Cu Nanoparticles/Fluorine-Doped Tin Oxide (FTO) Nanocomposites for Photocatalytic H2 Evolution under Visible Light Irradiation
Abstract
:1. Introduction
2. Results
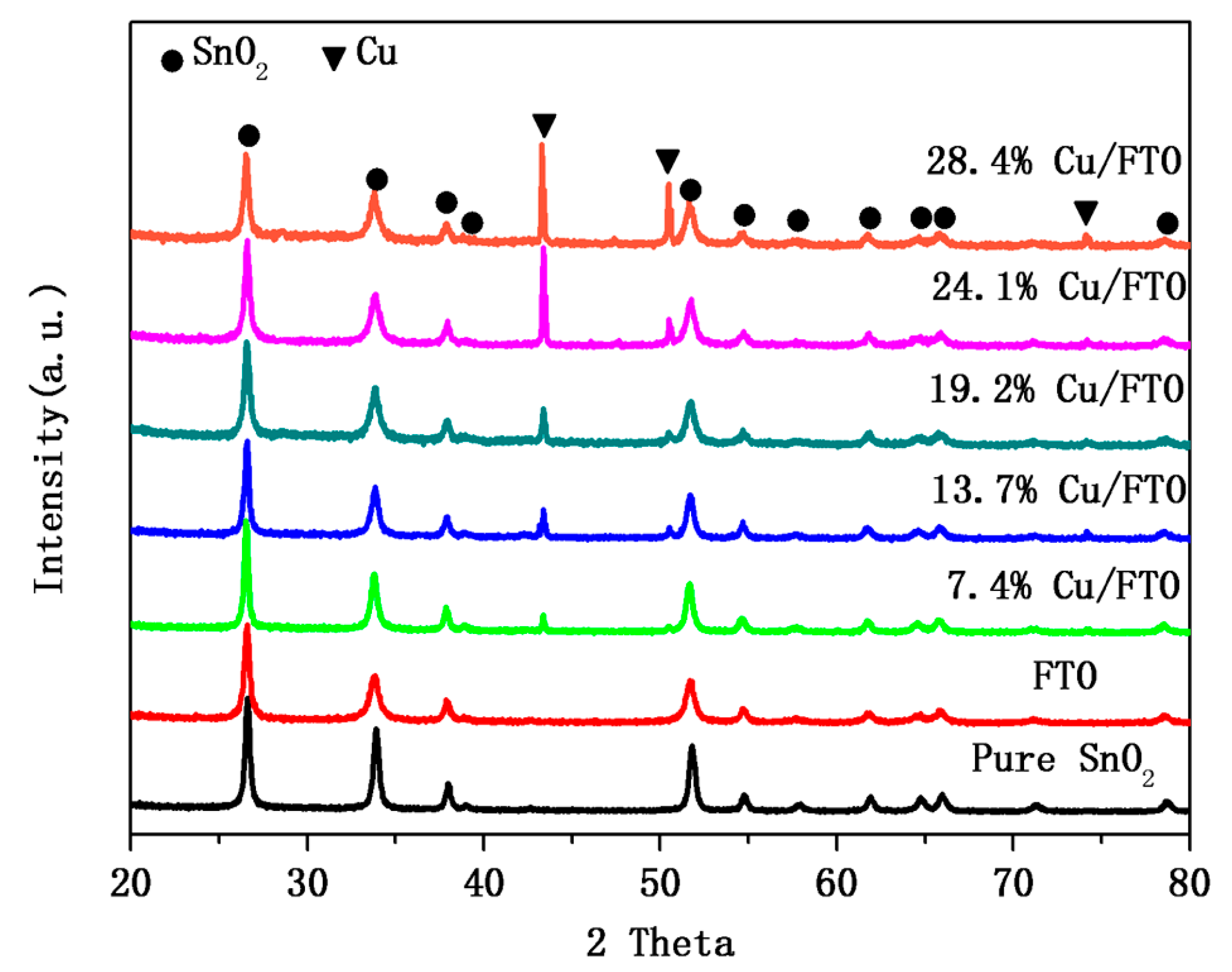
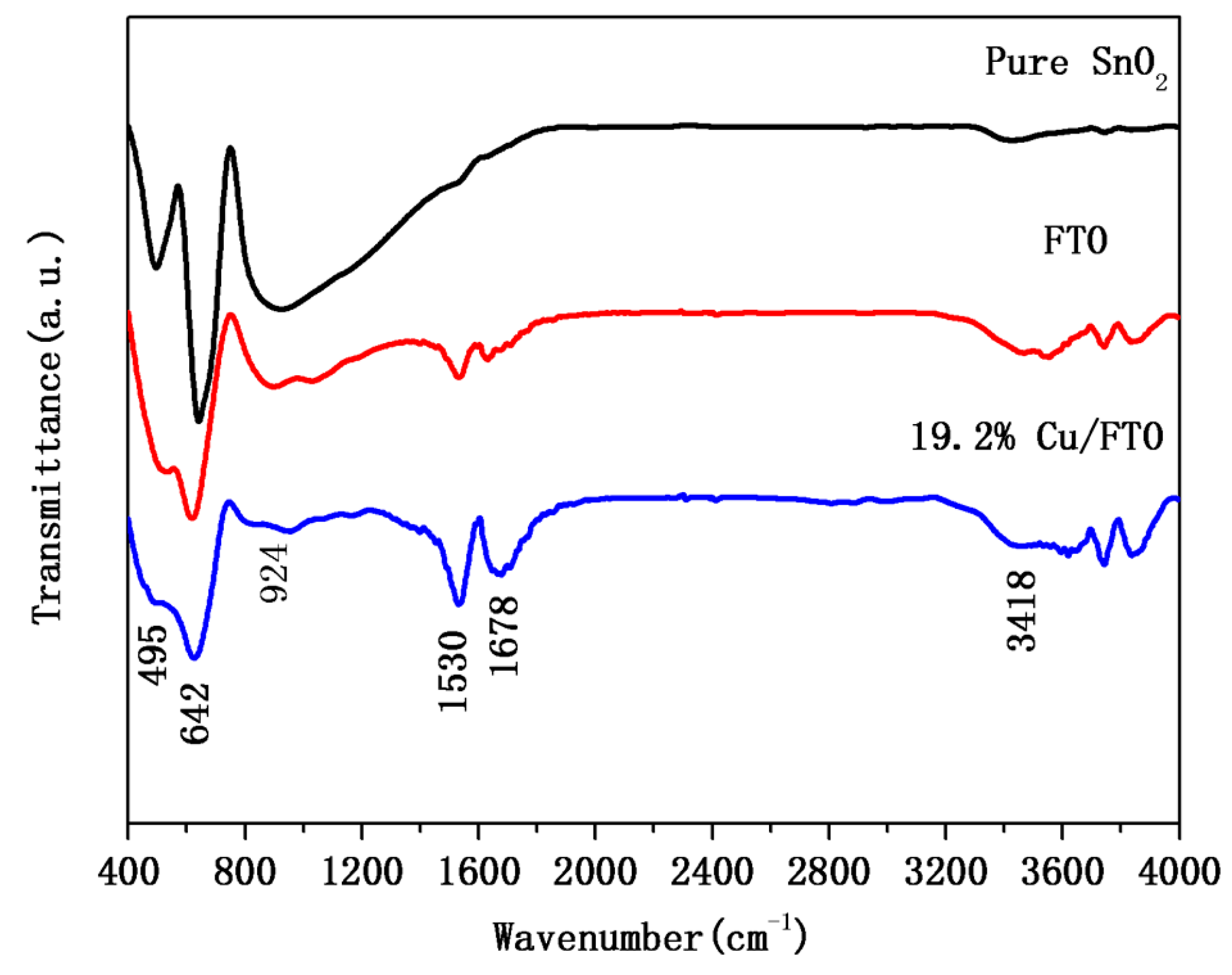
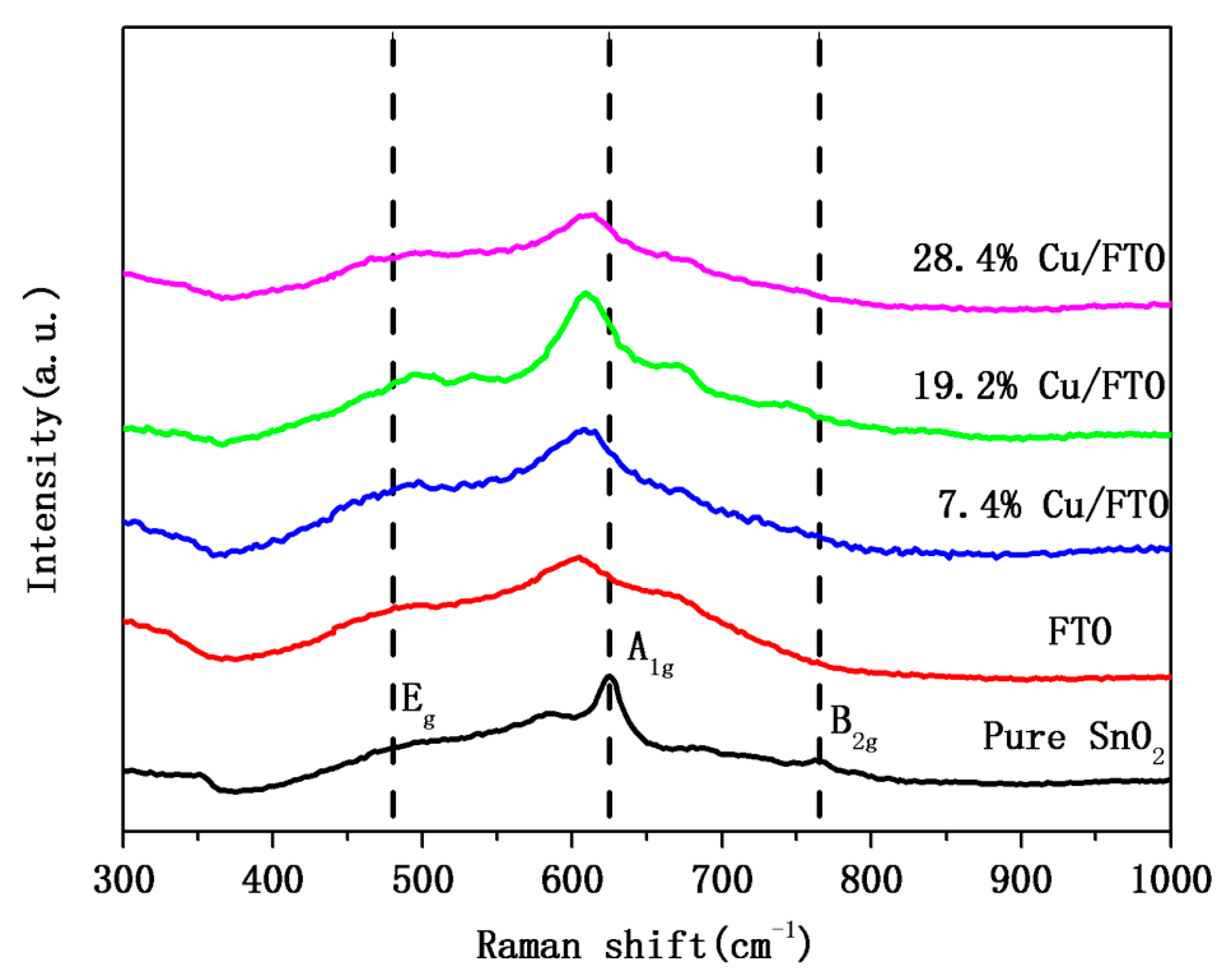
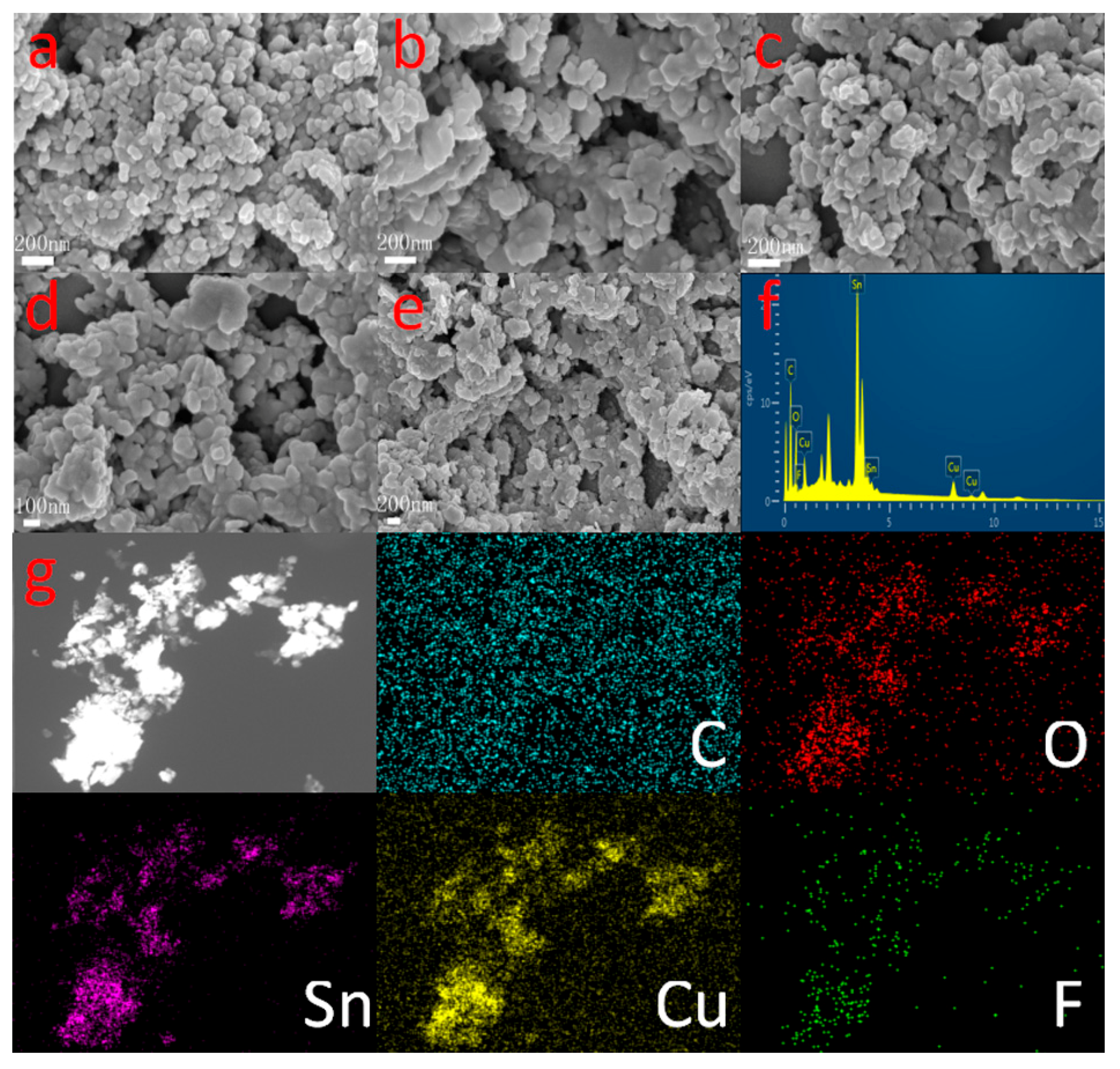
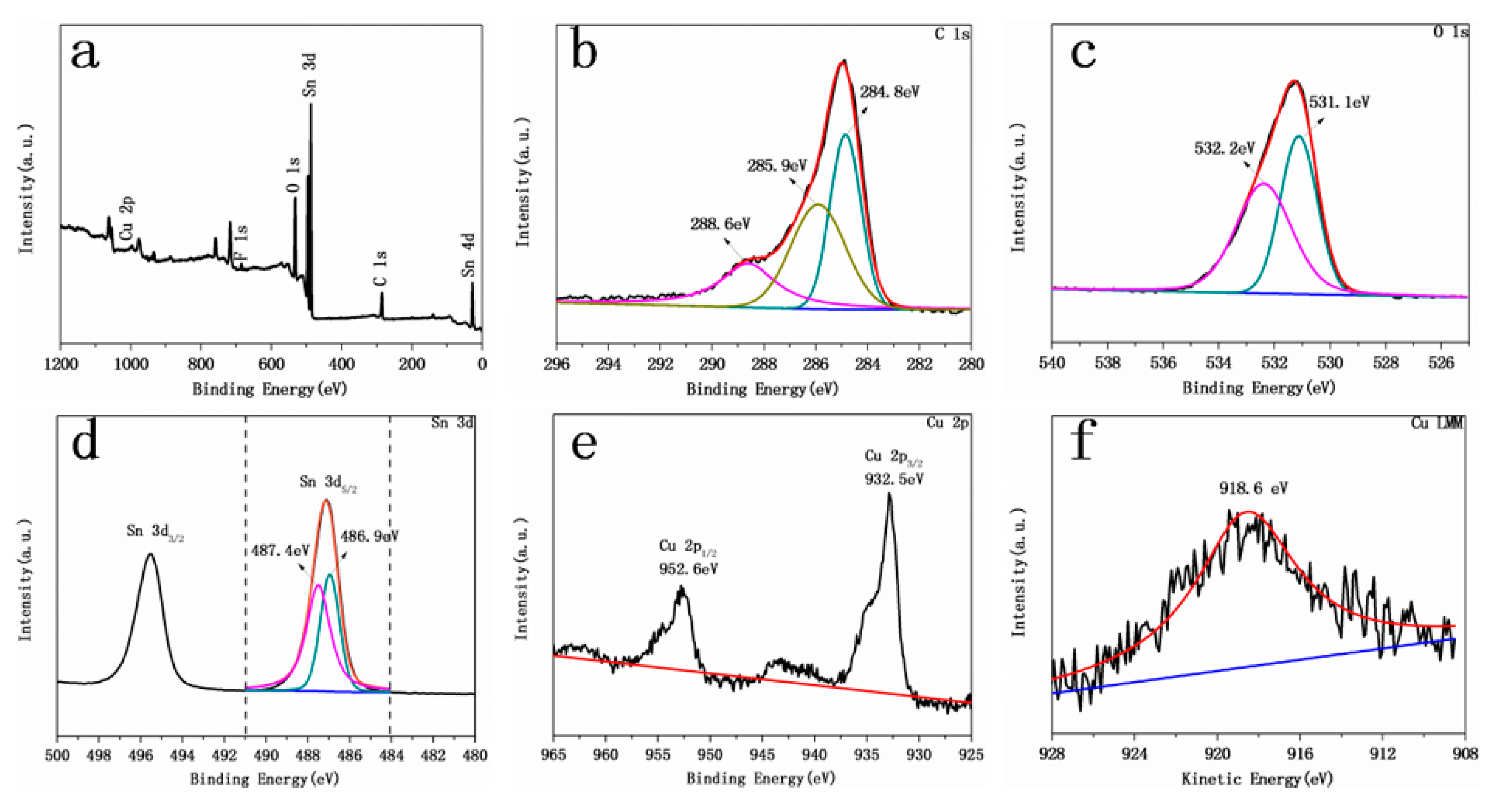
2.1. Crystal Structure and Composition
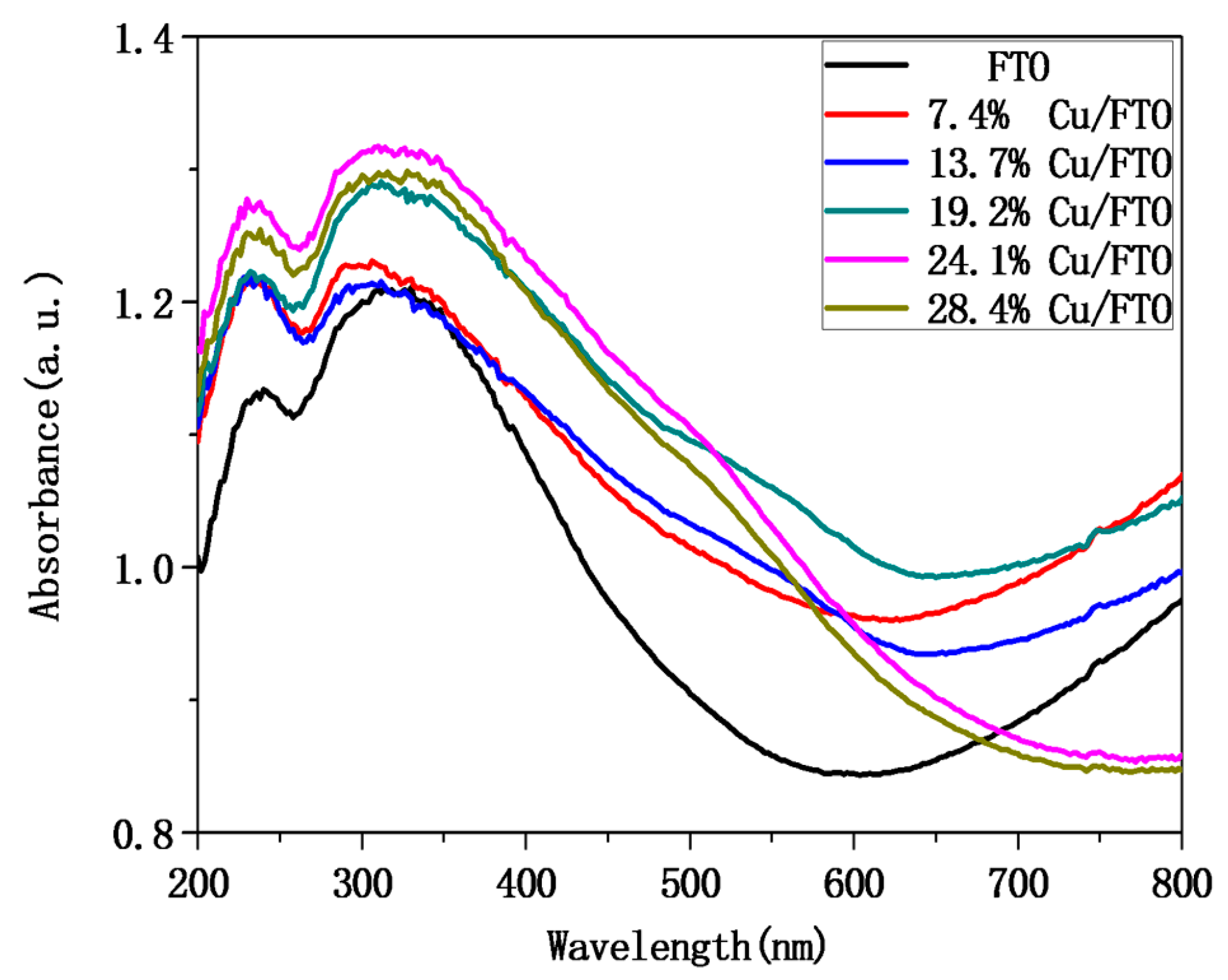
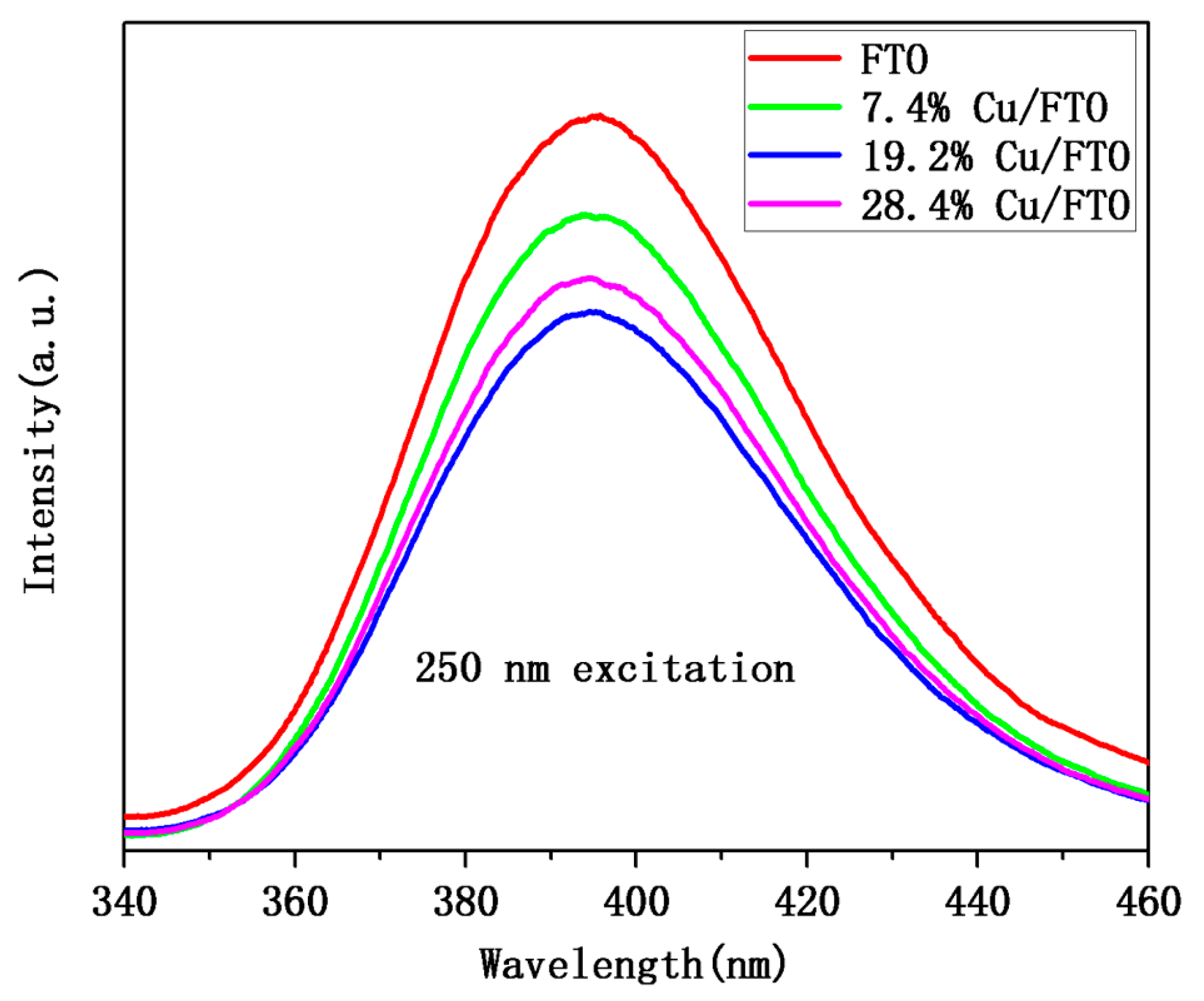
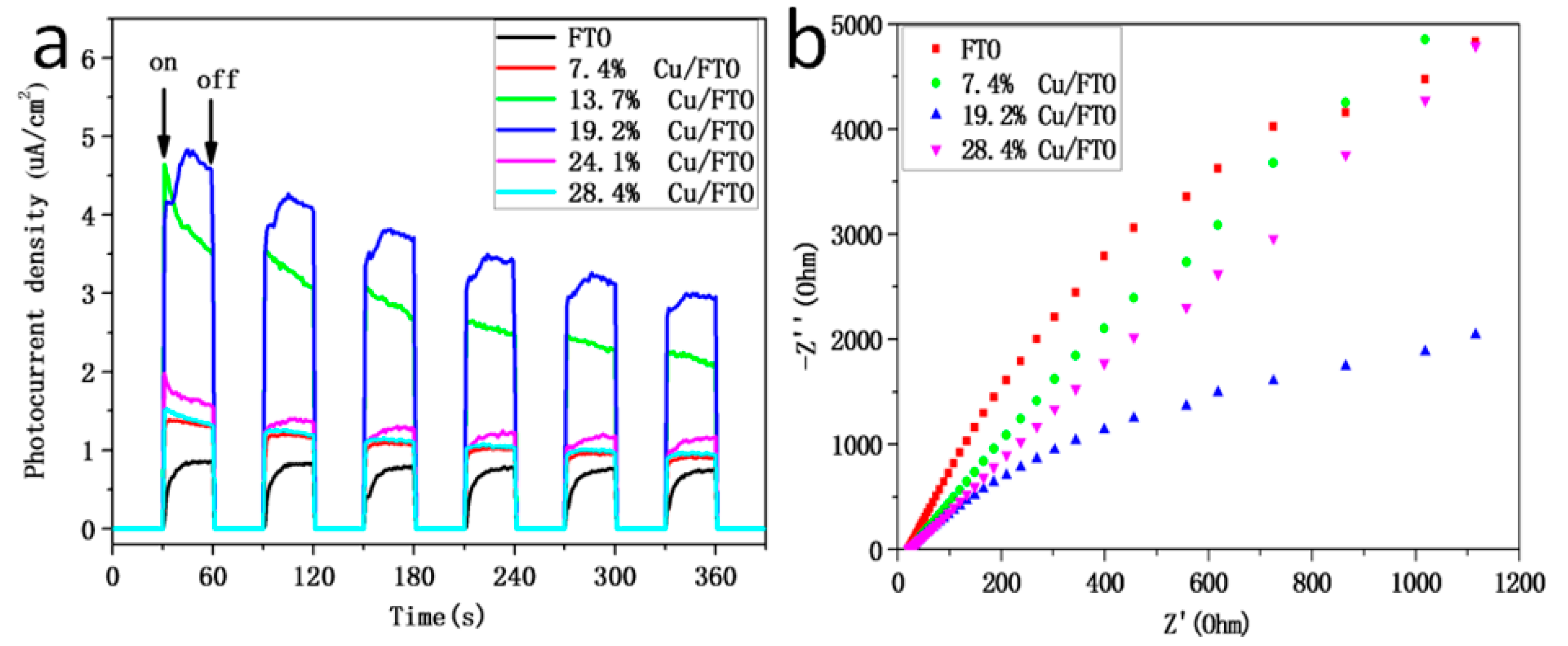
2.2. Optical and Photoelectrochemical Properties
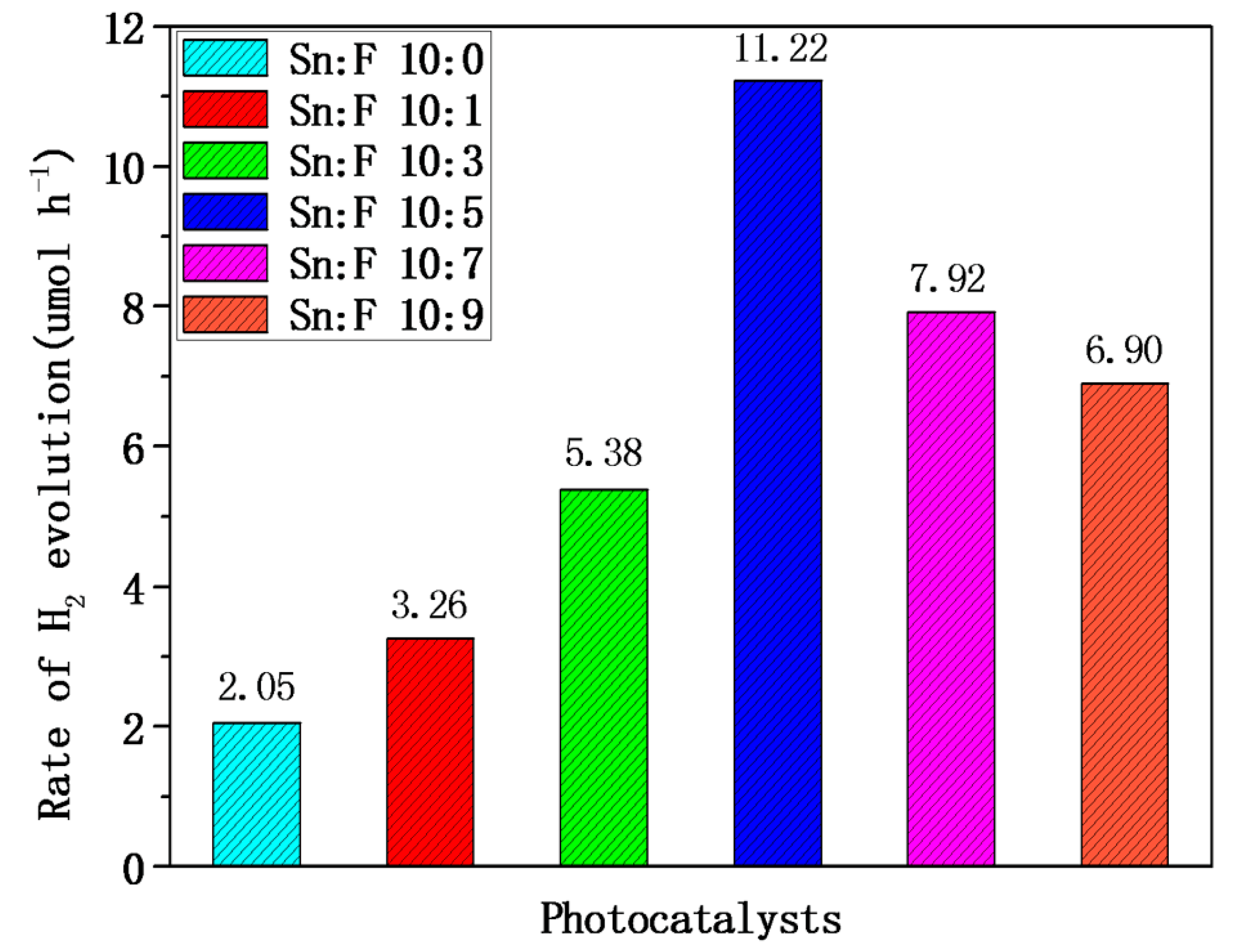
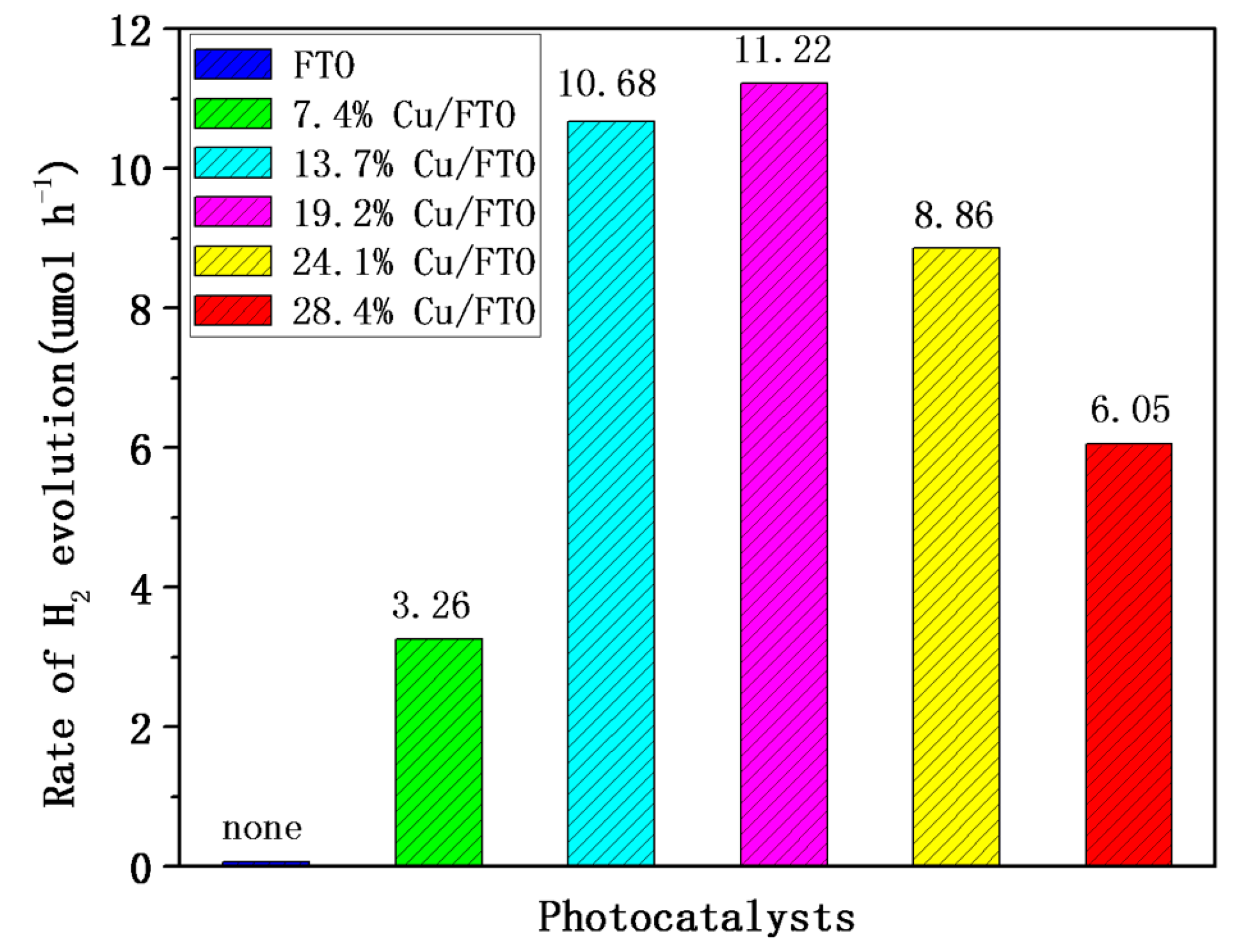
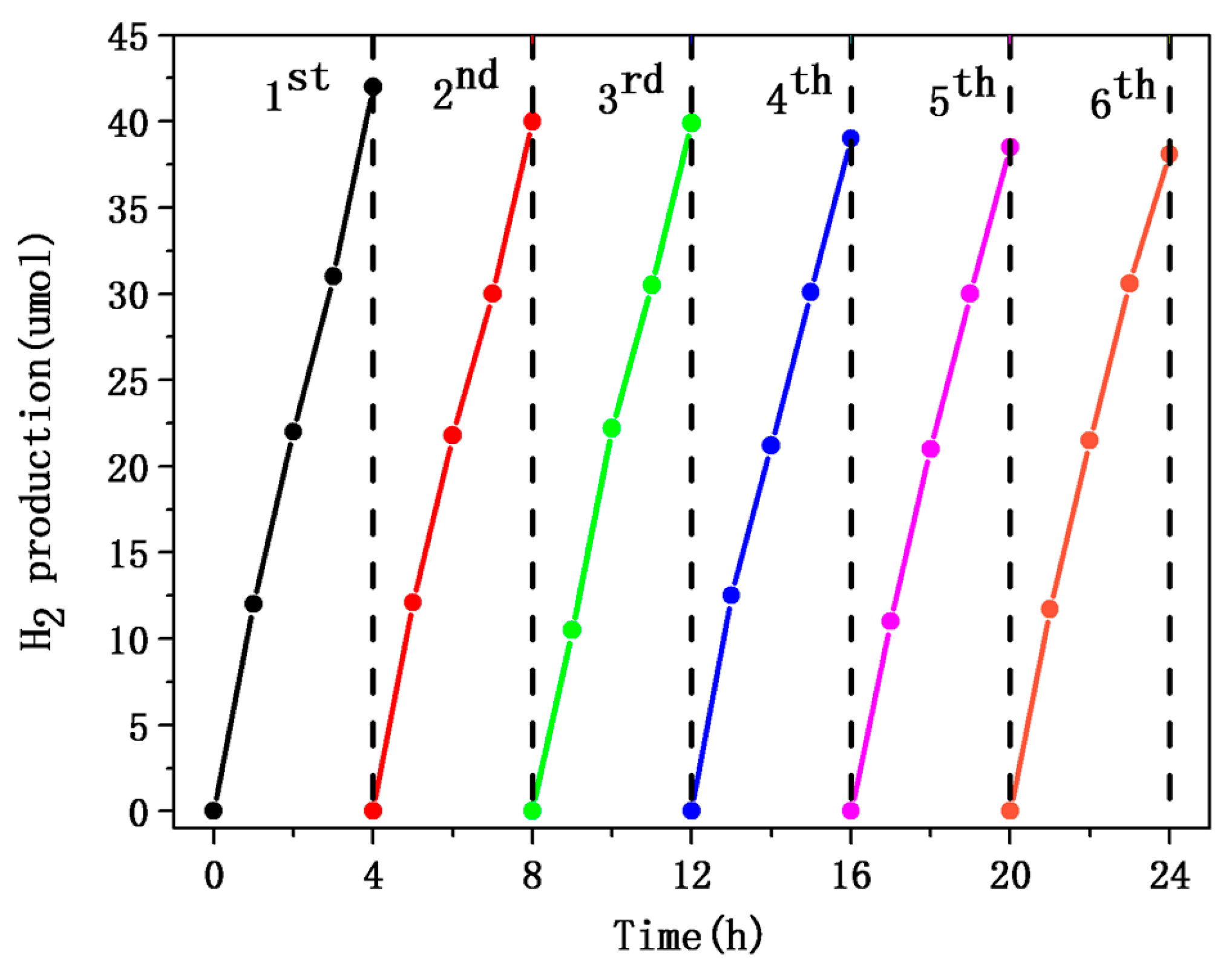
2.3. Photocatalytic Hydrogen Evolution
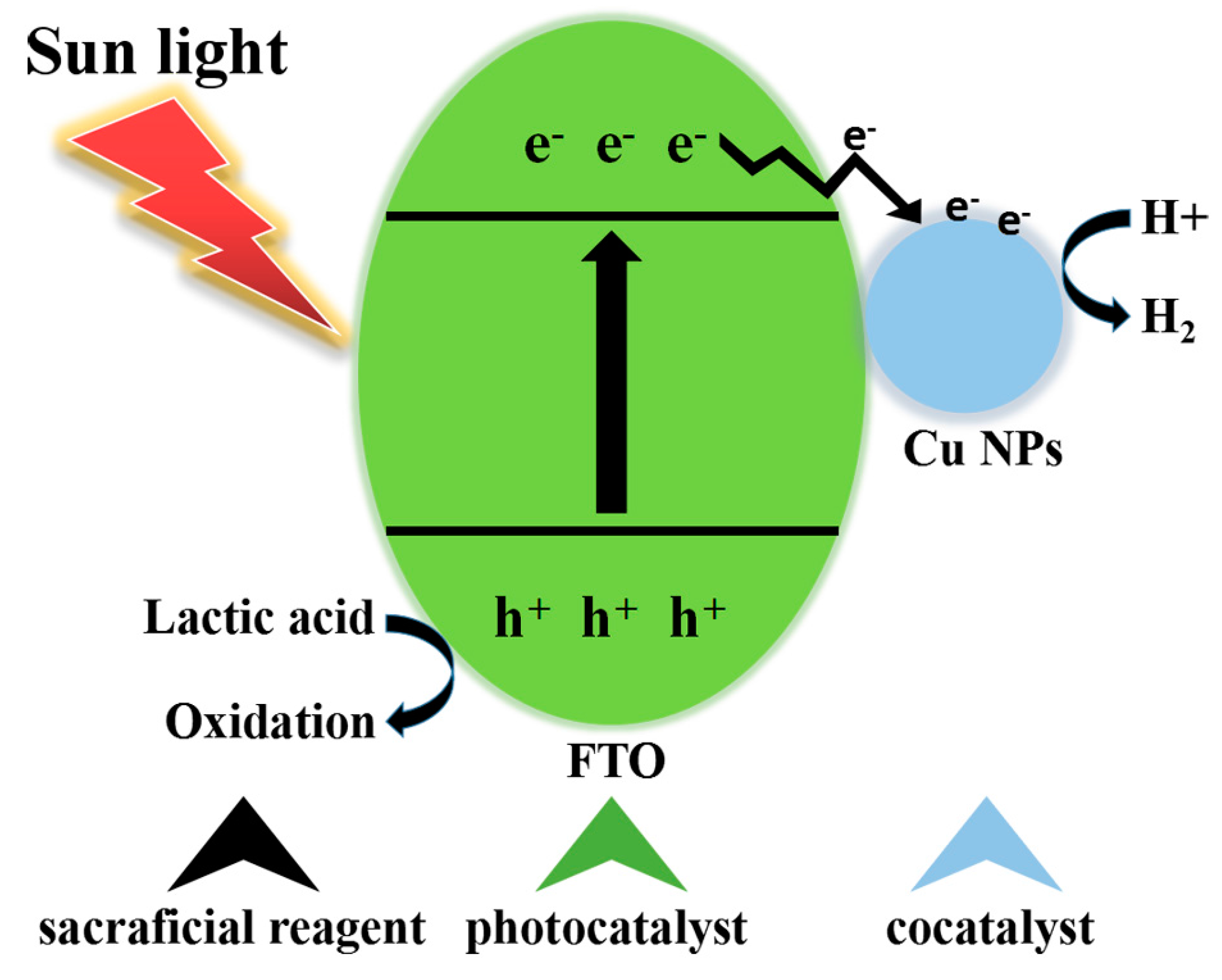
2.4. Mechanism
3. Materials and Methods
3.1. Materials
3.2. Synthesis of FTO Nanopowders
3.3. Synthesis of Cu/FTO Nanocomposites
3.4. Characterization
3.5. Photocatalytic Hydrogen Evolution Experiment
3.6. Photoelectrochemical Measurements
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, Y.; Wang, Q.; Zhan, X.; Wang, F.; Safdar, M.; He, J. Visible light driven type II heterostructures and their enhanced photocatalysis properties: A review. Nanoscale 2013, 5, 8326–8339. [Google Scholar] [CrossRef] [PubMed]
- Jafari, T.; Moharreri, E.; Amin, A.; Miao, R.; Song, W.Q.; Suib, S. Photocatalytic Water Splitting—The Untamed Dream: A Review of Recent Advances. Molecules 2016, 21, 900. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.P.; Zeng, H.P. A novel triphenylamine functionalized bithiazole–metal complex with C60 for photocatalytic hydrogen production under visible light irradiation. J. Mater. Chem. A 2015, 3, 6258–6264. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Photolysis-decomposition of water at the surface of an irradiated semiconductor. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.P.; Fang, L.T.; Lei, Y.L.; Zeng, G.C.; Zeng, H.P. Facile preparation of yttrium and aluminum co-doped ZnO via a sol–gel route for photocatalytic hydrogen production. J. Mater. Chem. A 2014, 2, 11040–11044. [Google Scholar] [CrossRef]
- Qin, J.Y.; Huo, J.P.; Zhang, P.Y.; Zeng, J.; Wang, T.T.; Zeng, H.P. Improving the photocatalytic hydrogen production of Ag/g-C3N4 nanocomposites by dye-sensitization under visible light irradiation. Nanoscale 2016, 8, 2249–2259. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.W.; Pan, D.Y.; Li, Z.; Jiao, Z.; Wu, M.H.; Shek, C.; Wu, C.M.L.; Lai, J.K.L. Recent Advances in Tin Dioxide Materials: Some Developments in Thin Films, Nanowires, and Nanorods. Chem. Rev. 2014, 114, 7442–7486. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Ansari, S.A.; Khan, M.E.; Ansari, M.O.; Min, B.K. Visible light-induced enhanced photoelectrochemical and photocatalytic studies of gold decorated SnO2 nanostructures. New J. Chem. 2015, 39, 2758–2766. [Google Scholar] [CrossRef]
- Babu, B.; Kadam, A.N.; Ravikumar, R.; Chan, B. Enhanced visible light photocatalytic activity of Cu-doped SnO2 quantum dots by solution combustion synthesis. J. Alloys Compd. 2017, 703, 330–336. [Google Scholar] [CrossRef]
- Ghimbeu, C.M.; Lumbreras, M.; Siadat, M.; van Landschoot, R.C.; Schoonman, J. Electrostatic sprayed SnO2 and Cu-doped SnO2 films for H2S detection. Sens. Actuators B Chem. 2008, 133, 694–698. [Google Scholar] [CrossRef]
- Chandra, S.; George, G.; Ravichandran, K.; Thirumurugan, K. Influence of simultaneous cationic (Mn) and anionic (F) doping on the magnetic and certain other properties of SnO2 thin films. Surf. Interface 2017, 7, 39–46. [Google Scholar] [CrossRef]
- Seema, H.; Christian, K.K.; Chandra, V.; Kim, K.S. Graphene-SnO2 composites for highly efficient photocatalytic degradation of methylene blue under sunlight. Nanotechnology 2012, 23, 355705. [Google Scholar] [CrossRef] [PubMed]
- Kent, C.A.; Concepcion, J.J.; Dares, C.J.; Torelli, D.A.; Rieth, A.J.; Miller, A.S.; Meyer, T.J. Water Oxidation and Oxygen Monitoring by Cobalt-Modified Fluorine-Doped Tin Oxide Electrodes. J. Am. Chem. Soc. 2013, 135, 8432–8435. [Google Scholar] [CrossRef] [PubMed]
- Samad, W.Z.; Goto, M.; Kanda, H.; Nordin, N.; Liew, K.H.; Yarmo, M.A.; Yusop, M.R. Fluorine-doped tin oxide catalyst for glycerol conversion to methanol in sub-critical water. J. Supercrit. Fluids 2017, 120, 366–378. [Google Scholar] [CrossRef]
- Zhang, P.Y.; Song, T.; Wang, T.T.; Zeng, H.P. In-situ synthesis of Cu nanoparticles hybridized with carbon quantum dots as a broad spectrum photocatalyst for improvement of photocatalytic H2 evolution. Appl. Catal. B Environ. 2017, 206, 328–335. [Google Scholar] [CrossRef]
- He, W.; Kim, H.K.; Wamer, W.G.; Melka, D.; Callahan, J.H. Photogenerated Charge Carriers and Reactive Oxygen Species in ZnO/Au Hybrid Nanostructures with Enhanced Photocatalytic and Antibacterial Activity. J. Am. Chem. Soc. 2014, 136, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Logar, M.; Jancar, B.; Sturm, S.; Suvorov, D. Weak polyion multilayer-assisted in situ synthesis as a route toward a plasmonic Ag/TiO2 photocatalyst. Langmuir 2010, 26, 12215–12224. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, Y.; Kofuji, Y.; Kanazawa, S.; Sakamoto, H.; Ichikawa, S.; Tanaka, S.; Hirai, T. Platinum nanoparticles strongly associated with graphitic carbon nitride as efficient co-catalysts for photocatalytic hydrogen evolution under visible light. Chem. Commun. 2014, 50, 15255–15258. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.L.; Kang, S.; Li, X.Q.; Qin, L.X.; Mu, J. TiO2 nanosheets loaded with Cu: A low-cost efficient photocatalytic system for hydrogen evolution from water. Int. J. Hydrog. Energy 2014, 39, 15403–15410. [Google Scholar] [CrossRef]
- Han, C.; Yang, M.Q.; Zhang, N.; Xu, Y.J. Enhancing the visible light photocatalytic performance of ternary CdS–(graphene–Pd) nanocomposites via a facile interfacial mediator and co-catalyst strategy. J. Mater. Chem. A 2014, 2, 19156–19166. [Google Scholar] [CrossRef]
- Liu, E.Z.; Qi, L.L.; Bian, J.J.; Chen, Y.H.; Hu, X.Y.; Fan, J.; Liu, H.C.; Zhu, C.J.; Wang, Q.P. A facile strategy to fabricate plasmonic Cu modified TiO2 nano-flower films for photocatalytic reduction of CO2 to methanol. Mater. Res. Bull. 2015, 68, 203–209. [Google Scholar] [CrossRef]
- Huo, J.P.; Zeng, H.P. Copper nanoparticles embedded in the triphenylamine functionalized bithiazole–metal complex as active photocatalysts for visible light-driven hydrogen evolution. J. Mater. Chem. A 2015, 3, 17201–17208. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, D.; Wu, Q.Y.; Diao, P. Cu2O/CuO Bilayered Composite as a High-Efficiency Photocathode for Photoelectrochemical Hydrogen Evolution Reaction. Sci. Rep. 2016, 6, 35158. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cui, S.S.; Sun, Z.J.; Du, P.W. Copper oxide nanomaterials synthesized from simple copper salts as active catalysts for electrocatalytic water oxidation. Electrochim. Acta 2015, 160, 202–208. [Google Scholar] [CrossRef]
- Zhang, P.Y.; Wang, T.T.; Zeng, H.P. Design of Cu-Cu2O/g-C3N4 nanocomponent photocatalysts for hydrogen evolution under visible light irradiation using water-soluble Erythrosin B dye sensitization. Appl. Surf. Sci. 2017, 391, 404–414. [Google Scholar] [CrossRef]
- Liu, H.Y.; Wang, T.T.; Zeng, H.P. CuNPs for Efficient Photocatalytic Hydrogen Evolution. Part. Part. Syst. Charact. 2015, 32, 869–873. [Google Scholar] [CrossRef]
- Zheng, L.R.; Zheng, Y.H.; Chen, C.Q.; Zhan, Y.Y.; Lin, X.Y.; Zheng, Q.; Wei, K.M.; Zhu, J.F. Network Structured SnO2/ZnO Heterojunction Nanocatalyst with High Photocatalytic Activity. Inorg. Chem. 2009, 48, 1819–1825. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.J.; Meng, Q.N.; Wang, L.; Zhang, J.H.; Song, Y.B.; Jin, H.; Zhang, K.; Sun, H.C.; Wang, H.Y.; Yang, B. Highly Photoluminescent Carbon Dots for Multicolor Patterning, Sensors, and Bioimaging. Angew. Chem. Int. Ed. 2013, 52, 3953–3957. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Tian, Y.; Zhang, J.X.; Cai, W. The characterization of fluorine doped tin oxide films by Fourier Transformation Infrared spectrum. Appl. Phys. Lett. 2011, 98, 021906. [Google Scholar] [CrossRef]
- Noor, N.; Parkin, I.P. Enhanced transparent-conducting fluorine-doped tin oxide films formed by Aerosol-Assisted Chemical Vapour Deposition. J. Mater. Chem. C 2013, 1, 984–996. [Google Scholar] [CrossRef]
- Liu, E.; Kang, L.; Yang, Y.; Sun, T.; Hu, X. Plasmonic Ag deposited TiO2 nano-sheet film for enhanced photocatalytic hydrogen production by water splitting. Nanotechnology 2014, 25, 165401–165410. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.L.; Handoko, A.D.; Do, Y.H.; Xi, S.B.; Yeo, B.S. In Situ Raman Spectroscopy of Copper and Copper Oxide Surfaces during Electrochemical Oxygen Evolution Reaction: Identification of CuIII Oxides as Catalytically Active Species. ACS Catal. 2016, 6, 2473–2481. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, B.; Yang, S.; Wu, H.; Wu, Y. In situ construction of an SnO2/g-C3N4 heterojunction for enhanced visible-light photocatalytic activity. RSC Adv. 2015, 5, 68953–68963. [Google Scholar] [CrossRef]
- Song, T.; Huo, J.P.; Liao, T.; Zeng, J.; Qin, J.Y.; Zeng, H.P. Fullerene [C60] modified Cr2−xFexO3 nanocomposites for enhanced photocatalytic activity under visible light irradiation. Chem. Eng. J. 2016, 287, 359–366. [Google Scholar] [CrossRef]
- Wu, S.S.; Yuan, S.; Shi, L.Y.; Zhao, Y.; Fang, J.H. Preparation, characterization and electrical properties of fluorine-doped tin dioxide nanocrystals. J. Colloid Interface Sci. 2010, 346, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.M.; Zhang, C.Y.; Yang, P.; Du, Y.K.; Lu, C. WS2 as an Effective Noble-Metal Free Cocatalyst Modified TiSi2 for Enhanced Photocatalytic Hydrogen Evolution under Visible Light Irradiation. Catalysts 2016, 6, 136. [Google Scholar] [CrossRef]
- Yu, J.C.; Yu, J.G.; Ho, W.K.; Jiang, Z.T.; Zhang, L.Z. Effects of F− Doping on the Photocatalytic Activity and Microstructures of Nanocrystalline TiO2 Powders. Chem. Mater. 2002, 14, 3808–3816. [Google Scholar] [CrossRef]
- Chao, Y.G.; Zheng, J.F.; Chen, J.Z.; Wang, Z.J.; Jia, S.P.; Zhang, H.X.; Zhu, Z.P. Highly efficient visible light-driven hydrogen production of precious metal-free hybrid photocatalyst: CdS@NiMoS core–shell nanorods. Catal. Sci. Technol. 2017, 7, 2798–2804. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Wang, A.; Sun, Q.; Wang, T.; Zeng, H. Cu Nanoparticles/Fluorine-Doped Tin Oxide (FTO) Nanocomposites for Photocatalytic H2 Evolution under Visible Light Irradiation. Catalysts 2017, 7, 385. https://doi.org/10.3390/catal7120385
Liu H, Wang A, Sun Q, Wang T, Zeng H. Cu Nanoparticles/Fluorine-Doped Tin Oxide (FTO) Nanocomposites for Photocatalytic H2 Evolution under Visible Light Irradiation. Catalysts. 2017; 7(12):385. https://doi.org/10.3390/catal7120385
Chicago/Turabian StyleLiu, Hui, An Wang, Quan Sun, Tingting Wang, and Heping Zeng. 2017. "Cu Nanoparticles/Fluorine-Doped Tin Oxide (FTO) Nanocomposites for Photocatalytic H2 Evolution under Visible Light Irradiation" Catalysts 7, no. 12: 385. https://doi.org/10.3390/catal7120385



