Effect of Different Nano-Sized Silica Sols as Supports on the Structure and Properties of Cu/SiO2 for Hydrogenation of Dimethyl Oxalate
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physicochemical Properties
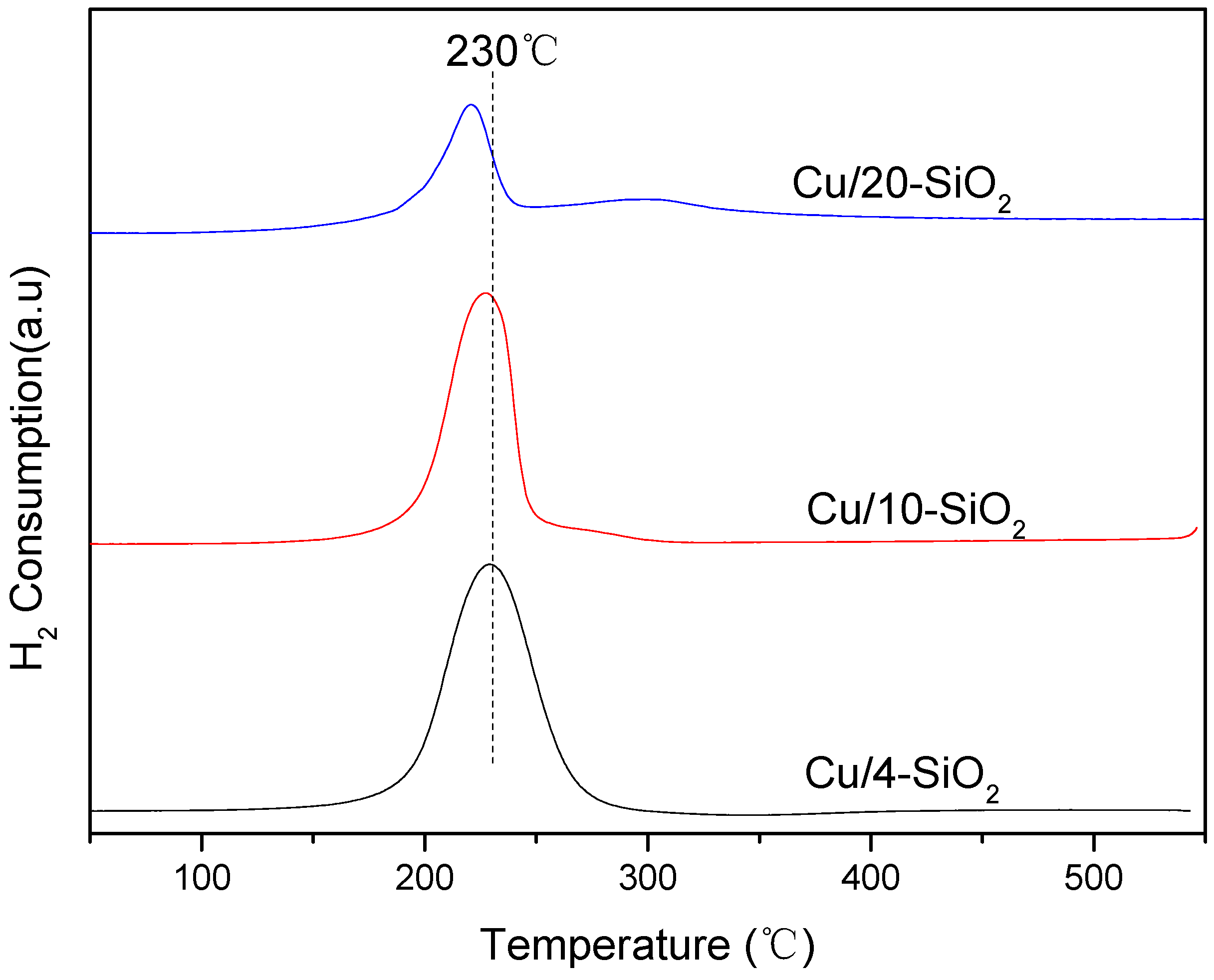
2.2. Reducibility of the Catalysts
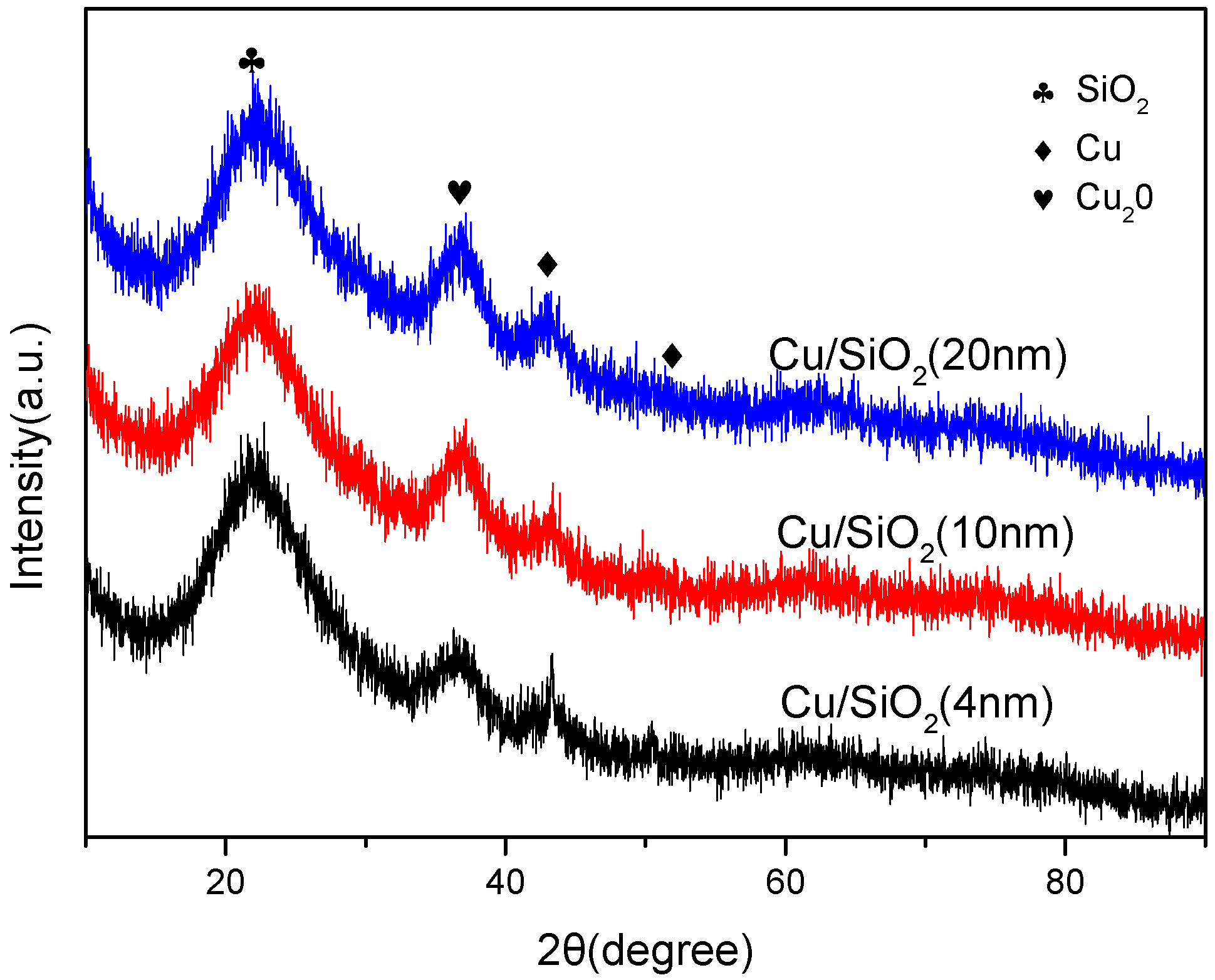
2.3. Morphology and Crystalline Phase
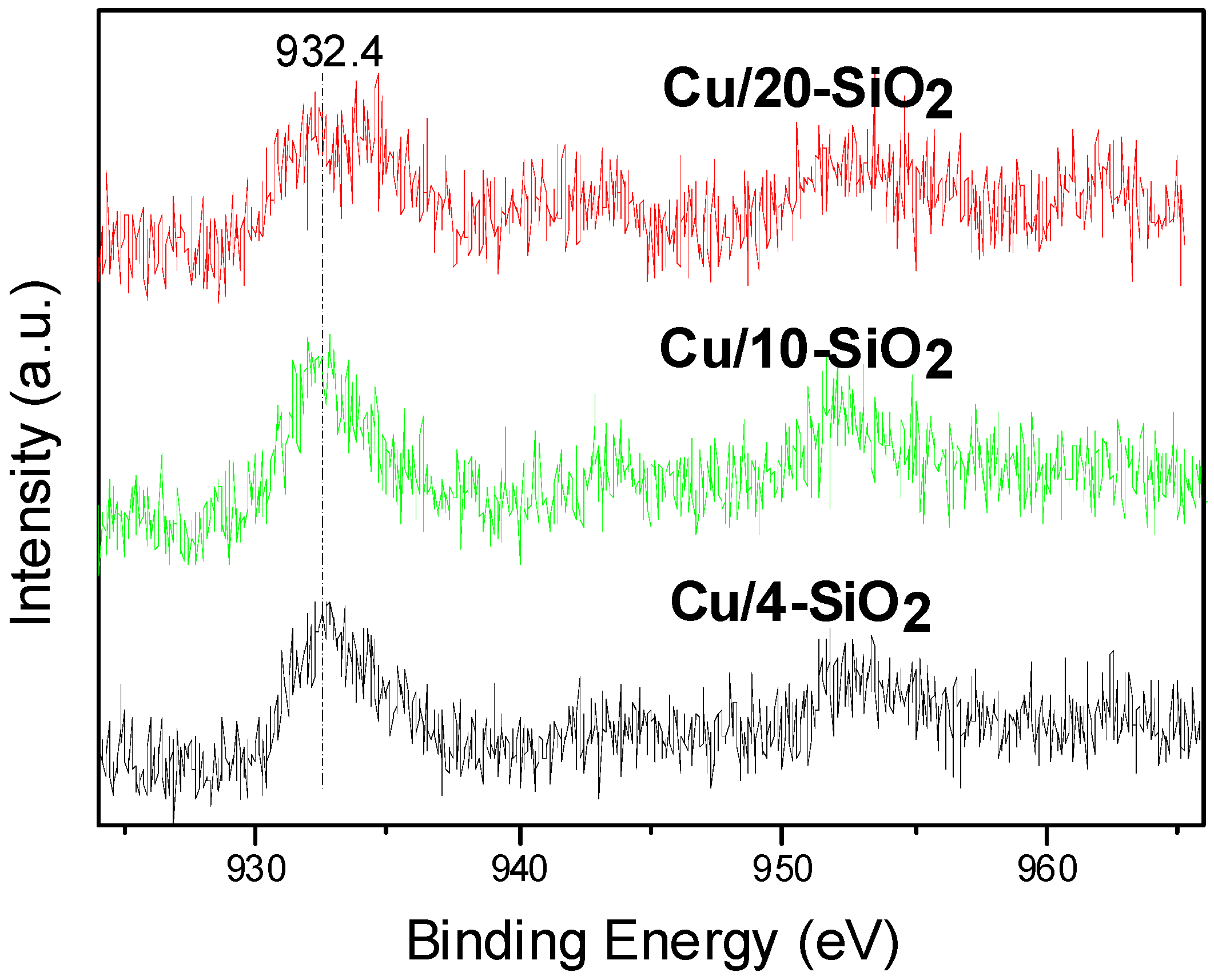
2.4. Surface Composition and Chemical States
2.5. Catalytic Activities
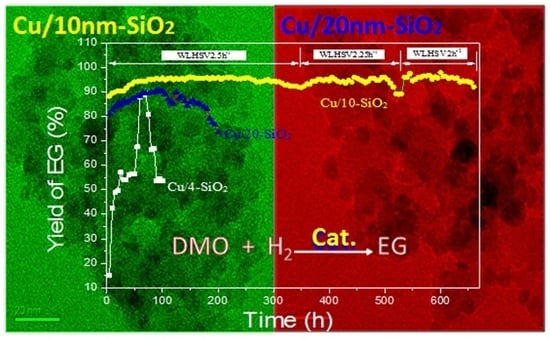
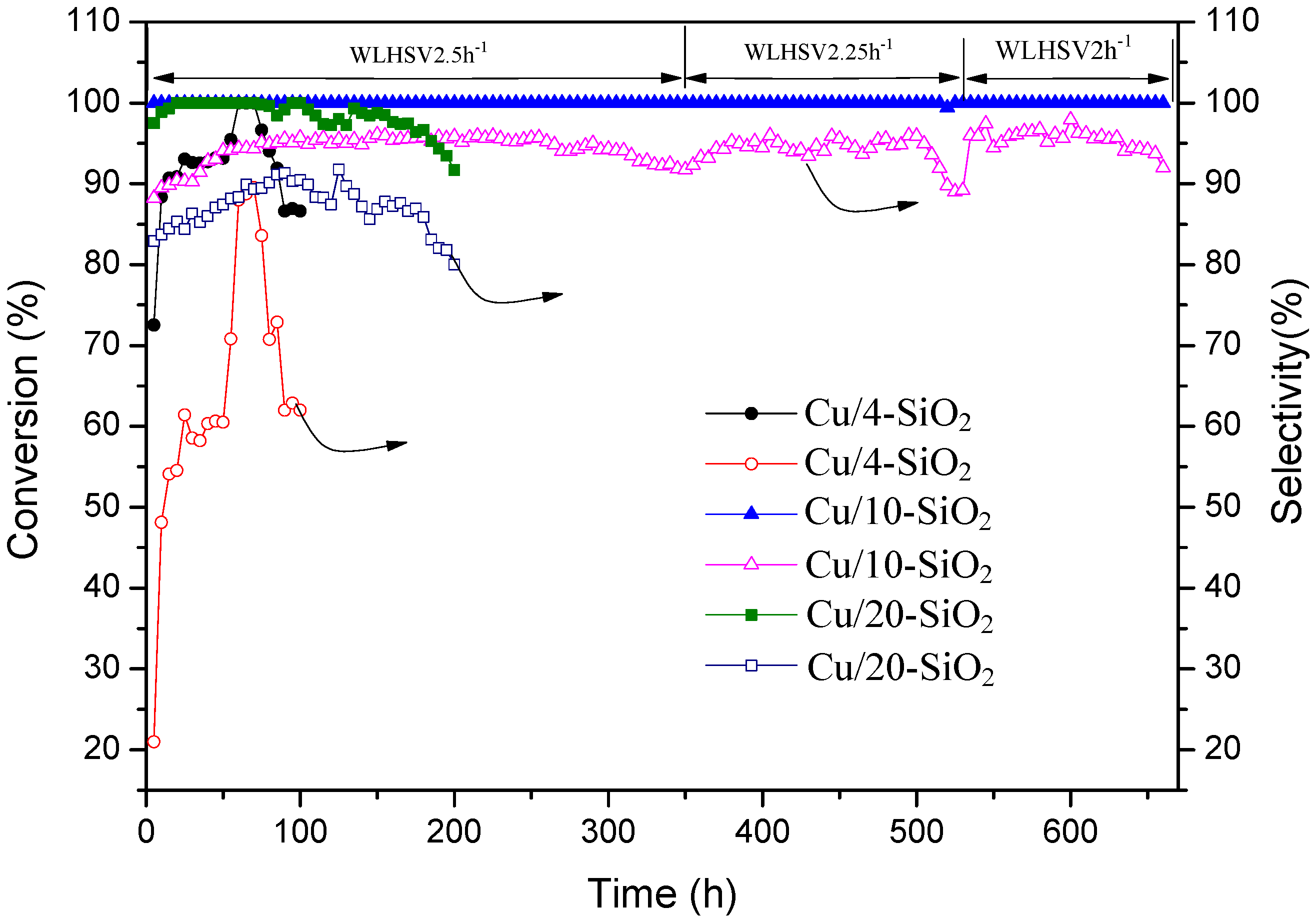
2.6. Long-Term Experiments
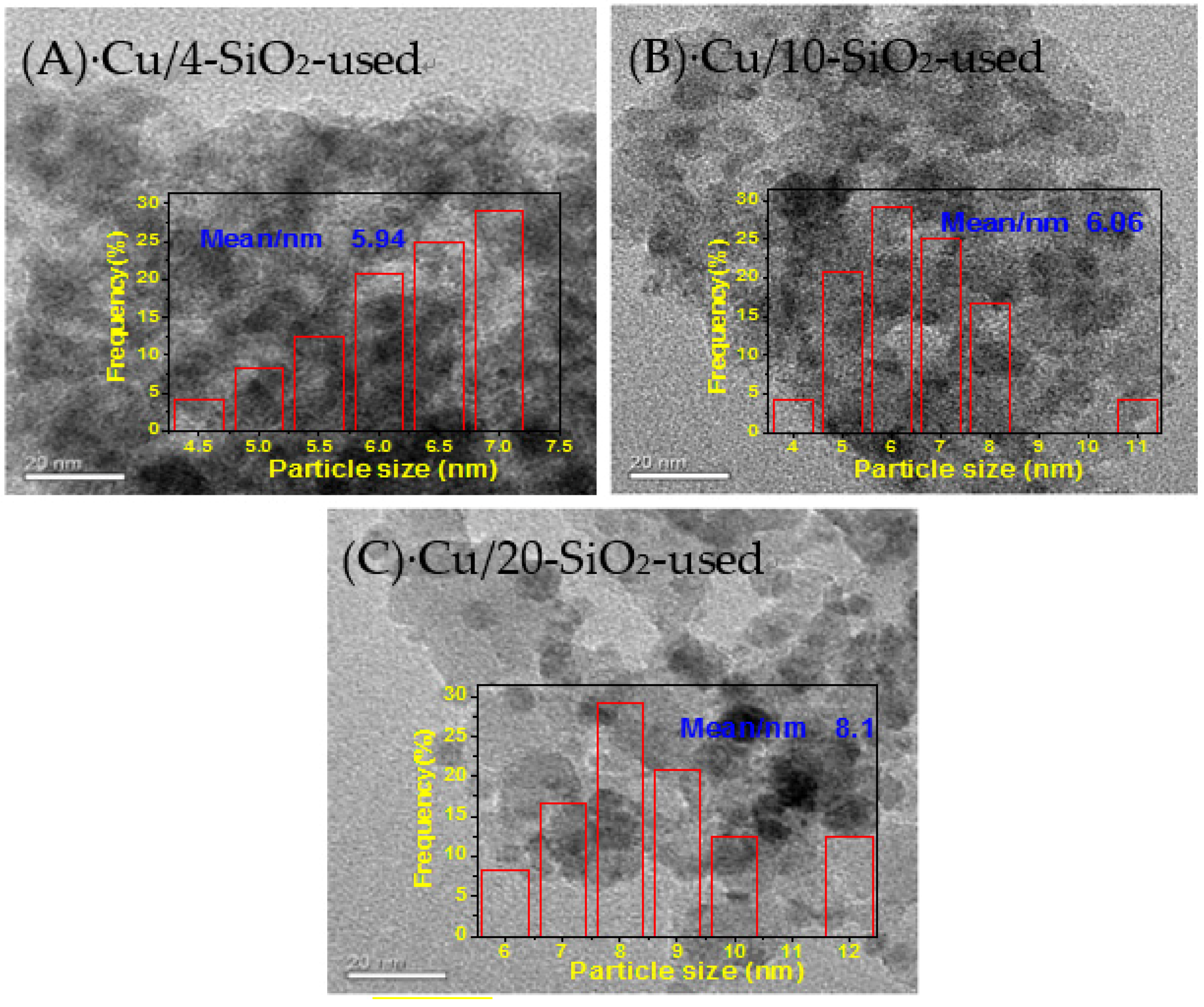
2.7. Characterization of the Used Catalysts
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Characterization
3.3. Activity Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Chakraborty, S.; Dai, H.; Bhattacharya, P.; Fairweather, N.T.; Gibson, M.S.; Krause, J.A.; Guan, H. Iron-Based Catalysts for the Hydrogenation of Esters to Alcohols. J. Am. Chem. Soc. 2014, 136, 7869–7872. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Koehler, C.; Tan, R.; Annibale, V.T.; Song, D. Ester hydrogenation catalyzed by Ru-CNN pincer complexes. Chem. Commun. 2011, 47, 8349. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.-L.; Guo, C.-L.; Zhang, J.-L. Highly active and stable CeO2-SiO2 supported Cu catalysts for the hydrogenation of methyl acetate to ethanol. Fuel Process.Technol. 2016, 143, 219–224. [Google Scholar] [CrossRef]
- Yue, H.; Ma, X.; Gong, J. An alternative synthetic approach for efficient catalytic conversion of syngas to ethanol. Accounts Chem. Res. 2014, 47, 1483–1492. [Google Scholar] [CrossRef] [PubMed]
- Zehner, L.R. Synthesis of Oxalate esters from Carbon Monoxide and Acetals or Ketals. U.S. Patent US4,005,131 A, 25 January 1977. [Google Scholar]
- Chen, L.; Guo, P.; Qiao, M.; Yan, S.; Li, H.; Shen, W.; Xu, H.; Fan, K. Cu/SiO2 catalysts prepared by the ammonia-evaporation method: Texture, structure, and catalytic performance in hydrogenation of dimethyl oxalate to ethylene glycol. J. Catal. 2008, 257, 172–180. [Google Scholar] [CrossRef]
- Yue, H.; Zhao, Y.; Zhao, S.; Wang, B.; Ma, X.; Gong, J. A copper-phyllosilicate core-sheath nanoreactor for carbon-oxygen hydrogenolysis reactions. Nat. Commun. 2013, 4, 2339. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Li, F.; Cui, Y.; Dai, W.-L.; Fan, K. Investigation of the structural evolution and catalytic performance of the CuZnAl catalysts in the hydrogenation of dimethyl oxalate to ethylene glycol. Catal. Today 2013, 10, 75. [Google Scholar] [CrossRef]
- Zhu, Y.; Kong, X.; Cao, D.-B.; Cui, J.; Zhu, Y.; Li, Y.-W. The Rise of Calcination Temperature Enhances the Performance of Cu Catalysts: Contributions of Support. ACS Catal. 2014, 4, 3675–3681. [Google Scholar] [CrossRef]
- Wen, C.; Yin, A.; Cui, Y.; Yang, X.; Dai, W.-L.; Fan, K. Enhanced catalytic performance for SiO2-TiO2 binary oxide supported Cu-based catalyst in the hydrogenation of dimethyloxalate. Appl. Catal. A Gen. 2013, 458, 82–89. [Google Scholar] [CrossRef]
- Yin, A.; Wen, C.; Dai, W.-L.; Fan, K. Ag/MCM-41 as a highly efficient mesostructured catalyst for the chemoselective synthesis of methyl glycolate and ethylene glycol. Appl. Catal. B Environ. 2011, 108–109, 90–99. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, Y.; Ding, G.; Zhu, S.; Zheng, H.; Li, Y. Highly selective synthesis of ethylene glycol and ethanol via hydrogenation of dimethyl oxalate on Cu catalysts: Influence of support. Appl. Catal. A Gen. 2013, 468, 296–304. [Google Scholar] [CrossRef]
- Yin, A.; Wen, C.; Dai, W.-L.; Fan, K. Surface modification of HMS material with silica sol leading to a remarkable enhanced catalytic performance of Cu/SiO2. Appl. Surf. Sci. 2011, 257, 5844–5849. [Google Scholar] [CrossRef]
- Ma, X.; Chi, H.; Yue, H.; Zhao, Y.; Xu, Y.; Lv, J.; Wang, S.; Gong, J. Hydrogenation of dimethyl oxalate to ethylene glycol over mesoporous Cu-MCM-41 catalysts. Aiche J. 2013, 59, 2530–2539. [Google Scholar] [CrossRef]
- Lin, H.; Zheng, X.; He, Z.; Zheng, J.; Duan, X.; Yuan, Y. Cu/SiO2 hybrid catalysts containing HZSM-5 with enhanced activity and stability for selective hydrogenation of dimethyl oxalate to ethylene glycol. Appl. Catal. A Gen. 2012, 445–446, 287–296. [Google Scholar] [CrossRef]
- Guo, X.; Yin, A.; Dai, W.-L.; Fan, K. One Pot Synthesis of Ultra-High Copper Contented Cu/SBA-15 Material as Excellent Catalyst in the Hydrogenation of Dimethyl Oxalate to Ethylene Glycol. Catal. Lett. 2009, 132, 22–27. [Google Scholar] [CrossRef]
- Chang, F.W.; Yang, H.C.; Roselin, L.S.; Kuo, W.Y. Ethanol dehydrogenation over copper catalysts on rice husk ash prepared by ion exchange. Appl. Catal. A Gen. 2006, 304, 30–39. [Google Scholar] [CrossRef]
- Marchi, A.J.; Fierro, J.L.G.; Santamaria, J.; Monzon, A. Dehydrogenation of isopropylic alcohol on a Cu/SiO2 catalyst: A study of the activity evolution and reactivation of the catalyst. Appl. Catal. A 1996, 142, 375–386. [Google Scholar] [CrossRef]
- Poels, E.K.; Brands, D.S. Modification of Cu/ZnO/SiO2 catalysts by high temperature reduction. Appl. Catal. A 2000, 191, 83. [Google Scholar] [CrossRef]
- Yin, A.; Guo, X.; Dai, W.-L.; Fan, K. Effect of initial precipitation temperature on the structural evolution and catalytic behavior of Cu/SiO2 catalyst in the hydrogenation of dimethyloxalate. Catal. Commun. 2011, 12, 412–416. [Google Scholar] [CrossRef]
- Ma, X.; Yang, Z.; Liu, X.; Tan, X.; Ge, Q. Dynamic redox cycle of Cu0 and Cu+ over Cu/SiO2 catalyst in ester hydrogenation. RSC Adv. 2015, 5, 37581. [Google Scholar] [CrossRef]
- LaGrow, A.P.; Ward, M.R.; Lloyd, D.C.; Gai, P.L.; Boyes, E.D. Visualizing the Cu/Cu2O Interface Transition in Nanoparticles with Environmental Scanning Transmission Electron Microscopy. J. Am. Chem. Soc. 2017, 139, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Yue, H.; Zhao, Y.; Zhao, S.; Zhao, L.; Lv, J.; Wang, S.; Ma, X. Synthesis of Ethanol via Syngas on Cu/SiO2Catalysts with Balanced Cu0–Cu+Sites. J. Am. Chem. Soc. 2012, 134, 13922–13925. [Google Scholar] [CrossRef] [PubMed]
- Yin, A.; Wen, C.; Guo, X.; Dai, W.-L.; Fan, K. Influence of Ni species on the structural evolution of Cu/SiO2 catalyst for the chemoselective hydrogenation of dimethyl oxalate. J. Catal. 2011, 280, 77–88. [Google Scholar] [CrossRef]
- He, Z.; Lin, H.; He, P.; Yuan, Y. Effect of boric oxide doping on the stability and activity of a Cu–SiO2 catalyst for vapor-phase hydrogenation of dimethyl oxalate to ethylene glycol. J. Catal. 2011, 277, 54–63. [Google Scholar] [CrossRef]
- Huang, Y.; Ariga, H.; Zheng, X.; Duan, X.; Takakusagi, S.; Asakura, K.; Yuan, Y. Silver-modulated SiO2-supported copper catalysts for selective hydrogenation of dimethyl oxalate to ethylene glycol. J. Catal. 2013, 307, 74–83. [Google Scholar] [CrossRef]
- Miyazaki, H.; Uda, T.; Hirai, K.; Nakamura, Y.; Ikezawa, H.; Tsuchie, T. Process for Producing Ethylene Glycol and/or Glycolic Acid Ester, Catalyst Composition Used Therefor, and Process for Production Thereof. U.S. Patent US4,585,890, 29 April 1986. [Google Scholar]
- Zhao, S.; Yue, H.; Zhao, Y.; Wang, B.; Geng, Y.; Lv, J.; Wang, S.; Gong, J.; Ma, X. Chemoselective synthesis of ethanol via hydrogenation of dimethyl oxalate on Cu/SiO2: Enhanced stability with boron dopant. J. Catal. 2013, 297, 142–150. [Google Scholar] [CrossRef]

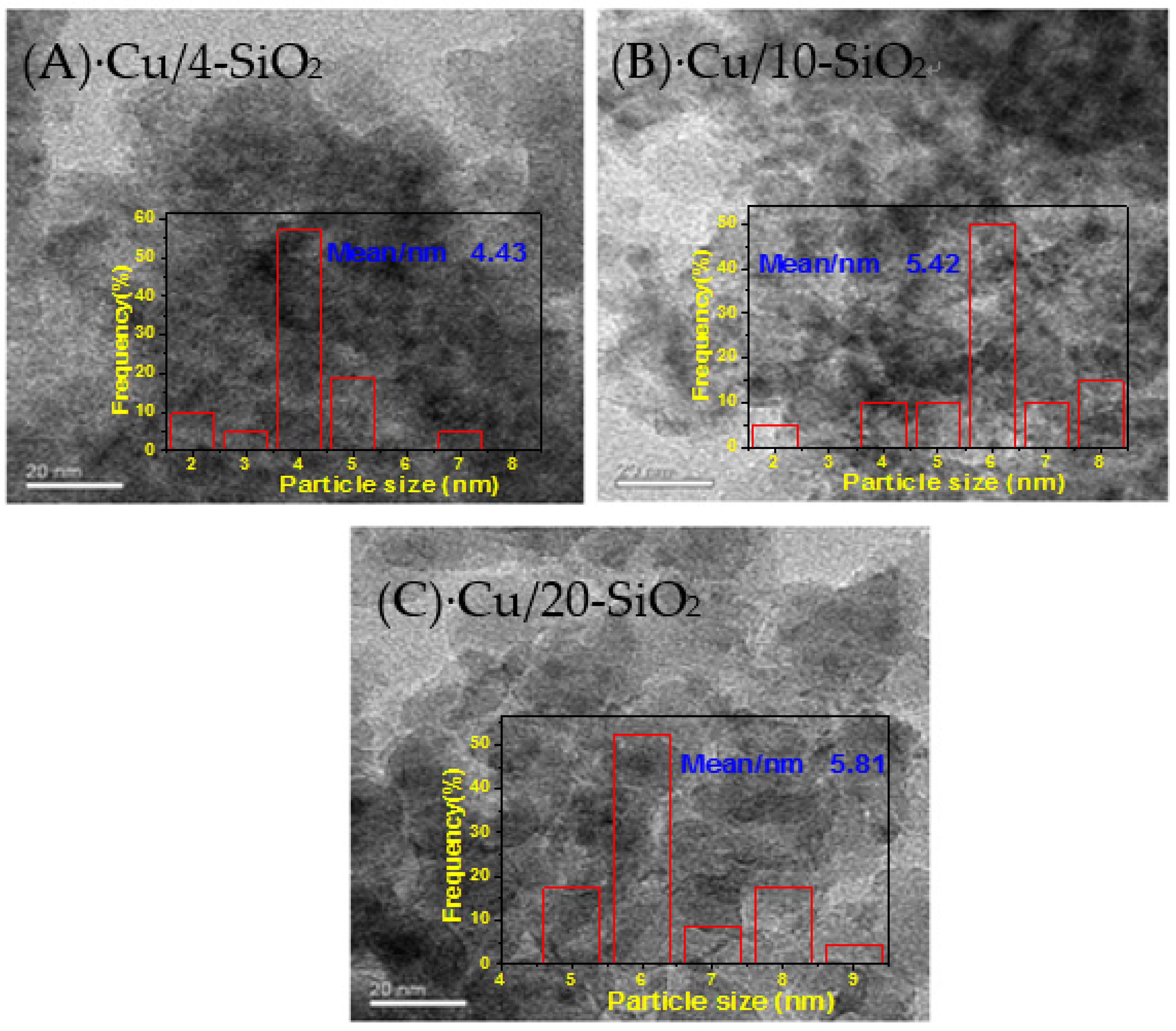

| Sample | Cu loading (wt %) a | Cu Dispersion (%) b | SCub (m2·g−1) | SBET (m2·g−1) | Vp (cm3·g−1) | Dp (nm) | dCu2O(111) (nm) c | dCu (nm) d |
|---|---|---|---|---|---|---|---|---|
| Cu/4-SiO2 | 17.5 | 25.7 | 30.5 | 472.7 | 1.05 | 4.6 | 3.3 | 4.4 |
| Cu/10-SiO2 | 16.8 | 23.9 | 27.2 | 349.4 | 0.77 | 8.8 | 3.8 | 5.4 |
| Cu/20-SiO2 | 16.1 | 20.1 | 21.9 | 244.9 | 0.59 | 9.7 | 4.5 | 5.8 |
| Cu/4-SiO2-used | 17.3 | - | - | 274.3 | 0.69 | 6.5 | - | 5.9 |
| Cu/10-SiO2-used | 16.7 | - | - | 257.9 | 0.61 | 9.7 | - | 6.1 |
| Cu/20-SiO2-used | 16.0 | - | - | 181.5 | 0.50 | 10.6 | - | 8.1 |
| Catalyst | Cu/Si (mol/mol) | BE of Cu 2p3/2 (eV) |
|---|---|---|
| Cu/4-SiO2 | 0.134 | 932.4 |
| Cu/10-SiO2 | 0.165 | 932.5 |
| Cu/20-SiO2 | 0.151 | 932.8 |
| Samples | Temp (°C) | WLHSV(DMO) (h−1) | Conversion (%) | Selectivity (%) | TOF b (h−1) | |||
|---|---|---|---|---|---|---|---|---|
| Ethanol | MG | EG | Others a | |||||
| Cu/4-SiO2 | 200 | 2.5 | 98.2 | 1.8 | 27.0 | 70.2 | 1.0 | - |
| 195 | 6.0 | 9.8 | 0 | 94.5 | 5.5 | 0 | 7.0 | |
| Cu/10-SiO2 | 200 | 2.5 | 100 | 1.3 | 0 | 94.7 | 4.0 | - |
| 195 | 6.0 | 38.1 | 0 | 80.4 | 19.6 | 0 | 30.6 | |
| Cu/20-SiO2 | 200 | 2.5 | 100 | 1.9 | 4.4 | 90.7 | 3.0 | - |
| 195 | 6.0 | 23.0 | 0 | 81.6 | 18.4 | 0 | 22.9 | |
| Catalyst | Cu Loading (wt %) | Reaction Conditions | Catalytic Activity | Life Time (h) | Ref. |
|---|---|---|---|---|---|
| Cu/SiO2-3 HZ-38 | 27.6 | p = 3 MPa, T = 190 °C, H2/DMO = 80, WLHSV(DMO) = 1.5 h−1. | Conversion 99.8%, Selectivity 94.4% | 300 | [11] |
| Cu3Ni/HMS | 20 | 2.5 MPa H2, 200 °C, H2/DMO ratio 100, LHSV = 1.0 h−1 | Conversion100%, Selectivity98% | 150 | [24] |
| 1B-Cu-SiO2 | 30 | T = 190 °C, p(H2) = 3.0 MPa, H2/DMO = 80. WLHSV(DMO) = 0.75·h−1. | conversion > 99%, Selectivity93% | 300 | [25] |
| Cu1-Ag0.05/SiO2 | 10 | T = 190 °C, p(H2) = 3.0 MPa, H2/DMO = 80, WLHSV(DMO) = 0.6 h−1. | conversion 99%, Selectivity 97% | 150 | [26] |
| CuSiZr1-850 | ~35 | T = 190 °C, 3 MPa, H2/DMO = 150, WLHSV(DMO) = 0.3 h−1. | EG yield of > 96% | 600 | [6] |
| Cu/10-SiO2 | 16.8 | T = 200 °C, 2.5 MPa, H2/DMO = 120, WLHSV(DMO) = 2~2.5 h−1. | conversion > 99%, Selectivity94% | 660 | This work |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Wang, D.; Zhu, M.; Yu, F.; Dai, B. Effect of Different Nano-Sized Silica Sols as Supports on the Structure and Properties of Cu/SiO2 for Hydrogenation of Dimethyl Oxalate. Catalysts 2017, 7, 75. https://doi.org/10.3390/catal7030075
Zhang C, Wang D, Zhu M, Yu F, Dai B. Effect of Different Nano-Sized Silica Sols as Supports on the Structure and Properties of Cu/SiO2 for Hydrogenation of Dimethyl Oxalate. Catalysts. 2017; 7(3):75. https://doi.org/10.3390/catal7030075
Chicago/Turabian StyleZhang, Chuancai, Denghao Wang, Mingyuan Zhu, Feng Yu, and Bin Dai. 2017. "Effect of Different Nano-Sized Silica Sols as Supports on the Structure and Properties of Cu/SiO2 for Hydrogenation of Dimethyl Oxalate" Catalysts 7, no. 3: 75. https://doi.org/10.3390/catal7030075