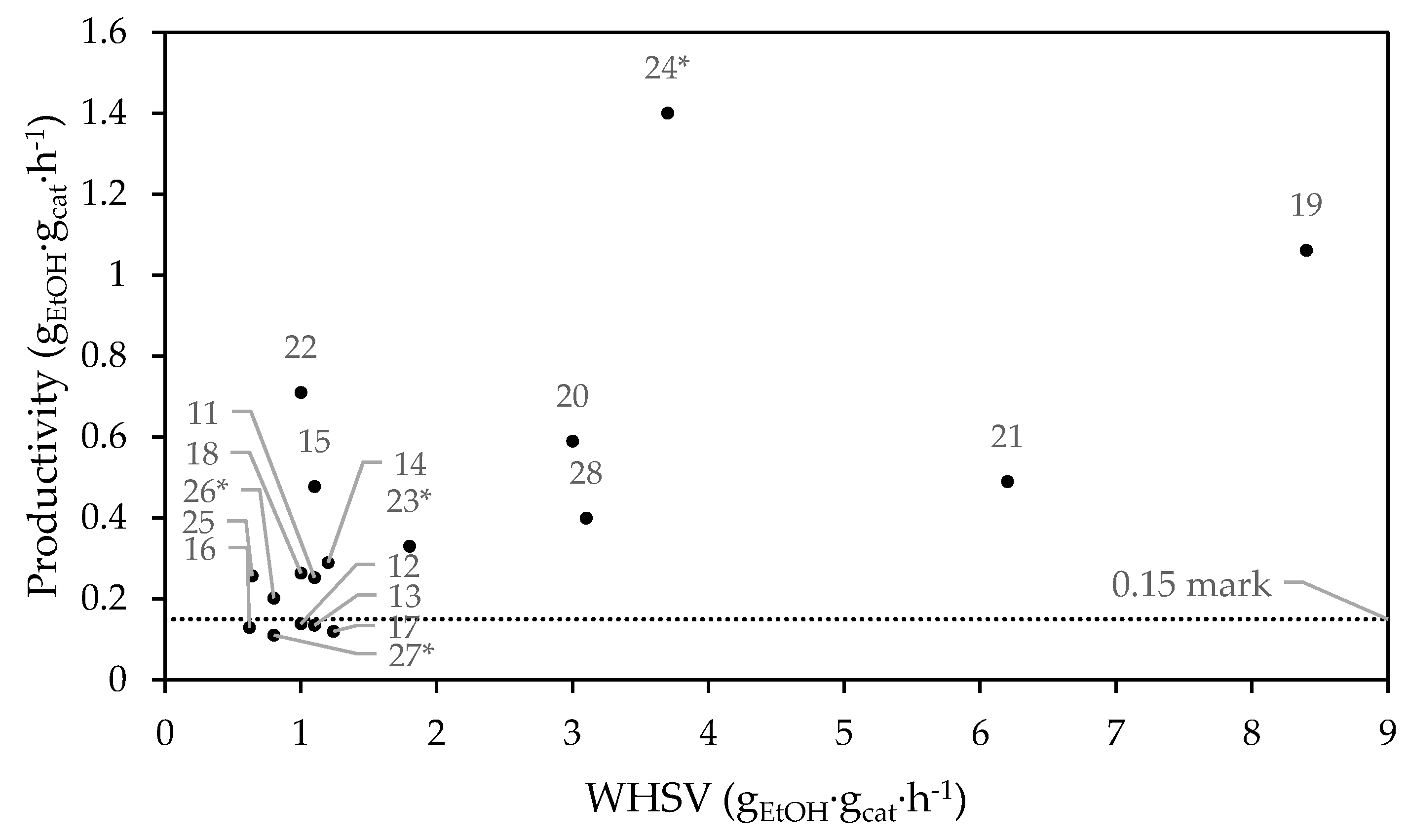
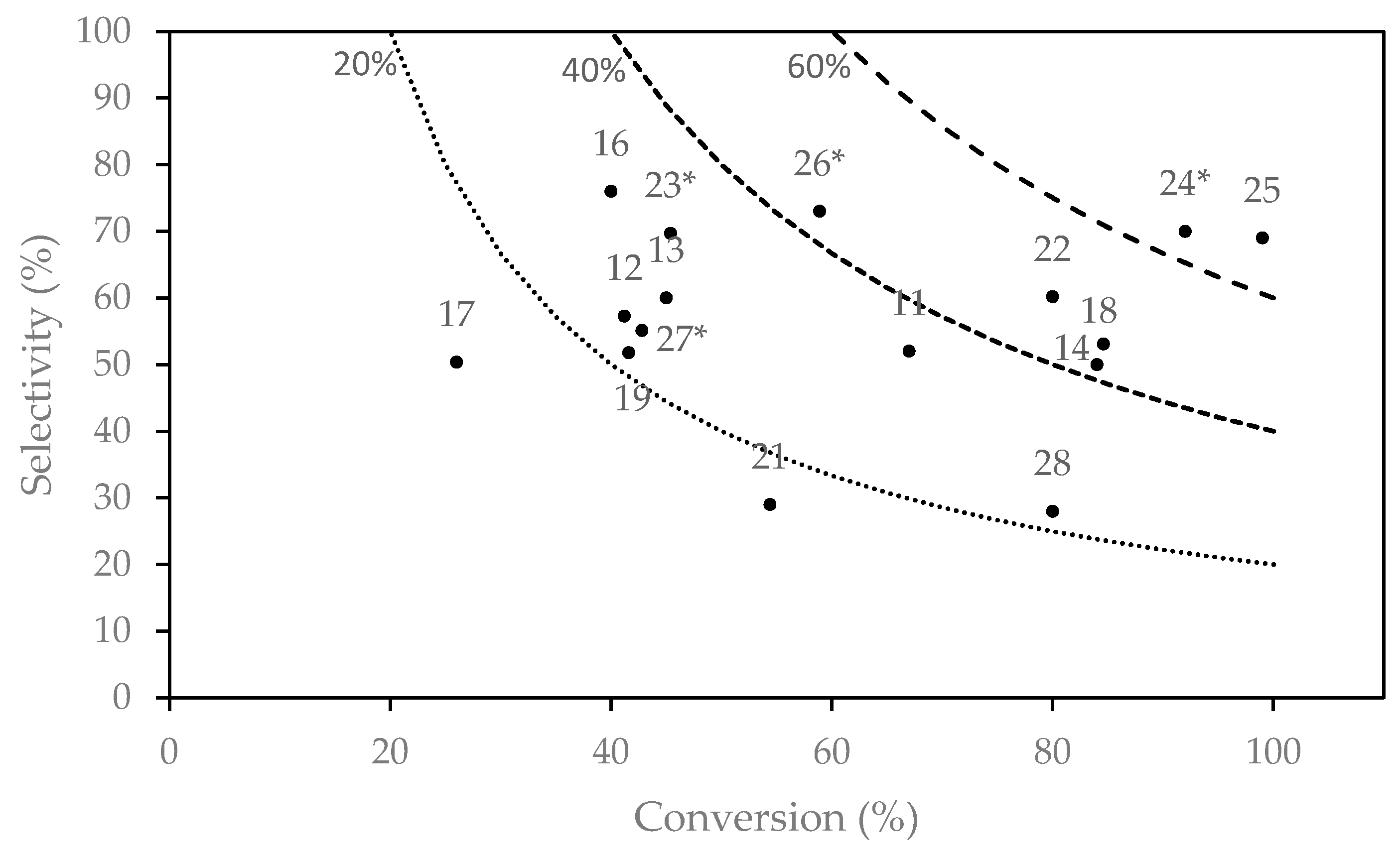
| ID | Catalyst | T (K) | WHSV (h−1) | EtOH/AA | TOS (h) | XEtOH (%) | YBD (%) | PBD | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Old catalytic systems | |||||||||
| 1 | Wet-kneaded MgO-SiO2 | 623 | 0.15 | - | - | 50 | 42 | 0.06 | [8] |
| 2 | Commercial MgO-SiO2 | 713 | 0.3 | - | - | 70 | 48 | 0.06 | [8] |
| 3 | 2% Cr2O3-59% MgO-39% SiO2 | 673 | 0.4 | - | - | 68 | 38 | 0.08 | [8] |
| 4 | 3% CuO-56% MgO-42% SiO2 | 673 | 0.7 | - | - | 86 | 44 | 0.22 | [8] |
| 5 | 40% ZnO-60% Al2O3 | 698 | 1.5 | - | - | 94 | 56 | 0.50 | [26] |
| 6 | 20% MgO-80% Al2O3 | 698 | 1.5 | - | - | - | 48 | 0.40 | [26] |
| 7 | 40% Cr2O3-60% Al2O3 | 698 | 1.5 | - | - | - | 47 | 0.40 | [26] |
| 8 | 9.5% ZrO2-90.5% SiO2 | 698 | 1.0 | - | - | - | 23 | 0.13 | [8] |
| 9 | Ag/ZrO2/SiO2 | 598 | 0.3 | - | - | 34 | 24 | 0.04 | [16] |
| 10 | 40% ZrO2-60% Fe2O3 | 698 | 1.5 | - | - | - | 40 | 0.34 | [26] |
| Recent MgO-SiO2 catalysts | |||||||||
| 11 | MgO-SiO2 (WK) | 698 | 1.1 | - | 4 | ~67 a | 35 | 0.25 | [27] |
| 12 | MgO-SiO2 (MC) | 673 | 1.0 | - | - | 41.2 | 23.6 a | 0.14 | [28] |
| 13 | 3% Au/MgO-SiO2 | 573 | 1.1 | - | 3.3 | 45 | 27 a | 0.14 | [3] |
| 14 | 1% Ag/MgO-SiO2 | 753 | 1.2 | - | 3.3 | 84 | 42 | 0.29 | [29] |
| 15 | 1% CuO/MgO-SiO2 | 698 | 1.1 | - | 4 | 74 | 74 a | 0.48 a | [25] |
| 16 | 1.5% Zr-1% Zn/MgO-SiO2 | 648 | 0.62 | - | 3 | 40 | 30.4 | 0.13 | [30] |
| 17 | 1.2% K/ZrZn/MgO-SiO2 | 648 | 1.24 | - | 3 | 26 | 13.1 | 0.12 | [30] |
| 18 | 2% ZnO/MgO-SiO2 | 648 | 1.0 | - | 3 | 84.6 | 45 | 0.26 a | [31] |
| 19 | 1.2% Zn-Talc | 673 | 8.4 a | - | 7 | 41.6 | 21.5 | 1.1 a | [19] |
| Recent Zr-containing catalysts | |||||||||
| 20 | 3.5% Ag/Zr/BEA | 593 | 1.2–3.0 | - | 3 | - | - | 0.59 | [32] |
| 21 | 2000 ppm Na/Zn1Zr10On | 623 | 6.2 | - | - | 54.4 | 15.2 a | 0.49 | [33] |
| 22 | 2% ZnO-7% La2O3/SiO2-2% ZrO2 | 648 | 1.0 | - | 3 | 80.0 | 60.0 | 0.71 | [34] |
| 23 | 2% ZrO2/SiO2 b | 593 | 1.8 | 3.5 | - | 45.4 | 31.6 | 0.33 a | [35] |
| 24 | 4.7% Cu/MCF + 2.7% Zr/MCF b | 673 | 3.7 | 0.7–1.6 | 15 | 92 | 64.4 a | 1.4 | [36] |
| Other recent catalytic systems | |||||||||
| 25 | HM-Hf/SiO2 | 633 | 0.64 | - | 10 | 99 | 68.8 | 0.26 | [37] |
| 26 | 3% Ta/BEA b | 623 | 0.8 | 3.7 | 4 | 58.9 | 43.1 | 0.20 | [38] |
| 27 | 0.7% Nb/BEA b | 623 | 0.8 | 2.7 | 4 | 42.8 | 23.6 | 0.11 | [39] |
| 28 | 1.4% Cr-16% Ba/Al-MCM-41 | 723 | 3.1 | - | 10 | 80 | 22.4 | 0.40 a | [40] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pomalaza, G.; Capron, M.; Ordomsky, V.; Dumeignil, F. Recent Breakthroughs in the Conversion of Ethanol to Butadiene. Catalysts 2016, 6, 203. https://doi.org/10.3390/catal6120203
Pomalaza G, Capron M, Ordomsky V, Dumeignil F. Recent Breakthroughs in the Conversion of Ethanol to Butadiene. Catalysts. 2016; 6(12):203. https://doi.org/10.3390/catal6120203
Chicago/Turabian StylePomalaza, Guillaume, Mickaël Capron, Vitaly Ordomsky, and Franck Dumeignil. 2016. "Recent Breakthroughs in the Conversion of Ethanol to Butadiene" Catalysts 6, no. 12: 203. https://doi.org/10.3390/catal6120203








