Low-Temperature Sol-Gel Synthesis of Nitrogen-Doped Anatase/Brookite Biphasic Nanoparticles with High Surface Area and Visible-Light Performance
Abstract
:1. Introduction
2. Results and Discussion
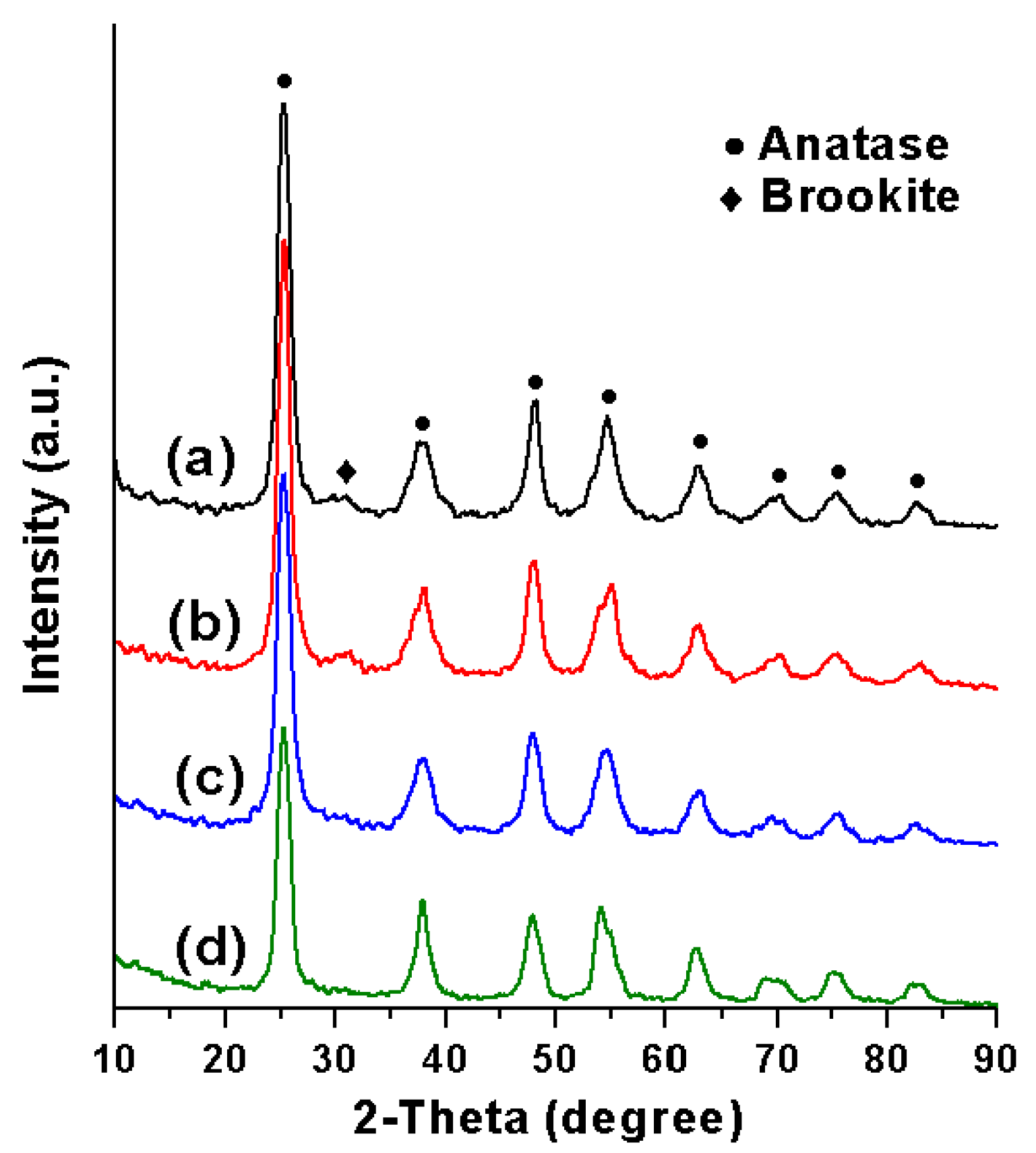
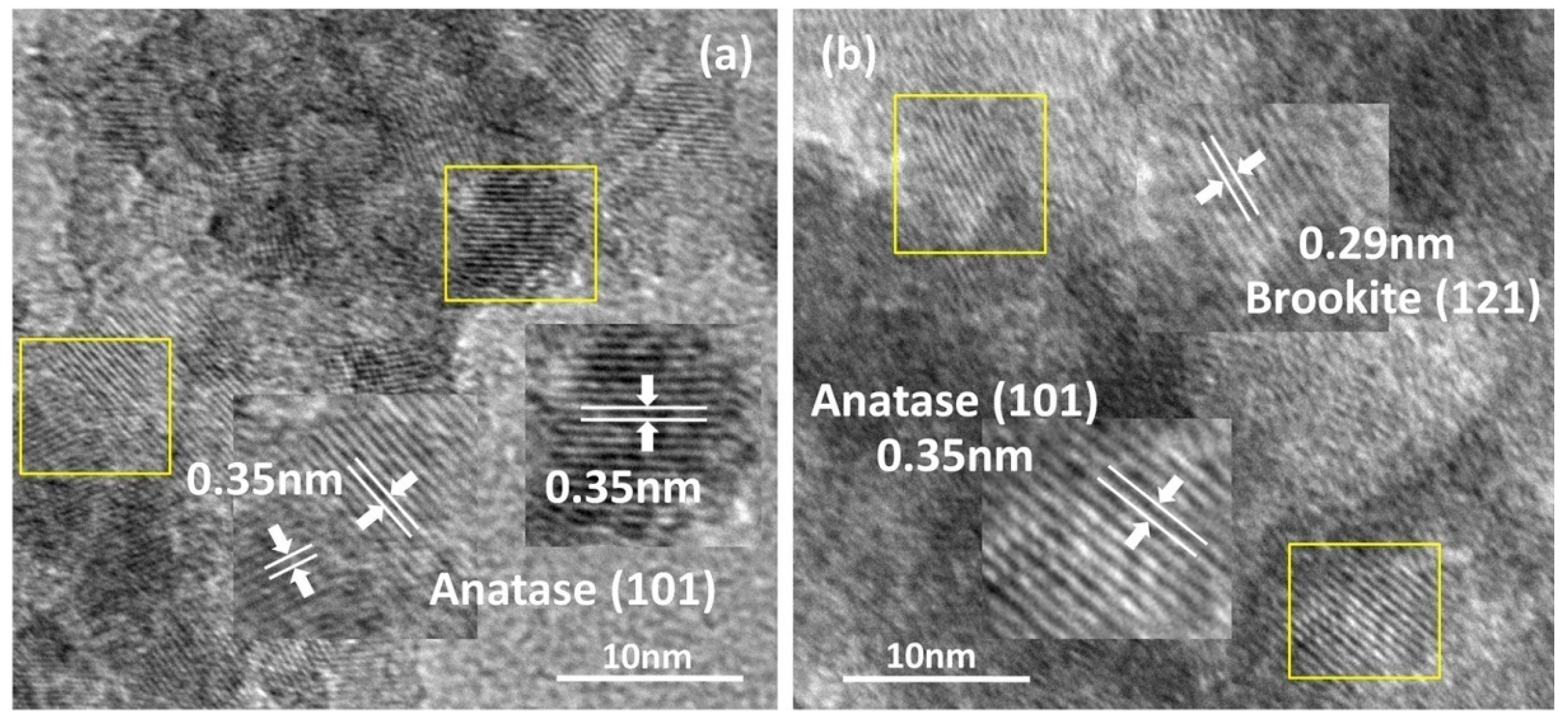
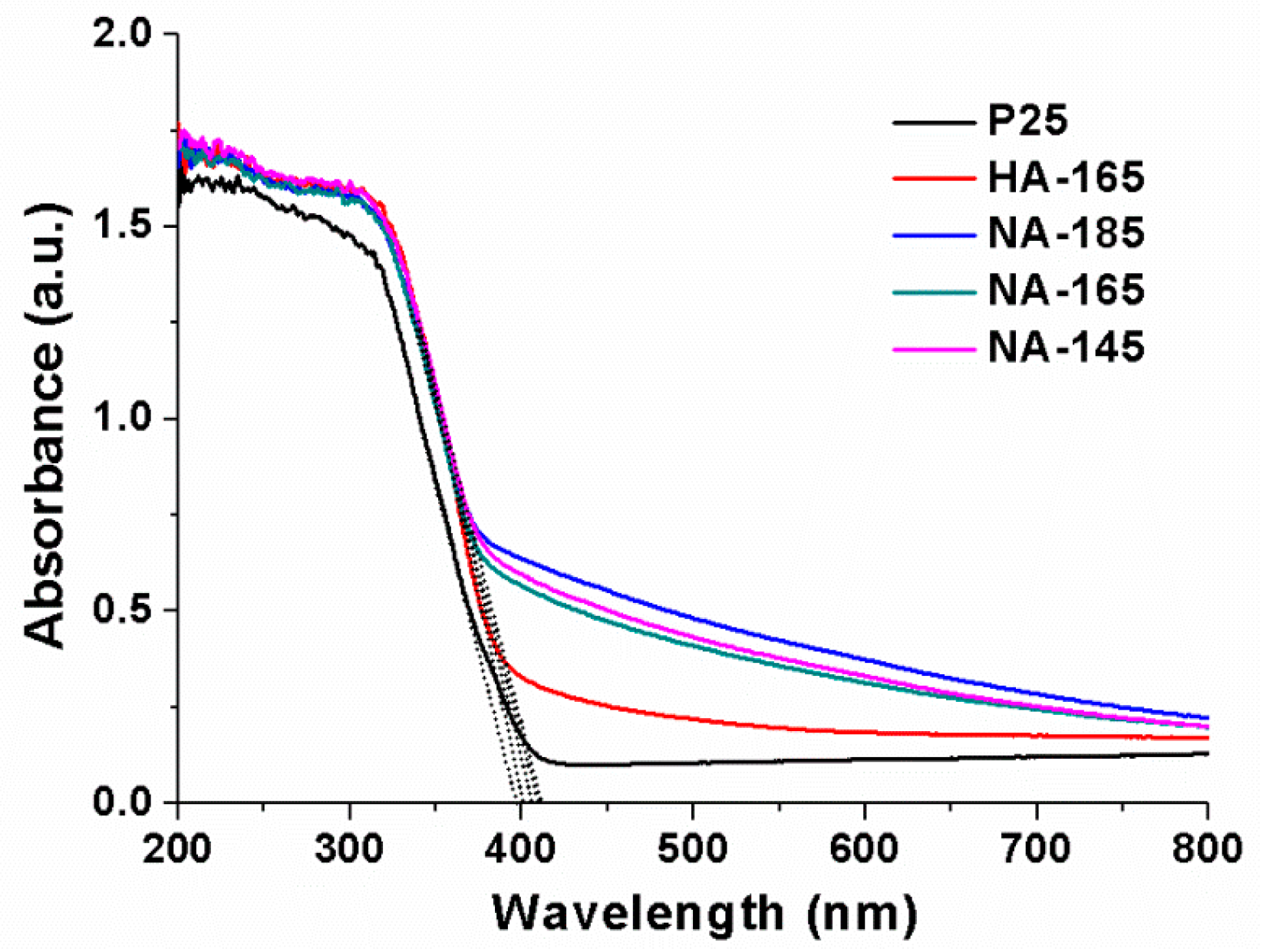
2.1. Syntheses and Characterizations
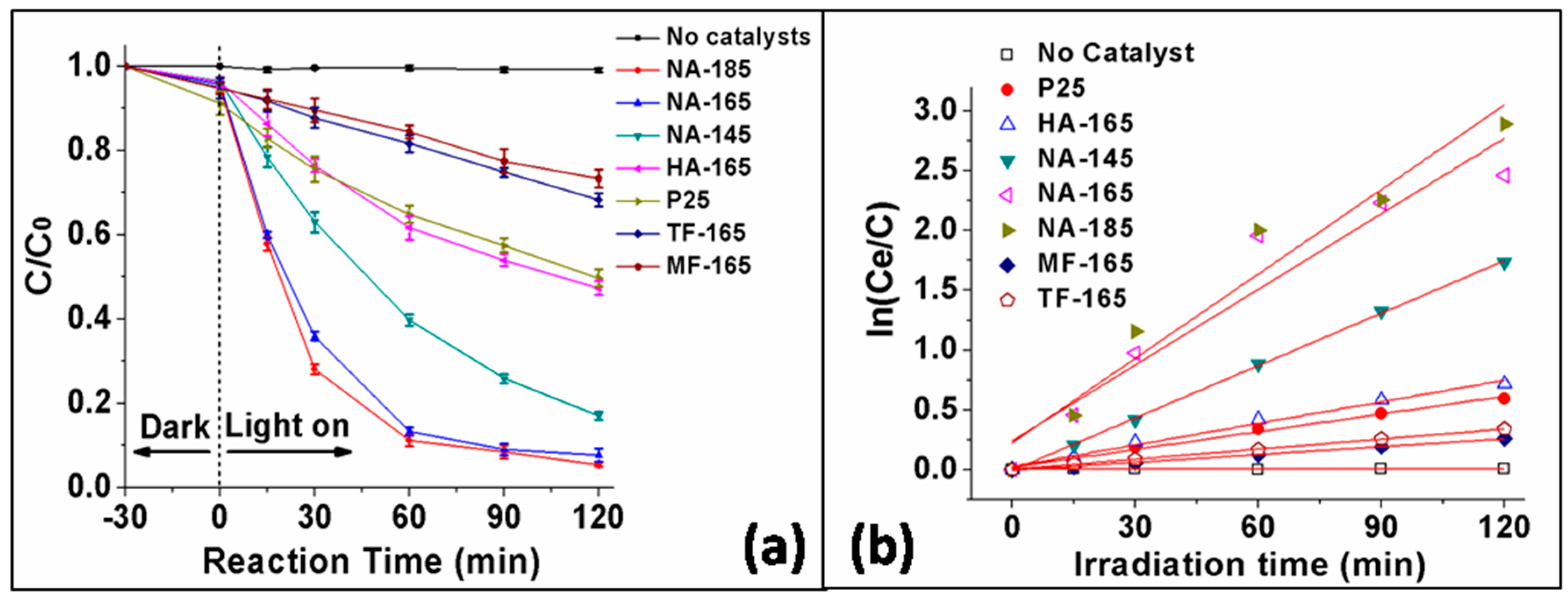
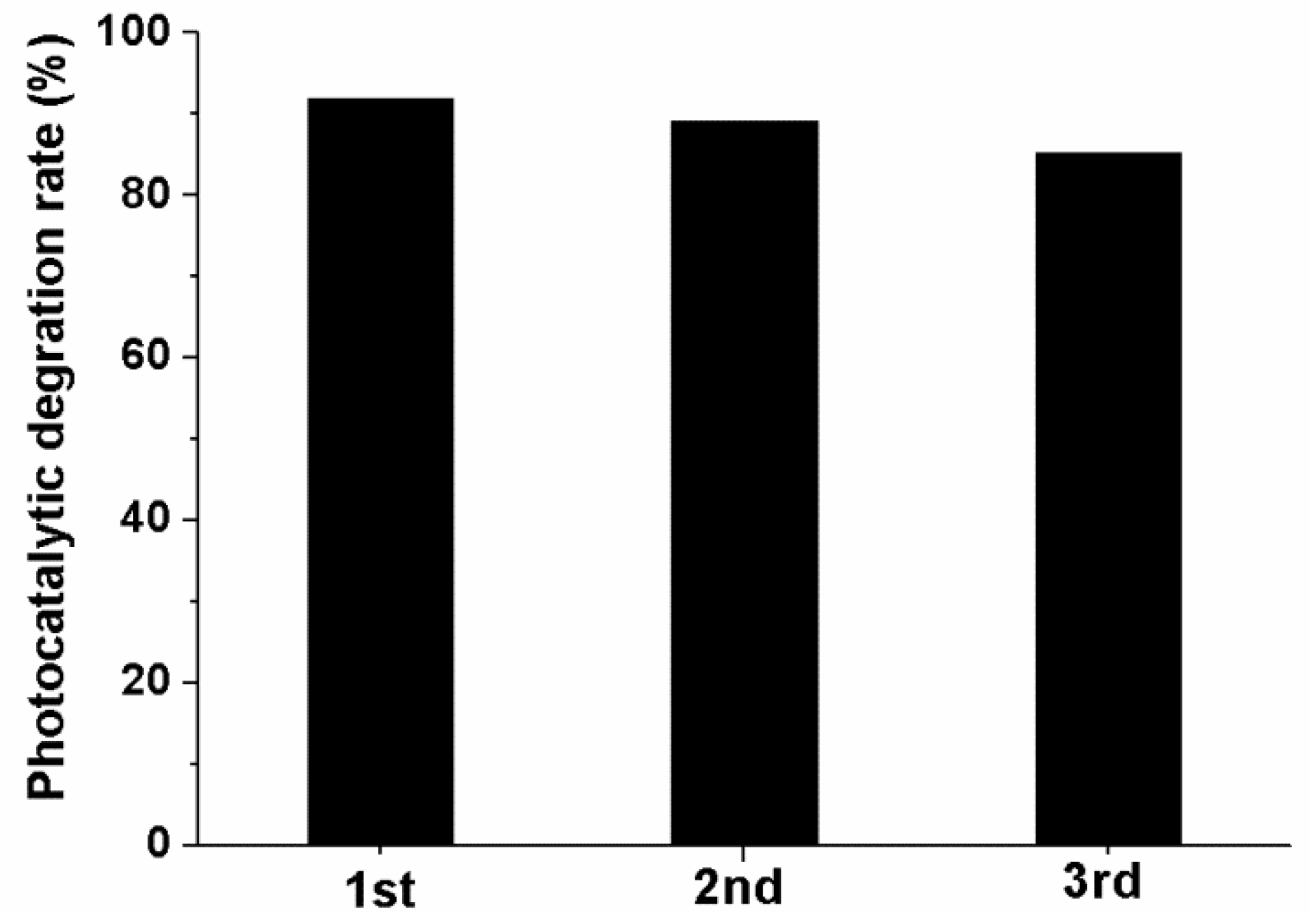
2.2. Photocatalytic Activity
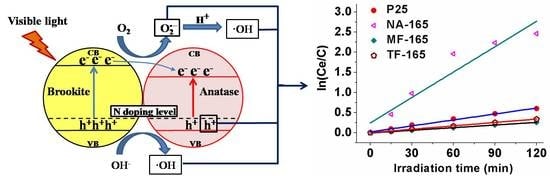
2.3. Possible Reasons for the Enhancement of the Visible-Light Performance
3. Materials and Methods
3.1. Synthesis
3.2. Photocatalytic Activity
3.3. Characterizations
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, W.; Zou, L.; Wang, L. Photocatalytic TiO2/adsorbent nanocomposites prepared via wet chemical impregnation for wastewater treatment: A review. Appl. Catal. A 2009, 371, 1–9. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Long, J.; Gu, Q.; Ding, Z.; Fu, X. Nitrogen-doped titanium dioxide visible light photocatalyst: Spectroscopic identification of photoactive centers. J. Catal. 2010, 276, 201–214. [Google Scholar] [CrossRef]
- Ryu, J.; Choi, W. Substrate-specific photocatalytic activities of TiO2 and multiactivity test for water treatment application. Environ. Sci. Technol. 2008, 42, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Valentin, C.D.; Finazzi, E.; Pacchioni, G.; Selloni, A.; Livraghi, S.; Paganini, M.C.; Giamello, E. N-doped TiO2: Theory and experiment. Chem. Phys. 2007, 339, 44–56. [Google Scholar] [CrossRef]
- Wang, H.; Gao, X.; Duan, G.; Yang, X.; Liu, X. Facile preparation of anatase–brookite–rutile mixed-phase N-doped TiO2 with high visible-light photocatalytic activity. J. Environ. Chem. Eng. 2015, 3, 603–608. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Huo, Y. Highly Active TiO2N Photocatalysts Prepared by Treating TiO2 Precursors in NH3/Ethanol Fluid under Supercritical Conditions. J. Phys. Chem. B 2006, 110, 1559–1565. [Google Scholar] [CrossRef] [PubMed]
- Paola, A.D.; Bellardita, M.; Palmisano, L. Brookite, the Least Known TiO2 Photocatalyst. Catalysts 2013, 3, 36–73. [Google Scholar] [CrossRef]
- Reyes-Coronado, D.; Rodríguez-Gattorno, G.; Espinosa-Pesqueira, M.E.; Cab, C.; de Coss, R.; Oskam, G. Phase-pure TiO2 nanoparticles: Anatase, brookite and rutile. Nanotechnology 2008, 19, 145605. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yu, J.C.; Leung, M.K.P.; Ho, W.; Cheng, B.; Zhao, X.; Zhao, J. Effects of acidic and basic hydrolysis catalysts on the photocatalytic activity and microstructures of bimodal mesoporous titania. J. Catal. 2003, 217, 69–78. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, F.; Jiao, Y.; Yang, H.; Zhang, J. Ag0-loaded brookite/anatase composite with enhanced photocatalytic performance towards the degradation of methyl orange. J. Mol. Catal. A Chem. 2011, 348, 114–119. [Google Scholar] [CrossRef]
- Ozawa, T.; Iwasaki, M.; Tada, H.; Akita, T.; Tanaka, K.; Ito, S. Low-temperature synthesis of anatase–brookite composite nanocrystals: The junction effect on photocatalytic activity. J. Colloid Interface Sci. 2005, 281, 510–513. [Google Scholar] [CrossRef] [PubMed]
- Kandiel, T.A.; Feldhoff, A.; Robben, L.; Dillert, R.; Bahnemann, D.W. Tailored Titanium Dioxide Nanomaterials: Anatase Nanoparticles and Brookite Nanorods as Highly Active Photocatalysts. Chem. Mater. 2010, 22, 2050–2060. [Google Scholar] [CrossRef]
- Mutuma, B.K.; Shao, G.N.; Kim, W.D.; Kim, H.T. Sol–gel synthesis of mesoporous anatase–brookite and anatase–brookite–rutile TiO2 nanoparticles and their photocatalytic properties. J. Colloid Interface Sci. 2015, 442, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, D.O.; Dunnill, C.W.; Buckeridge, J.; Shevlin, S.A.; Logsdail, A.J.; Woodley, S.M.; Catlow, C.R.A.; Powell, M.J.; Palgrave, R.G.; Parkin, I.P.; et al. Band alignment of rutile and anatase TiO2. Nat. Mater. 2013, 12, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.A.; Salleh, W.N.W.; Jaafar, J.; Ismail, A.F.; Nor, N.A.M. Photodegradation of phenol by N-Doped TiO2 anatase/rutile nanorods assembled microsphere under UV and visible light irradiation. Mater. Chem. Phys. 2015, 162, 113–123. [Google Scholar] [CrossRef]
- Nie, X.; Zhuo, S.; Maeng, G.; Sohlberg, K. Doping of TiO2 Polymorphs for Altered Optical and Photocatalytic Properties. Int. J. Photoenergy 2009, 2009, 294042. [Google Scholar] [CrossRef]
- Yu, J.C.; Yu, J.; Ho, W.; Zhang, L. Preparation of highly photocatalytic active nano-sized TiO2 particles via ultrasonic irradiation. Chem. Commun. 2001, 19, 1942–1943. [Google Scholar] [CrossRef]
- Gai, L.; Duan, X.; Jiang, H.; Mei, Q.; Zhou, G.; Tian, Y.; Liu, H. One-pot synthesis of nitrogen-doped TiO2 nanorods with anatase/brookite structures and enhanced photocatalytic activity. CrystEngComm 2012, 14, 7662–7671. [Google Scholar] [CrossRef]
- El-Sheikh, S.M.; Khedr, T.M.; Zhang, G.; Vogiazi, V.; Ismail, A.A.; O’Shea, K.; Dionysiou, D.D. Tailored synthesis of anatase–brookite heterojunction photocatalysts for degradation of cylindrospermopsin under UV–Vis light. Chem. Eng. J. 2017, 310, 428–436. [Google Scholar] [CrossRef]
- Etacheri, V.; Seery, M.K.; Hinder, S.J.; Pillai, S.C. Highly Visible Light Active TiO2−xNx Heterojunction Photocatalysts. Chem. Mater. 2010, 22, 3843–3853. [Google Scholar] [CrossRef]
- Li, L.; Liu, C. Facile Synthesis of Anatase–Brookite Mixed-Phase N-Doped TiO2 Nanoparticles with High Visible-Light Photocatalytic Activity. Eur. J. Inorg. Chem. 2009, 25, 3727–3733. [Google Scholar] [CrossRef]
- Lee, H.U.; Lee, Y.; Lee, S.C.; Park, S.Y.; Son, B.; Lee, J.W.; Lim, C.; Choi, C.; Choi, M.; Lee, S.Y.; et al. Visible-light-responsive bicrystalline (anatase/brookite) nanoporous nitrogen-doped TiO2photocatalysts by plasma treatment. Chem. Eng. J. 2014, 254, 268–275. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Qin, W.; Zuo, S.; Yu, Y.; Hao, Z. Solvothermal-induced phase transition and visible photocatalytic activity of nitrogen-doped titania. J. Hazard. Mater. 2009, 163, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Huang, J.; Li, X.; Abass, M.A.; Zhang, S. Effective surface disorder engineering of metal oxide nanocrystals forimproved photocatalysis. Appl. Catal. B 2017, 203, 615–624. [Google Scholar] [CrossRef]
- Zimbone, M.; Cacciato, G.; Boutinguiza, M.; Privitera, V.; Grimaldi, M.G. Laser irradiation in water for the novel, scalable synthesis of black TiOx photocatalyst for environmental remediation. Beilstein J. Nanotechnol. 2017, 8, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Jiao, X.; Chen, D. Preparation of TiO2 aerogels by a sol-gel combined solvothermal route. J. Mater. Chem. 2009, 19, 3078–3083. [Google Scholar] [CrossRef]
- Wang, J.; Uma, S.; Klabunde, K.J. Visible light photocatalysis in transition metal incorporated titania-silica aerogels. Appl. Catal. B 2004, 48, 151–154. [Google Scholar] [CrossRef]
- Li, J.; Ishigaki, T.; Sun, X. Anatase, Brookite, and Rutile Nanocrystals via Redox Reactions under Mild Hydrothermal Conditions: Phase-Selective Synthesis and Physicochemical Properties. J. Phys. Chem. C 2007, 111, 4969–4976. [Google Scholar] [CrossRef]
- Jagadale, T.C.; Takale, S.P.; Sonawane, R.S.; Joshi, H.M.; Patil, S.I.; Kale, B.B.; Ogale, S.B. N-Doped TiO2 Nanoparticle Based Visible Light Photocatalyst by Modified Peroxide Sol−Gel Method. J. Phys. Chem. C 2008, 112, 14595–14602. [Google Scholar] [CrossRef]
- Li, J.; Xu, X.; Liub, X.; Qin, W.; Pan, L. Novel cake-like N-doped anatase/rutile mixed phase TiO2 derived from metal-organic frameworks for visible light photocatalysis. Ceram. Int. 2017, 43, 835–840. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Yang, M.; Zhang, G.; Dionysiou, D.D. HNO3-involved one-step low temperature solvothermal synthesis of N-doped TiO2 nanocrystals for efficient photocatalytic reduction of Cr(VI) in water. Appl. Catal. B 2013, 142, 249–258. [Google Scholar] [CrossRef]
- György, E.; del Pino, A.P.; Serra, P.; Morenza, J.L. Depth profiling characterisation of the surface layer obtained by pulsed Nd:YAG laser irradiation of titanium in nitrogen. Surf. Coat. Technol. 2003, 173, 265–270. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, L.; Andinobc, J.M.; Li, Y. Bicrystalline TiO2 with controllable anatase–brookite phase content for enhanced CO2 photoreduction to fuels. J. Mater. Chem. A 2013, 1, 8209–8216. [Google Scholar] [CrossRef]
- Wang, J.; Mao, B.; Gole, J.L.; Burda, C. Visible-light-driven reversible and switchable hydrophobic to hydrophilic nitrogen-doped titania surfaces: Correlation with photocatalysis. Nanoscale 2010, 2, 2257–2261. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; He, J.; Guo, L.; Li, Y.; Duan, D.; Chen, Y.; Li, J.; Yuan, F.; Wang, J. Biotemplated Mesoporous TiO2/SiO2 Composite Derived from Aquatic Plant Leaves for Efficient Dye Degradation. Catalysts 2017, 7, 82. [Google Scholar] [CrossRef]
- Miao, Y.; Zhai, Z.; Jiang, L.; Shi, Y.; Yan, Z.; Duan, D.; Zhen, K.; Wang, J. Facile and new synthesis of cobalt doped mesoporous TiO2 with high visible-light performance. Powder Technol. 2014, 266, 365–371. [Google Scholar] [CrossRef]
- Li, W.; Li, D.; Lin, Y.; Wang, P.; Chen, W.; Fu, X.; Shao, Y. Evidence for the Active Species Involved in the Photodegradation Process of Methyl Orange on TiO2. J. Phys. Chem. C 2012, 116, 3552–3560. [Google Scholar] [CrossRef]

| Samples | Anatase | Brookite | SBET (m2/g) | K (min−1) | ||
|---|---|---|---|---|---|---|
| Crystal Size a (nm) | Content b (%) | Crystal Size a (nm) | Content b (%) | |||
| NA-185 | 6.7 | 90 | 7.3 | 10 | 239 | 0.023 |
| NA-165 | 7.5 | 94 | 7.2 | 6 | 240 | 0.021 |
| NA-145 | 6.5 | 100 | - | - | 249 | 0.015 |
| HA-165 | 7.9 | 100 | - | - | 216 | 0.006 |
| TF-165 | - | - | - | - | 443 | 0.002 |
| MF-165 | - | - | - | - | 407 | 0.003 |
| P25 | - | - | - | - | 50 | 0.005 |
| Sample | Surface Area (m2/g) | Preparation Method | Application | The Times of K (min−1) to P25 | Reference |
|---|---|---|---|---|---|
| Nitrogen-doped TiO2 nanorods with anatase/brookite structures | 51.1 | Hydrothermal synthesis 200 °C, 48 h | Degradation of MO and 4-chlorophenol (4-CP) | 2.3, 2.7 | [19] |
| Nitrogen-doped anatase/brookite titania | 124.4 | Solvothermal synthesis 190 °C, 3 h | Degradation of MO | [24] | |
| Anatase–brookite mixed-phase N-doped TiO2 nanoparticles | 76.2 | Solvothermal synthesis 180 °C, 48 h | Degradation of Methylene blue (MB) | [22] | |
| Nitrogen-doped TiO2 film | Solvothermal synthesis 180 °C, 18 h | Degradation of MB | [35] | ||
| Bicrystalline (anatase/brookite) nanoporous nitrogen-doped TiO2 | 375.9 | Plasma treatment 0.5 h | Degradation of Rhodamine B (RhB) | [23] | |
| Nitrogen-doped anatase/brookite biphasic nanoparticles | 240 | Sol-gel synthesis 165 °C, 4 h | Degradation of MO | 4.2 | This work |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, L.; Li, Y.; Yang, H.; Yang, Y.; Liu, J.; Yan, Z.; Long, X.; He, J.; Wang, J. Low-Temperature Sol-Gel Synthesis of Nitrogen-Doped Anatase/Brookite Biphasic Nanoparticles with High Surface Area and Visible-Light Performance. Catalysts 2017, 7, 376. https://doi.org/10.3390/catal7120376
Jiang L, Li Y, Yang H, Yang Y, Liu J, Yan Z, Long X, He J, Wang J. Low-Temperature Sol-Gel Synthesis of Nitrogen-Doped Anatase/Brookite Biphasic Nanoparticles with High Surface Area and Visible-Light Performance. Catalysts. 2017; 7(12):376. https://doi.org/10.3390/catal7120376
Chicago/Turabian StyleJiang, Liang, Yizhou Li, Haiyan Yang, Yepeng Yang, Jun Liu, Zhiying Yan, Xiang Long, Jiao He, and Jiaqiang Wang. 2017. "Low-Temperature Sol-Gel Synthesis of Nitrogen-Doped Anatase/Brookite Biphasic Nanoparticles with High Surface Area and Visible-Light Performance" Catalysts 7, no. 12: 376. https://doi.org/10.3390/catal7120376