Novel Ni-Ce-Zr/Al2O3 Cellular Structure for the Oxidative Dehydrogenation of Ethane
Abstract
:1. Introduction
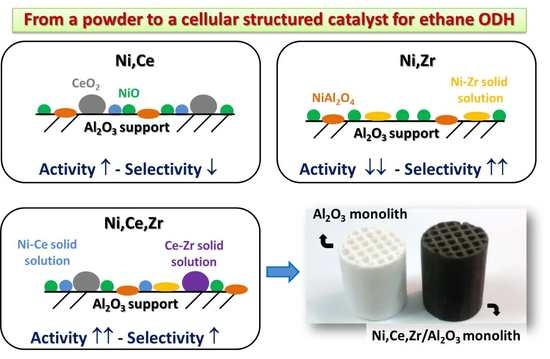
2. Results and Discussion
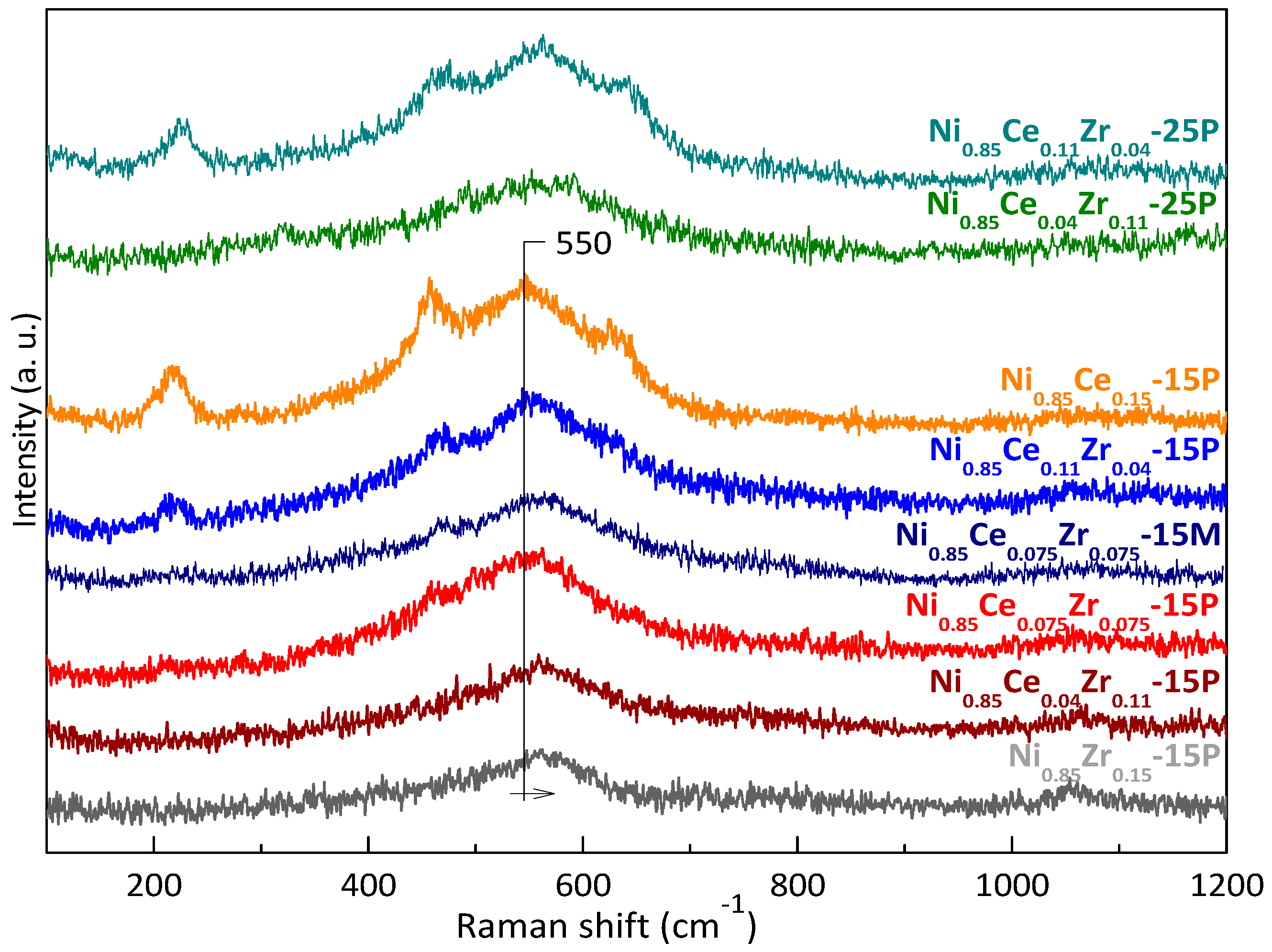
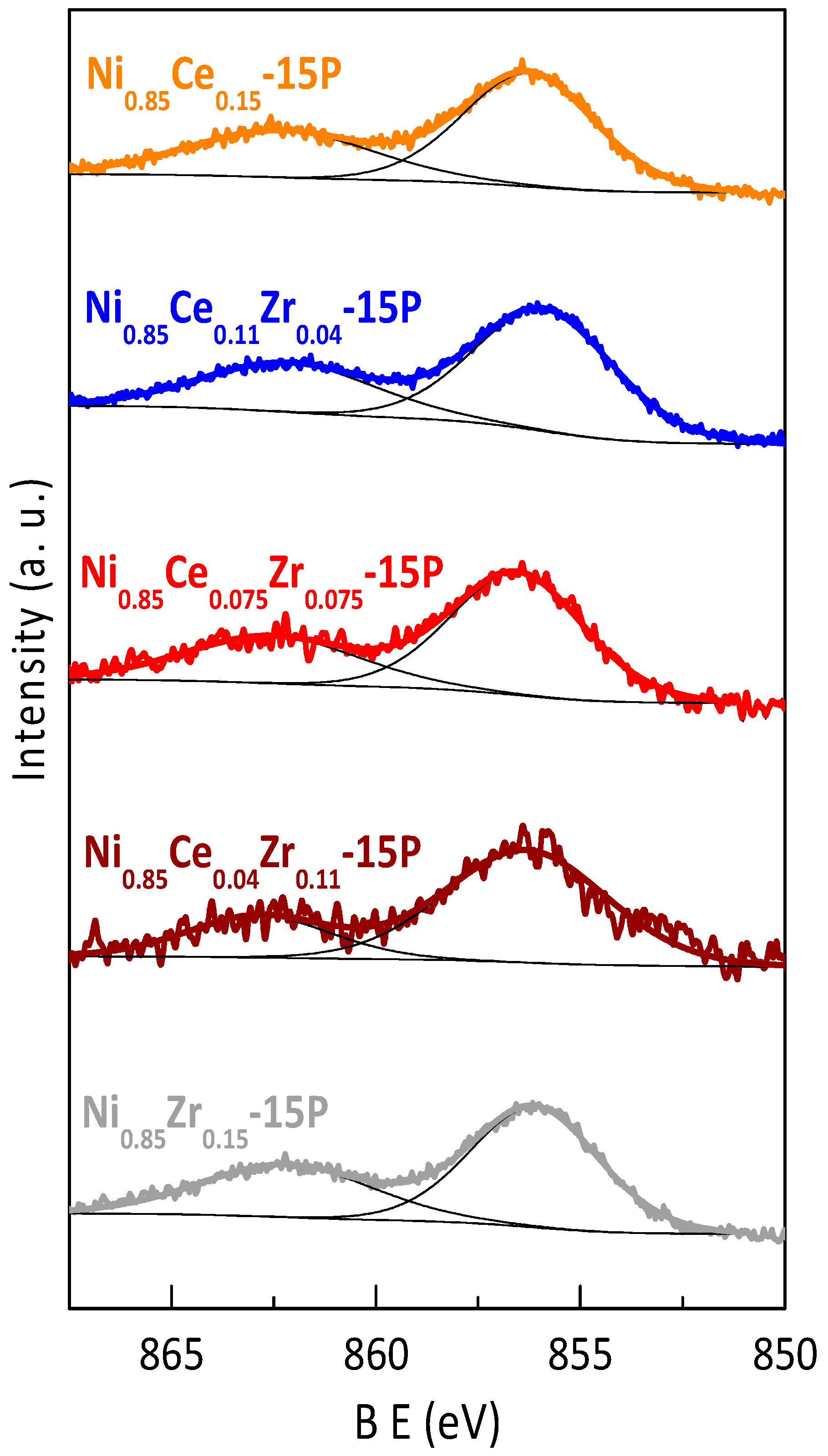
2.1. Characterization of Active Sites
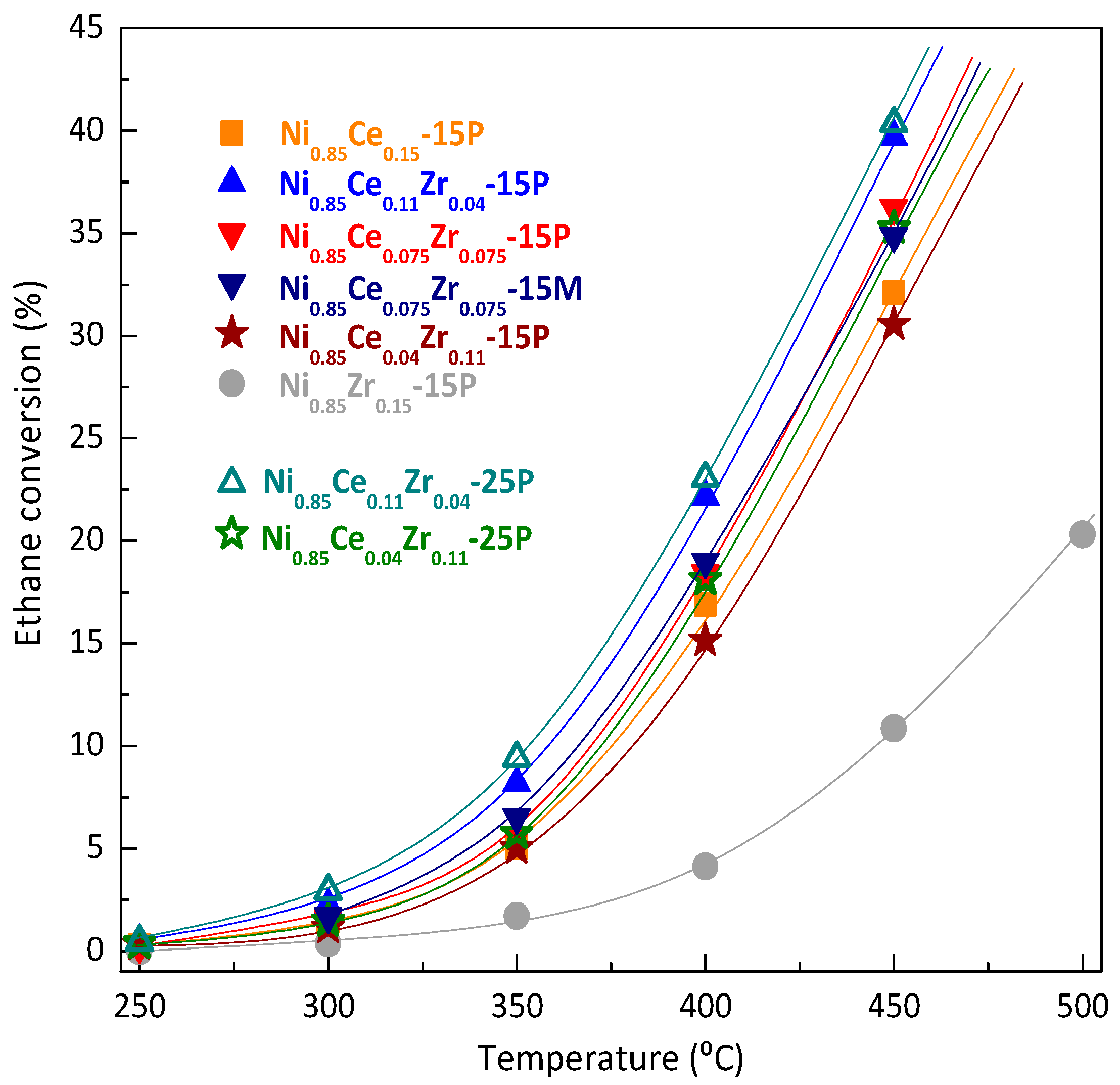
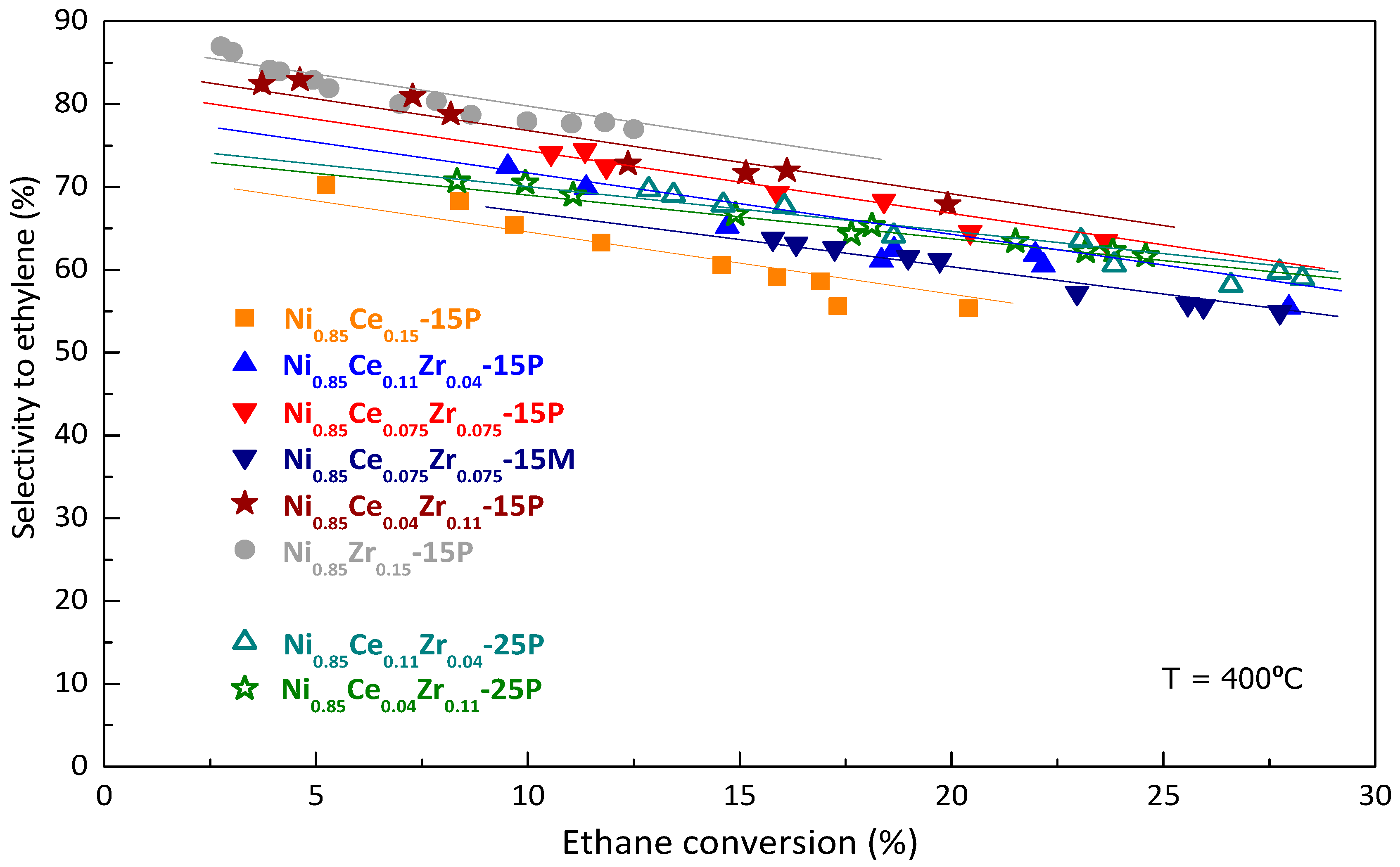
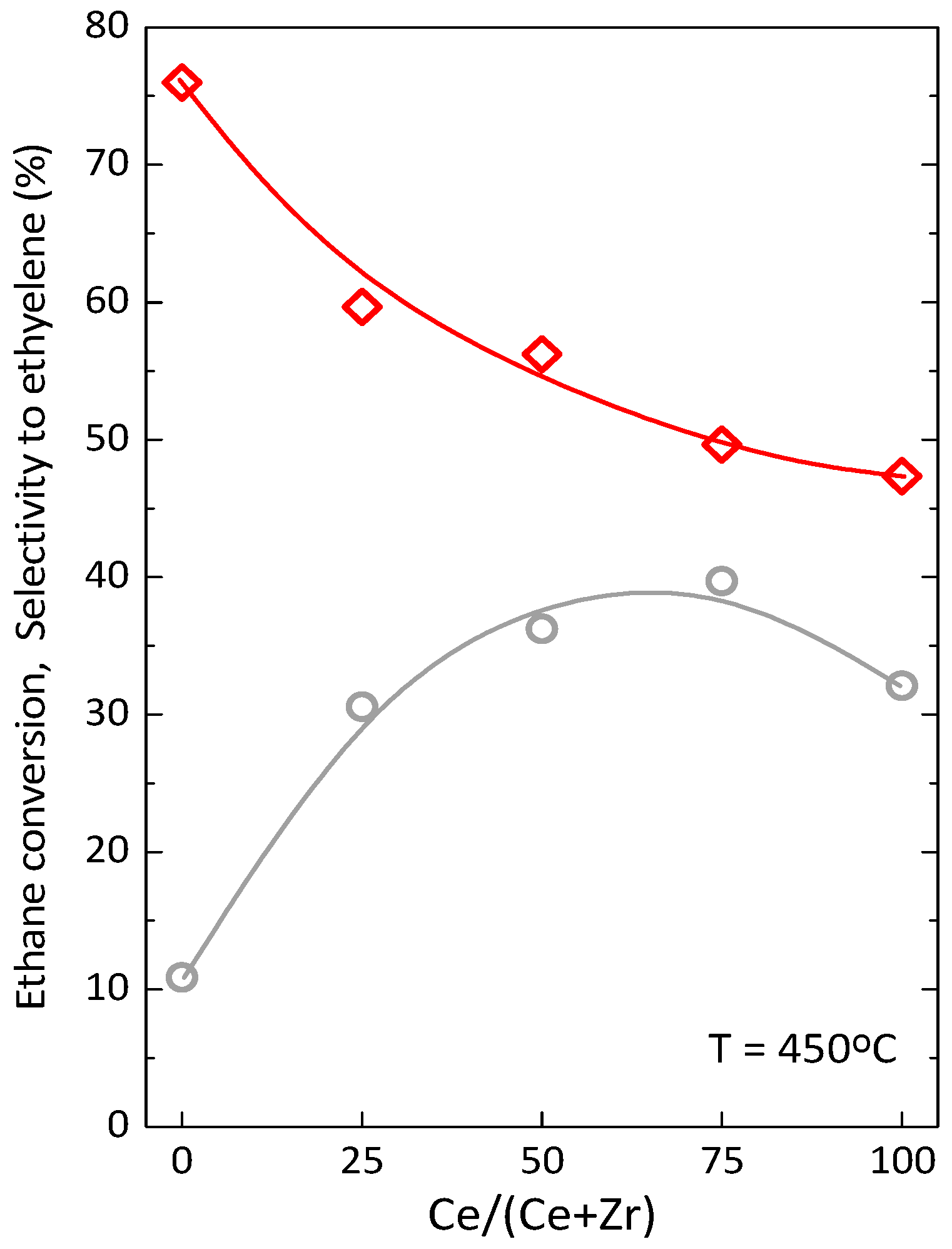
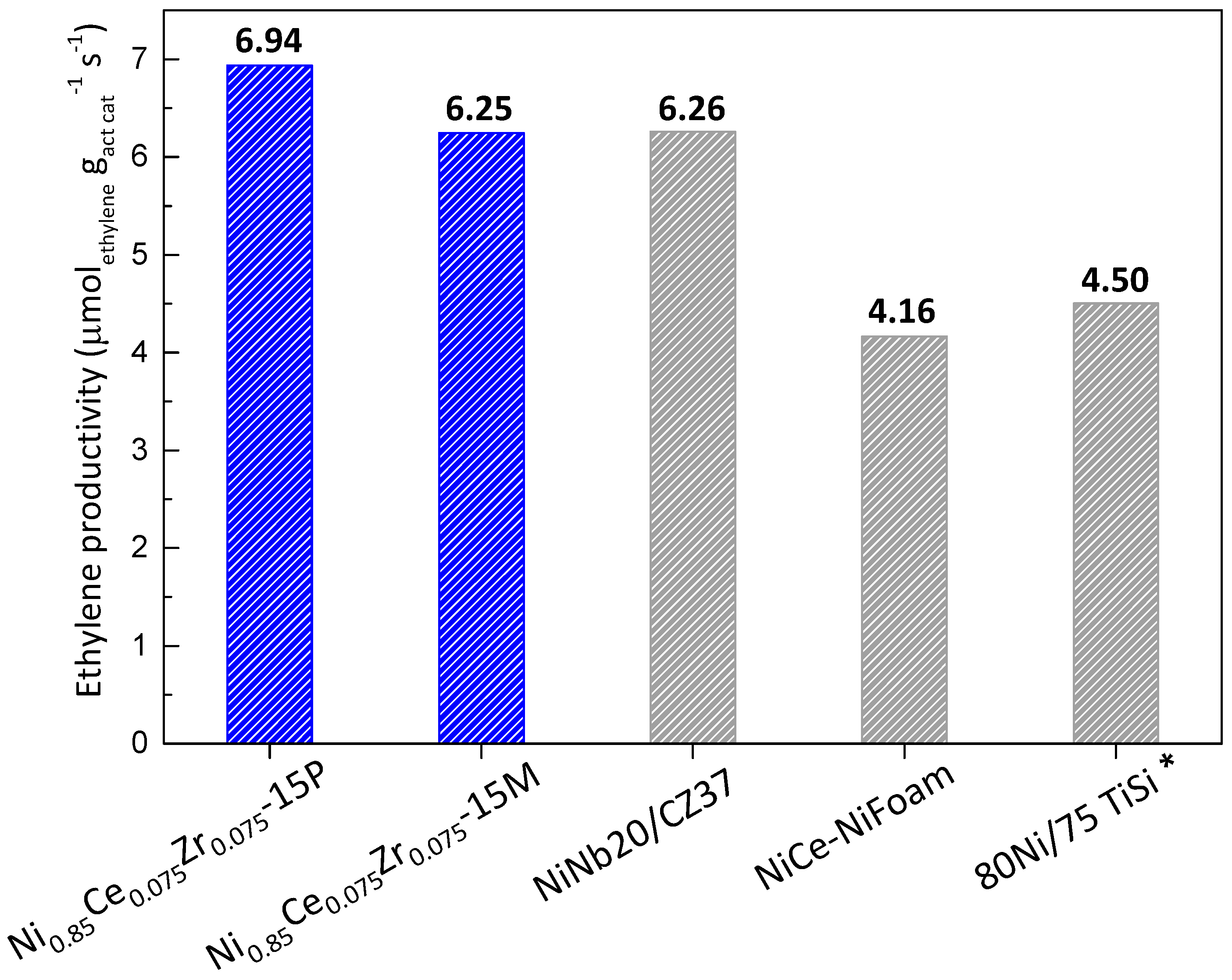
2.2. Catalytic Performance in ODHE: Powder and Monolithic Catalysts
3. Materials and Methods
3.1. Preparation of Powder Catalysts
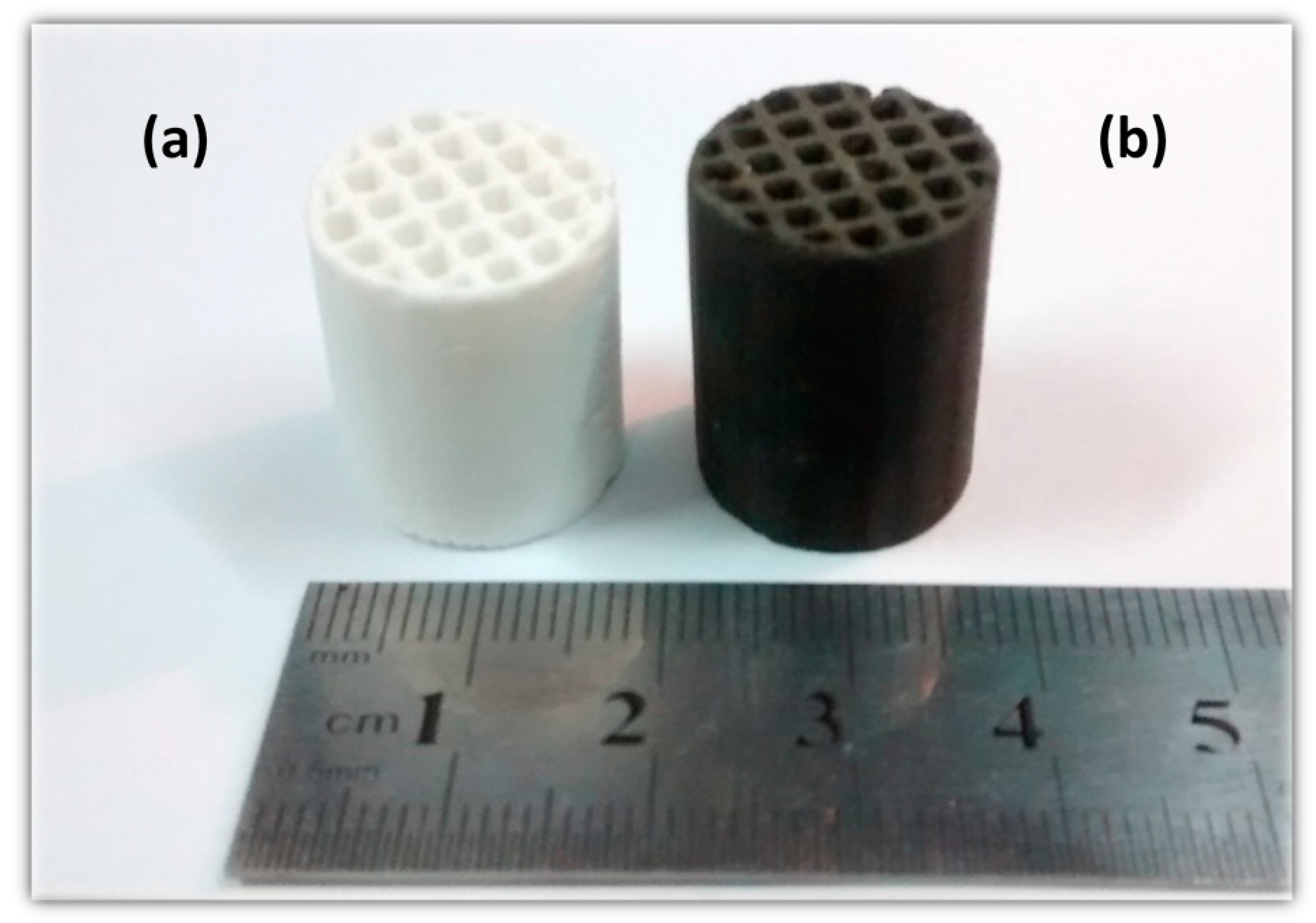
3.2. Preparation of Cellular Catalysts
3.3. Catalysts Characterization
3.4. Catalytic Tests
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chu, B.; Truter, L.; Nijhuis, T.A.; Cheng, Y. Oxidative dehydrogenation of ethane to ethylene over phase-pure M1 MoVNbTeOx catalysts in a micro-channel reactor. Catal. Sci. Technol. 2015, 5, 2807–2813. [Google Scholar] [CrossRef]
- Gärtner, C.A.; van Veen, A.C.; Lercher, J.A. Oxidative dehydrogenation of ethane: Common principles and mechanistic aspects. ChemCatChem 2013, 5, 3196–3217. [Google Scholar] [CrossRef]
- Védrine, J.C. Heterogeneous partial (amm)oxidation and oxidative dehydrogenation catalysis on mixed metal oxides. Catalysts 2016, 6, 22–48. [Google Scholar] [CrossRef]
- Heracleous, E.; Lee, A.F.; Wilson, K.; Lemonidou, A.A. Investigation of Ni-based alumina-supported catalysts for the oxidative dehydrogenation of ethane to ethylene: Structural characterization and reactivity studies. J. Catal. 2005, 231, 159–171. [Google Scholar] [CrossRef]
- Heracleous, E.; Lemonidou, A.A. Ni-Me-O mixed metal oxides for the effective oxidative dehydrogenation of ethane to ethylene - Effect of promoting metal Me. J. Catal. 2010, 270, 67–75. [Google Scholar] [CrossRef]
- Solsona, B.; López Nieto, J.M.; Agouram, S.; Soriano, M.D.; Dejoz, A.; Vázquez, M.I.; Concepción, P. Optimizing both catalyst preparation and catalytic behaviour for the oxidative dehydrogenation of ethane of Ni-Sn-O catalysts. Top. Catal. 2016, 59, 1564–1572. [Google Scholar] [CrossRef]
- Solsona, B.; Concepción, P.; Hernández, S.; Demicol, B.; López Nieto, J.M. Oxidative dehydrogenation of ethane over NiO-CeO2 mixed oxides catalysts. Catal. Today 2012, 180, 51–58. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, J.; He, Y.; Wu, T. Preparation and characterization of Ni-Zr-O nanoparticles and its catalytic behavior for ethane oxidative dehydrogenation. Appl. Surf. Sci. 2012, 258, 4922–4928. [Google Scholar] [CrossRef]
- Bortolozzi, J.P.; Gutierrez, L.B.; Ulla, M.A. Efficient structured catalysts for ethylene production through the ODE reaction: Ni and Ni-Ce on ceramic foams. Catal. Commun. 2014, 43, 197–201. [Google Scholar] [CrossRef]
- Smoláková, L.; Kout, M.; Koudelková, E.; Čapek, L. The role of Ni species distribution on the effect of Ce as a promoter in C2-ODH reaction. Top. Catal. 2015, 58, 843–853. [Google Scholar] [CrossRef]
- Smoláková, L.; Kout, M.; Koudelková, E.; Čapek, L. Effect of calcination temperature on the structure and catalytic performance of the Ni/Al2O3 and Ni-Ce/Al2O3 catalysts in oxidative dehydrogenation of ethane. Ind. Eng. Chem. Res. 2015, 54, 12730–12740. [Google Scholar] [CrossRef]
- Bortolozzi, J.P.; Banús, E.D.; Courtalón, N.L.; Ulla, M.A.; Milt, V.G.; Miró, E.E. Flexible NiZr-based structured catalysts for ethylene production through ODH of ethane: Catalytic performance enhancement. Catal. Today 2016, 273, 252–258. [Google Scholar] [CrossRef]
- Bortolozzi, J.P.; Banús, E.D.; Milt, V.G.; Miró, E.E. New formulations of Ni-containing ceramic papers to enhance the catalytic performance for the oxidative dehydrogenation of ethane. Ind. Eng. Chem. Res. 2014, 53, 17570–17579. [Google Scholar] [CrossRef]
- Lee, M.; Yun, Y.S.; Sung, J.; Lee, J.; Seo, Y.-J.; Song, I.K.; Yi, J. Enhanced ethylene productivity by the promotion of lattice oxygen in Ni-Nb-O/CexZr1−xO2 composite for oxidative dehydrogenation of ethane. Catal. Commun. 2017, 95, 58–62. [Google Scholar] [CrossRef]
- Kreutzer, M.T.; Kapteijn, F.; Moulijn, J.A. Shouldn’t catalysts shape up? Structured reactors in general and gas-liquid monolith reactors in particular. Catal. Today 2006, 111, 111–118. [Google Scholar] [CrossRef]
- Bortolozzi, J.P.; Banús, E.D.; Terzaghi, D.; Gutierrez, L.B.; Milt, V.G.; Ulla, M.A. Novel catalytic ceramic papers applied to oxidative dehydrogenation of ethane. Catal. Today 2013, 216, 24–29. [Google Scholar] [CrossRef]
- Brussino, P.; Bortolozzi, J.P.; Milt, V.G.; Banús, E.D.; Ulla, M.A. NiCe/γ-Al2O3 coated onto cordierite monoliths applied to oxidative dehydrogenation of ethane (ODE). Catal. Today 2016, 273, 259–265. [Google Scholar] [CrossRef]
- Bortolozzi, J.P.; Weiss, T.; Gutierrez, L.B.; Ulla, M.A. Comparison of Ni and Ni-Ce/Al2O3 catalysts in granulated and structured forms: Their possible use in the oxidative dehydrogenation of ethane reaction. Chem. Eng. J. 2014, 246, 343–352. [Google Scholar] [CrossRef]
- Rasmussen, S.B.; López-Medina, R.; Portela, R.; Mikolajska, E.; Daturi, M.; Ávila, P.; Bañares, M.A. Shaping up operando spectroscopy: Raman characterization of a working honeycomb monolith. Catal. Sci. Technol. 2015, 5, 4942–4945. [Google Scholar] [CrossRef]
- Zhang, Z.; Han, L.; Chai, R.; Zhang, Q.; Li, Y.; Zhao, G.; Liu, Y.; Lu, Y. Microstructured CeO2-NiO-Al2O3/Ni-foam catalyst for oxidative dehydrogenation of ethane to ethylene. Catal. Commun. 2017, 88, 90–93. [Google Scholar] [CrossRef]
- Ni, X.; Zhao, Q.; Zhou, F.; Zheng, H.; Cheng, J.; Li, B. Synthesis and characterization of NiO strips from a single source. J. Cryst. Growth 2006, 289, 299–302. [Google Scholar] [CrossRef]
- Savova, B.; Loridant, S.; Filkova, D.; Millet, J.M.M. Ni-Nb-O catalysts for ethane oxidative dehydrogenation. Appl. Catal. A Gen. 2010, 390, 148–157. [Google Scholar] [CrossRef]
- Yadav, S.K.; Jeevanandam, P. Synthesis of NiO-Al2O3 nanocomposites by sol-gel process and their use as catalyst for the oxidation of styrene. J. Alloys Compd. 2014, 610, 567–574. [Google Scholar] [CrossRef]
- Duan, W.J.; Lu, S.H.; Wu, Z.L.; Wang, Y.S. Size effects on properties of NiO nanoparticles grown in alkali salts. J. Phys. Chem. C 2012, 116, 26043–26051. [Google Scholar] [CrossRef]
- Liu, X.; Zuo, Y.; Li, L.; Huang, X.; Li, G. Heterosturcture NiO/Ce1−xNixO2: Synthesis and synergistic effect of simultaneous surface modification and internal doping for superior catalytic performance. RSC Adv. 2014, 4, 6397–6406. [Google Scholar] [CrossRef]
- Kosacki, I.; Suzuki, T.; Anderson, H.U.; Colomban, P. Raman scattering and lattice defects in nanocrystalline CeO2 thin films. Solid State Ion. 2002, 149, 99–105. [Google Scholar] [CrossRef]
- Kim, B.K.; Hamaguchi, H. Mode assignments of the Raman spectrum of monoclinic zirconia by isotopic exchange technique. Phys. Status Solidi 1997, 203, 557–563. [Google Scholar] [CrossRef]
- Yamamoto, T.; Tanaka, T.; Takenaka, S.; Yoshida, S.; Onari, T.; Takahashi, Y.; Kosaka, T.; Hasegawa, S.; Kudo, M. Structural analysis of iron and manganese species in iron- and manganese-promoted sulphated zirconia. J. Phys. Chem. B 1999, 103, 2385–2393. [Google Scholar] [CrossRef]
- Ali, T.T.; Narasimharao, K.; Ahmed, N.S.; Basahel, S.; Al-Thabaiti, S.; Mokhtar, M. Nanosized iron and nickel oxide zirconia supported catalysts forbenzylation of benzene: Role of metal oxide support interaction. Appl. Catal. A Gen. 2014, 486, 19–31. [Google Scholar] [CrossRef]
- Hoang, T.M.C.; Rao, N.K.; Lefferts, L.; Seshan, K. Investigation of Ce-Zr oxide-supported Ni catalysts in the steam reforming of meta-cresol as a model component for bio-derived tar. ChemCatChem 2015, 7, 468–478. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, C.-H.; Hanson, J.C.; Robinson, R.D.; Herman, I.P.; Chan, S.-W. Phases in ceria-zirconia binary oxide (1−x)CeO2–xZrO2 nanoparticles: The effect of particle size. J. Am. Ceram. Soc. 2006, 89, 1028–1036. [Google Scholar] [CrossRef]
- AbdelDayem, H.M.; Faiz, M.; Abdel-Samad, H.S.; Hassan, S.A. Rare earth oxides doped NiO/γ-Al2O3 catalyst for oxidative dehydrogenation of cyclohexane. J. Rare Earths 2015, 33, 611–618. [Google Scholar] [CrossRef]
- Ding, C.; Gao, W.; Zhao, Y.; Zhao, Y.; Zhou, H.; Li, J.; Jin, H. Effects of Co2+ doping on physicochemical behavior of hierarchical NiO nanostructure. Appl. Surf. Sci. 2016, 390, 890–896. [Google Scholar] [CrossRef]
- Jiménez-González, C.; Boukha, Z.; Rivas, B.; Delgado, J.J.; Cauqui, M.A.; González-Velasco, J.R.; Gutiérrez-Ortiz, J.I.; López-Fonseca, R. Structural characterization of Ni/alumina reforming catalysts activated at high temperatures. Appl. Catal. A Gen. 2013, 466, 9–20. [Google Scholar] [CrossRef]
- Kaminski, P.; Ziolek, M. Surface and catalytic properties of Ce-, Zr-, Au-, Cu-modified SBA-15. J. Catal. 2014, 312, 249–262. [Google Scholar] [CrossRef]
- Duprez, D. Study of surface reaction mechanisms by 16O/18O and H/D isotopic exchange. Catal. Today 2006, 112, 17–22. [Google Scholar] [CrossRef]
- Murota, T.; Hasegawa, T.; Aozasa, S.; Matsui, H.; Motoyama, M. Production method of cerium oxide with high storage capacity of oxygen and its mechanism. J. Alloys Compd. 1993, 193, 298–299. [Google Scholar] [CrossRef]
- Srinivas, D.; Satyanarayana, C.V.V.; Potdar, H.S.; Ratnasamy, P. Structural studies on NiO-CeO2-ZrO2 catalysts for steam reforming of ethanol. Appl. Catal. A Gen. 2003, 246, 323–334. [Google Scholar] [CrossRef]
- Pastor-Pérez, L.; Ramírez Reina, T.; Ivanova, S.; Centeno, M.A.; Odriozola, J.A.; Sepúlveda-Escribano, A. Ni-CeO2/C catalysts with enhanced OSC for the WGS reaction. Catalysts 2015, 5, 298–309. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wen, J.; Shen, M. Effect of interaction between Ce0.7Zr0.3O2 and Al2O3 on structural characteristics, thermal stability, and oxygen storage capacity. J. Phys. Chem. C 2008, 112, 5113–5122. [Google Scholar] [CrossRef]
- Azambre, B.; Zenboury, L.; Weber, J.V.; Burg, P. Surface characterization of acidic ceria-zirconia prepared by direct sulfation. Appl. Surf. Sci. 2010, 256, 4570–4581. [Google Scholar] [CrossRef]
- Si, Z.; Weng, D.; Wu, X.; Ma, Z.; Ma, J.; Ran, R. Lattice oxygen mobility and acidity improvements of NiO-CeO2-ZrO2 catalyst by sulfation for NOx reduction by ammonia. Catal. Today 2013, 201, 122–130. [Google Scholar] [CrossRef]
- López Nieto, J.M.; Solsona, B.; Grasselli, R.K.; Concepción, P. Promoted NiO catalysts for the oxidative dehydrogenation of ethane. Top. Catal. 2014, 57, 1248–1255. [Google Scholar] [CrossRef]
- Ykrelef, A.; Nadji, L.; Issaadi, R.; Agouram, S.; Rodríguez-Castellón, E.; Solsona, B.; López Nieto, J.M. Mixed oxide Ti-Si-O prepared by non-hydrolytic xerogel method as a diluter of nickel oxide for the oxidative dehydrogenation of ethane. Catal. Today 2017. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bortolozzi, J.P.; Portela, R.; Ávila, P.; Milt, V.; Miró, E. Novel Ni-Ce-Zr/Al2O3 Cellular Structure for the Oxidative Dehydrogenation of Ethane. Catalysts 2017, 7, 331. https://doi.org/10.3390/catal7110331
Bortolozzi JP, Portela R, Ávila P, Milt V, Miró E. Novel Ni-Ce-Zr/Al2O3 Cellular Structure for the Oxidative Dehydrogenation of Ethane. Catalysts. 2017; 7(11):331. https://doi.org/10.3390/catal7110331
Chicago/Turabian StyleBortolozzi, Juan Pablo, Raquel Portela, Pedro Ávila, Viviana Milt, and Eduardo Miró. 2017. "Novel Ni-Ce-Zr/Al2O3 Cellular Structure for the Oxidative Dehydrogenation of Ethane" Catalysts 7, no. 11: 331. https://doi.org/10.3390/catal7110331