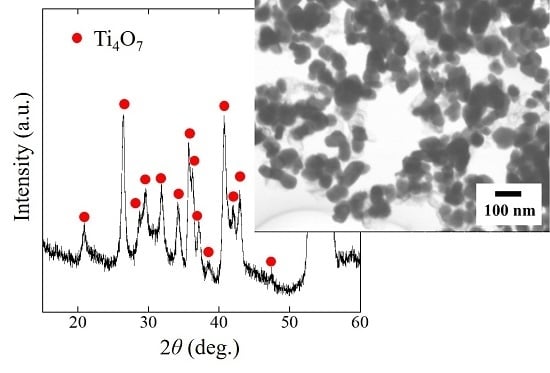
Synthesis of Ti4O7 Nanoparticles by Carbothermal Reduction Using Microwave Rapid Heating
Abstract
:1. Introduction
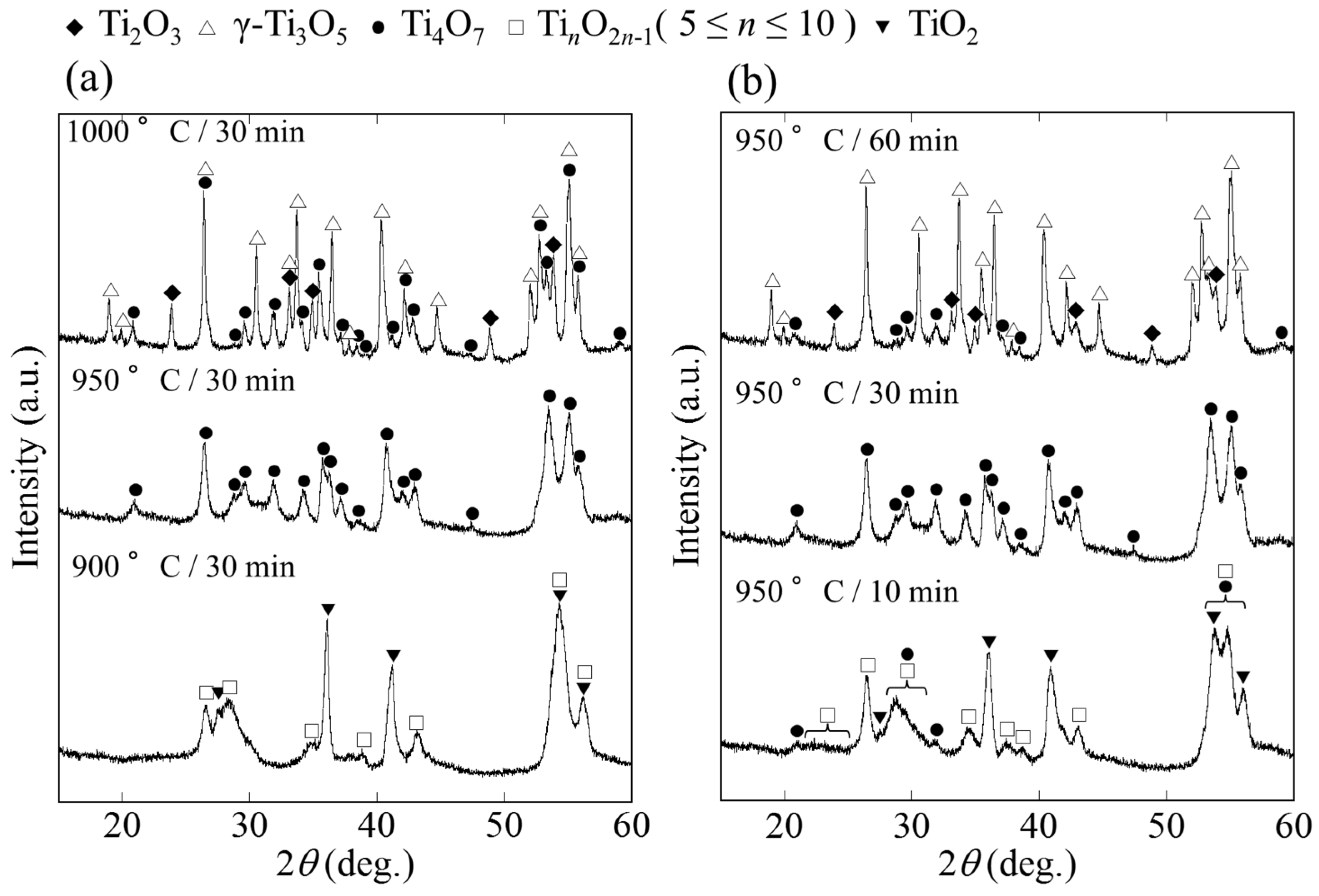
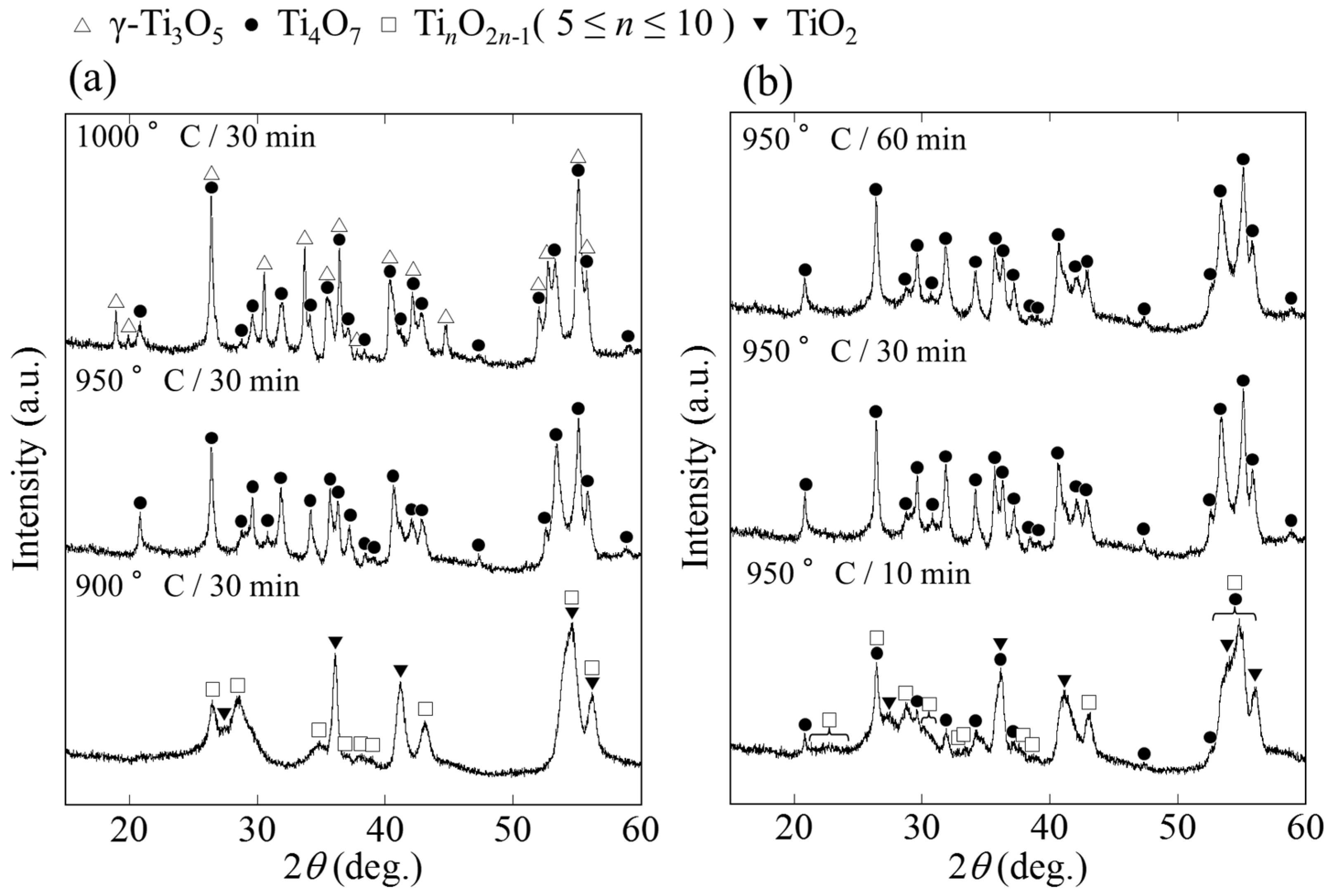
2. Results and Discussion
2.1. Phase and Reduction Behavior
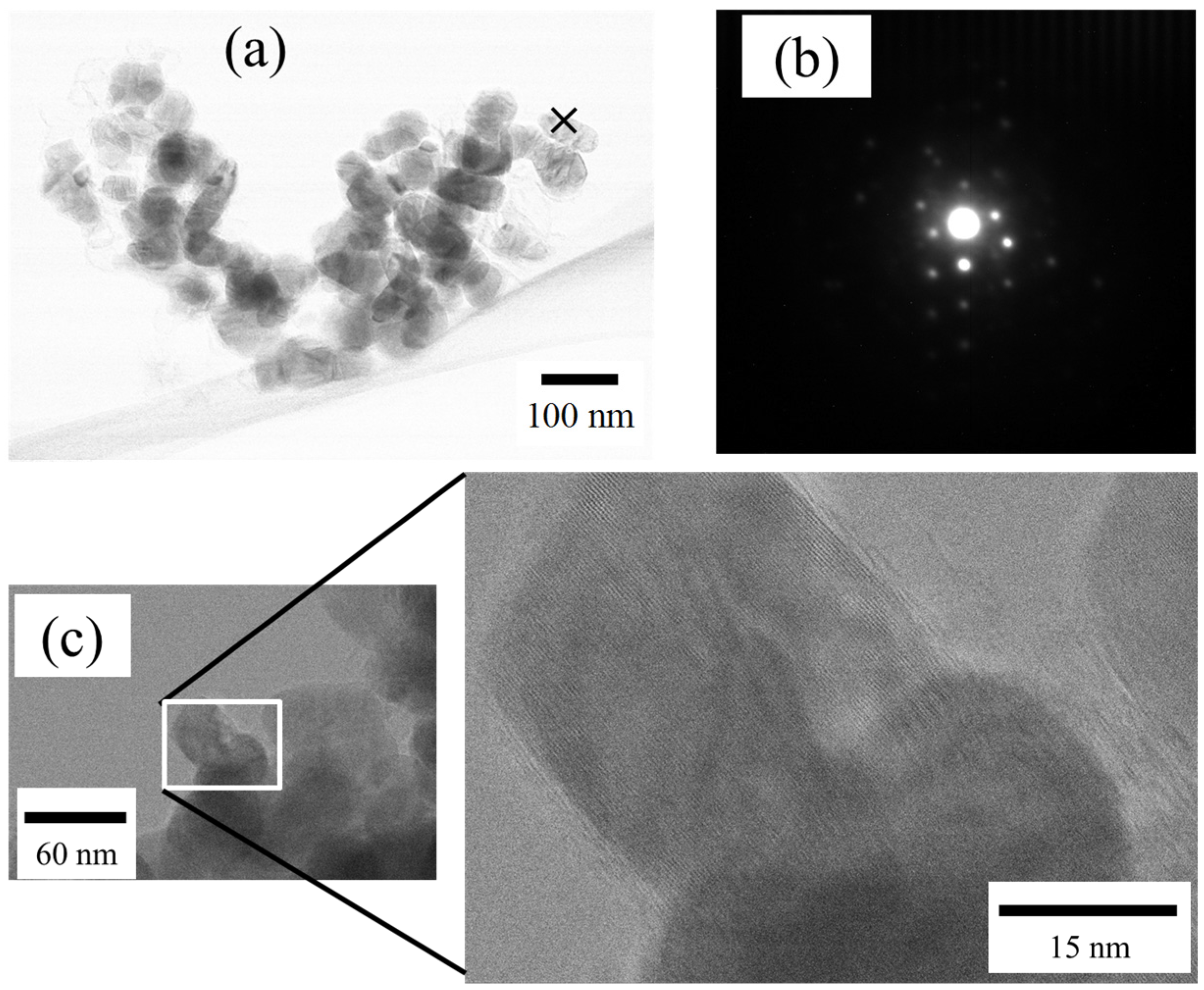
2.2. Size and Morphology of Nanoparticles
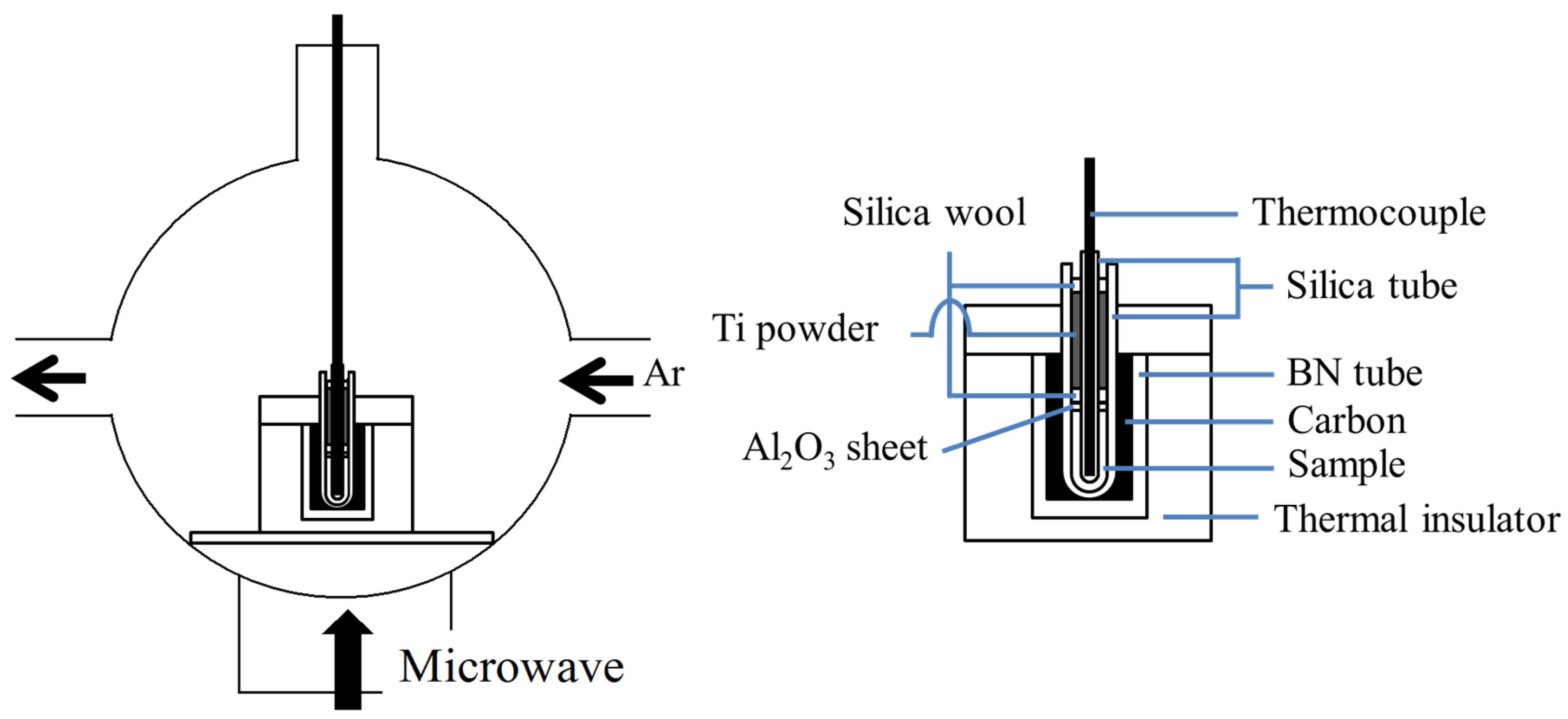
3. Experimental Section
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lori, O.; Elbaz, L. Advances in Ceramic Supports for Polymer Electrolyte Fuel Cells. Catalysts 2015, 5, 1445–1464. [Google Scholar] [CrossRef]
- Smith, J.R.; Walsh, F.C. Electrodes based on Magnéli phase titanium oxides: The properties and applications of Ebonex materials. J. Appl. Electrochem. 1998, 28, 1021–1033. [Google Scholar] [CrossRef]
- Ioroi, T.; Senoh, H.; Yamazaki, S.; Siroma, Z.; Fujiwara, N.; Yasuda, K. Stability of Corrosion-Resistant Magnéli-Phase Ti4O7-Supported PEMFC Catalysts at High Potentials. J. Electrochem. Soc. 2008, 155, B321–B326. [Google Scholar] [CrossRef]
- Tominaka, S.; Tsujimoto, Y.; Matsushita, Y.; Yamaura, K. Synthesis of nanostructured reduced titanium oxide: Crystal structure transformation maintaining nanomorphology. Angew. Chem. 2011, 50, 7418–7421. [Google Scholar] [CrossRef] [PubMed]
- Tominaka, S. Facile synthesis of nanostructured reduced titanium oxides using borohydride toward the creation of oxide-based fuel cell electrodes. Chem. Commun. 2012, 48, 7949–7951. [Google Scholar] [CrossRef] [PubMed]
- Portehault, D.; Maneeratana, V.; Candolfi, C.; Oeschler, N.; Veremchuk, I.; Grin, Y.; Sanchez, C.; Antonietti, M. Facile General Route toward Tunable Magnéli Nanostructures and Their Use As Thermoelectric Metal Oxide/Carbon Nanocomposites. ACS Nano 2011, 5, 9052–9061. [Google Scholar] [CrossRef] [PubMed]
- Conze, S.; Veremchuk, I.; Reibold, M.; Matthey, B.; Michaelis, A.; Grin, Y.; Kinski, I. Magnéli phases Ti4O7 and Ti8O15 and their carbon nanocomposites via the thermal decomposition-precursor route. J. Solid State Chem. 2015, 229, 235–242. [Google Scholar] [CrossRef]
- Hasegawa, G.; Sato, T.; Kanamori, K.; Nakano, K.; Yajima, T.; Kobayashi, Y.; Kageyama, H.; Abe, T.; Nakanishi, K. Hierarchically Porous Monoliths Based on N—Doped Reduced Titanium Oxides and Their Electric and Electrochemical Properties. Chem. Mater. 2013, 25, 3504–3512. [Google Scholar] [CrossRef]
- Pang, Q.; Kundu, D.; Cuisinier, M.; Nazar, L.F. Surface-enhanced redox chemistry of polysulphides on a metallic and polar host for lithium-sulphur batteries. Nat. Commun. 2014, 5, 4759. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M. Synthesis and development of titania with controlled structures. J. Ceram. Soc. Jpn. 2016, 124, 863–869. [Google Scholar] [CrossRef]
- Wu, H.B.; Chen, J.S.; Hng, H.H.; Lou, X.W. Nanostructured metal oxide-based materials as advanced anodes for lithium-ion batteries. Nanoscale 2012, 4, 2526–2542. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, W.; Terabayashi, O.; Murakami, Y.; Takasu, Y. Electrophoretic deposition of negatively charged tetratitanate nanosheets and transformation into preferentially oriented TiO2(B) film. J. Mater. Chem. 2002, 12, 3814–3818. [Google Scholar] [CrossRef]
- Zhu, R.; Liu, Y.; Ye, J.; Zhang, X. Magnéli phase Ti4O7 powder from carbothermal reduction method: Formation, conductivity and optical properties. J. Mater. Sci. Mater. Electron. 2013, 24, 4853–4856. [Google Scholar] [CrossRef]
- Toyoda, M.; Yano, T.; Tryba, B.; Mozia, S.; Tsumura, T.; Inagaki, M. Preparation of carbon-coated Magneli phases TinO2n−1 and their photocatalytic activity under visible light. Appl. Catal. B Environ. 2009, 88, 160–164. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Ye, J. Investigation of fabrication of Ti4O7 by carbothermal reduction in argon atmosphere and vacuum. J. Mater. Sci. Mater. Electron. 2016, 27, 3683–3692. [Google Scholar] [CrossRef]
- Dewan, M.A.R.; Zhang, G.; Ostrovski, O. Carbothermal Reduction of Titania in Different Gas Atmospheres. Metall. Mater. Trans. B 2009, 40, 62–69. [Google Scholar] [CrossRef]
- Peelamedu, R.D.; Fleming, M.; Agrawal, D.K.; Roy, R. Preparation of Titanium Nitride: Microwave-Induced Carbothermal Reaction of Titanium Dioxide. J. Am. Ceram. Soc. 2002, 85, 117–122. [Google Scholar] [CrossRef]
- Fukushima, J.; Kashimura, K.; Takayama, S.; Sato, M. Microwave-energy Distribution for Reduction and Decrystallization of Titanium Oxides. Chem. Lett. 2012, 41, 39–41. [Google Scholar] [CrossRef]

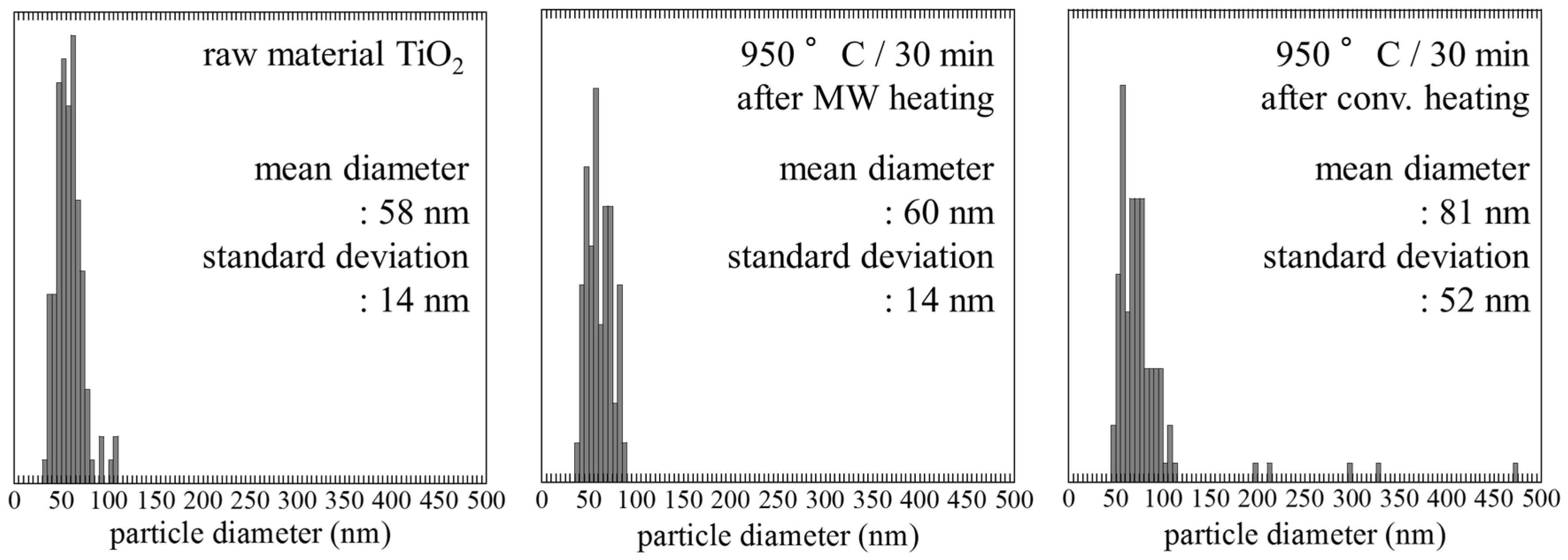
| After MW Heating | After Conv. Heating | After MW Heating | Miller Indices | Phase |
|---|---|---|---|---|
| (950 °C, 30 min) | (950 °C, 30 min) | (950 °C, 60 min) | ||
| 65(9) Å | 151(14) Å | 83(17) Å | (1 0 1) | Ti4O7 |
| 154(9) Å | 224(11) Å | 225(10) Å | (1 −2 −1) | |
| 143(17) Å | 233(21) Å | - | (2 0 6) | |
| - | - | 273(23) Å | (2 2 0) | γ-Ti3O5 |
| - | - | 330(20) Å | (1 1 −2) | |
| - | - | 297(21) Å | (3 1 0) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takeuchi, T.; Fukushima, J.; Hayashi, Y.; Takizawa, H. Synthesis of Ti4O7 Nanoparticles by Carbothermal Reduction Using Microwave Rapid Heating. Catalysts 2017, 7, 65. https://doi.org/10.3390/catal7020065
Takeuchi T, Fukushima J, Hayashi Y, Takizawa H. Synthesis of Ti4O7 Nanoparticles by Carbothermal Reduction Using Microwave Rapid Heating. Catalysts. 2017; 7(2):65. https://doi.org/10.3390/catal7020065
Chicago/Turabian StyleTakeuchi, Tomohiro, Jun Fukushima, Yamato Hayashi, and Hirotsugu Takizawa. 2017. "Synthesis of Ti4O7 Nanoparticles by Carbothermal Reduction Using Microwave Rapid Heating" Catalysts 7, no. 2: 65. https://doi.org/10.3390/catal7020065