Efficient Hydrolysis of Lignocellulose by Acidic Ionic Liquids under Low-Toxic Condition to Microorganisms
Abstract
:1. Introduction
2. Results and Discussion
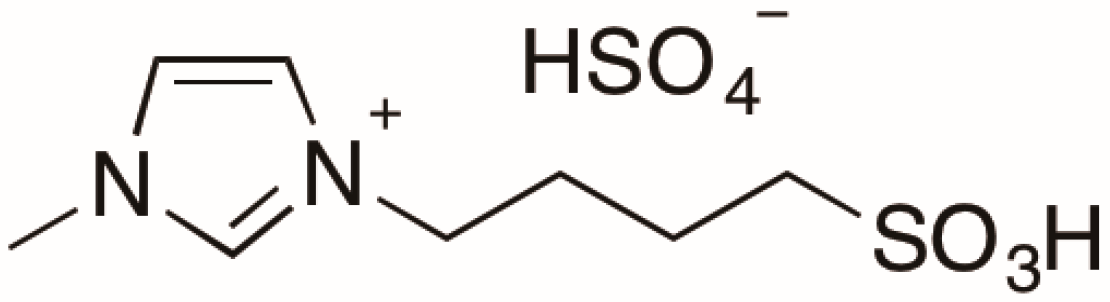
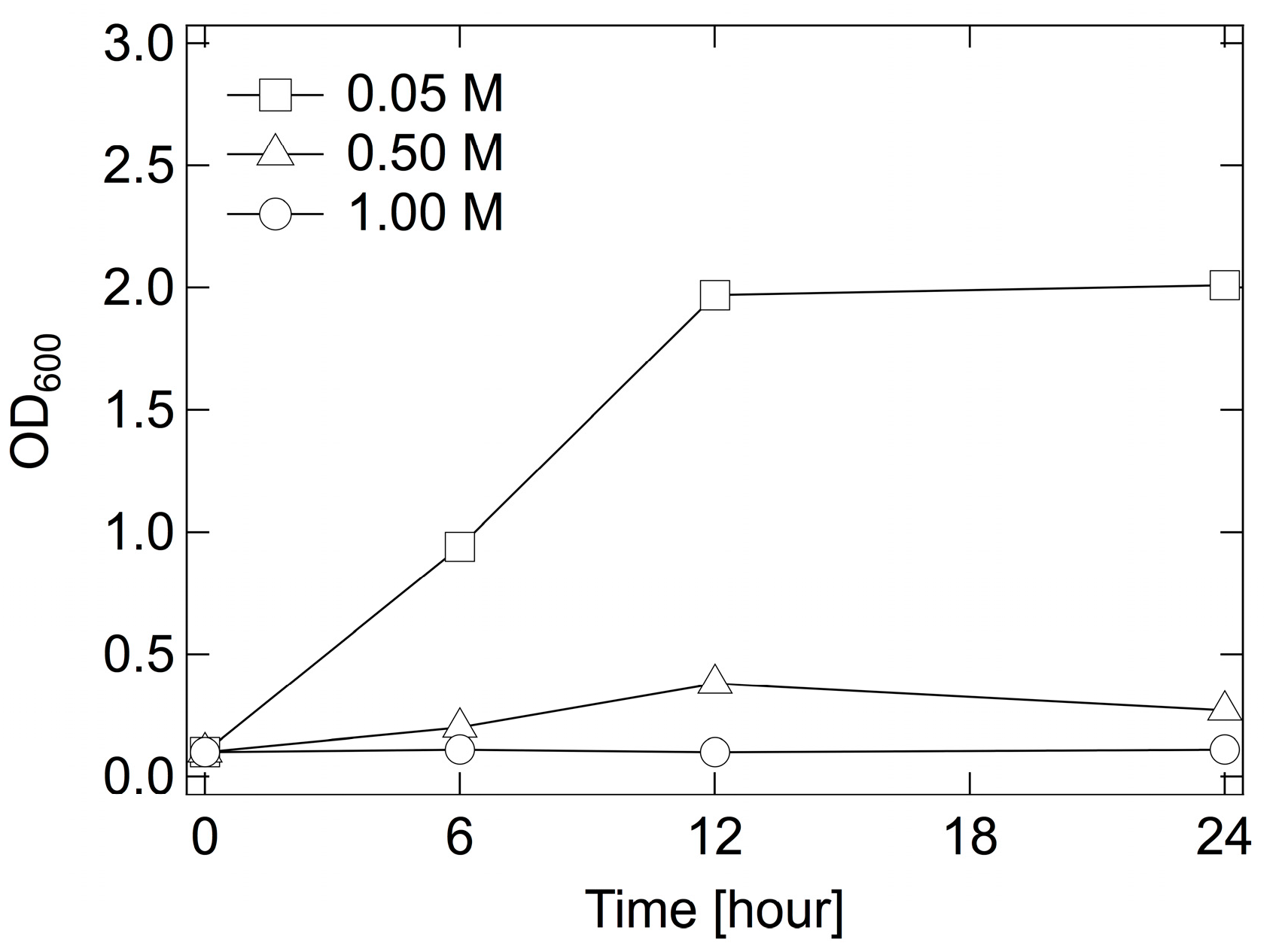
2.1. Effect of [Sbmim][HSO4] on E. coli Growth
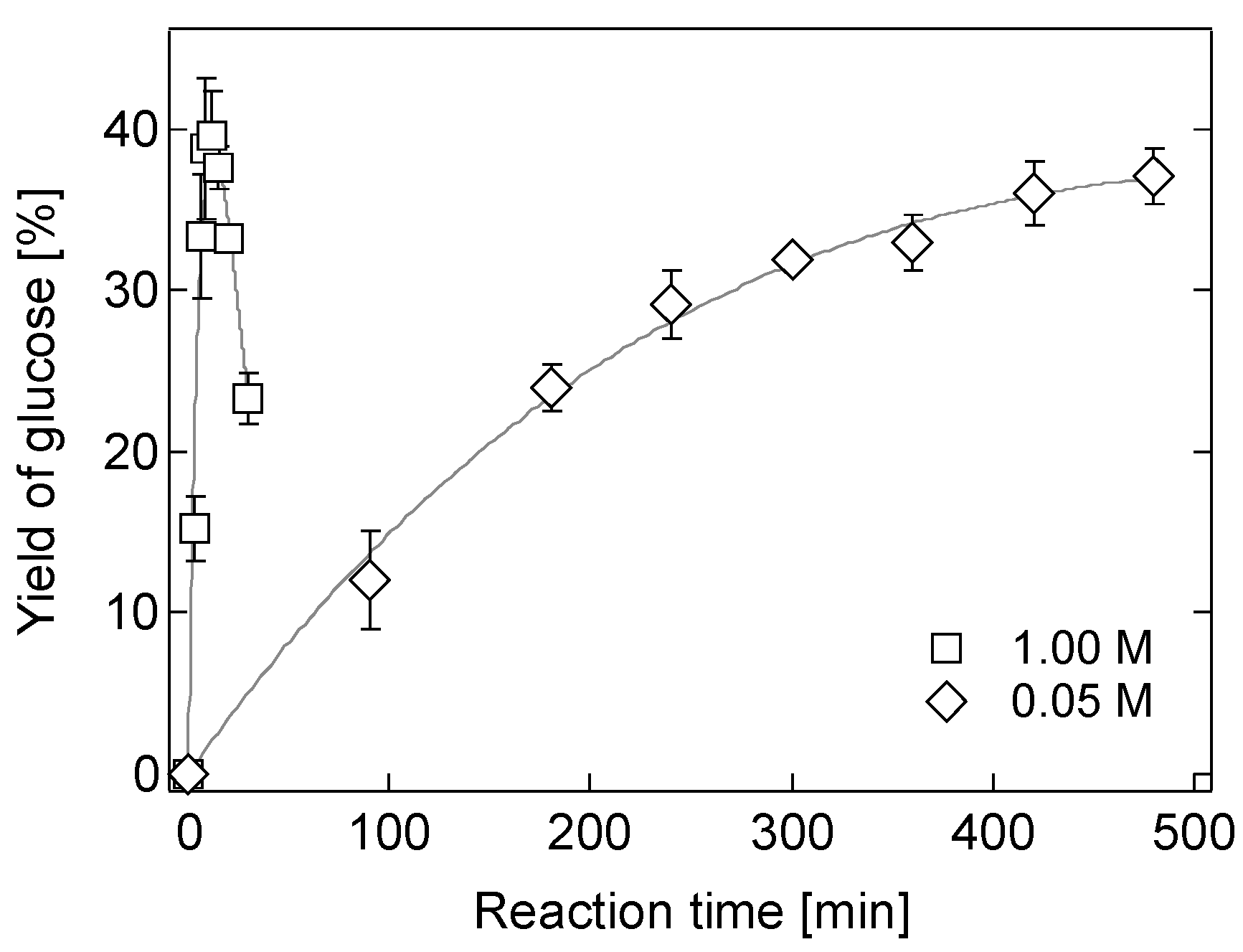
2.2. Hydrolysis of Bagasse in a 0.05 M [Sbmim][HSO4] Solution
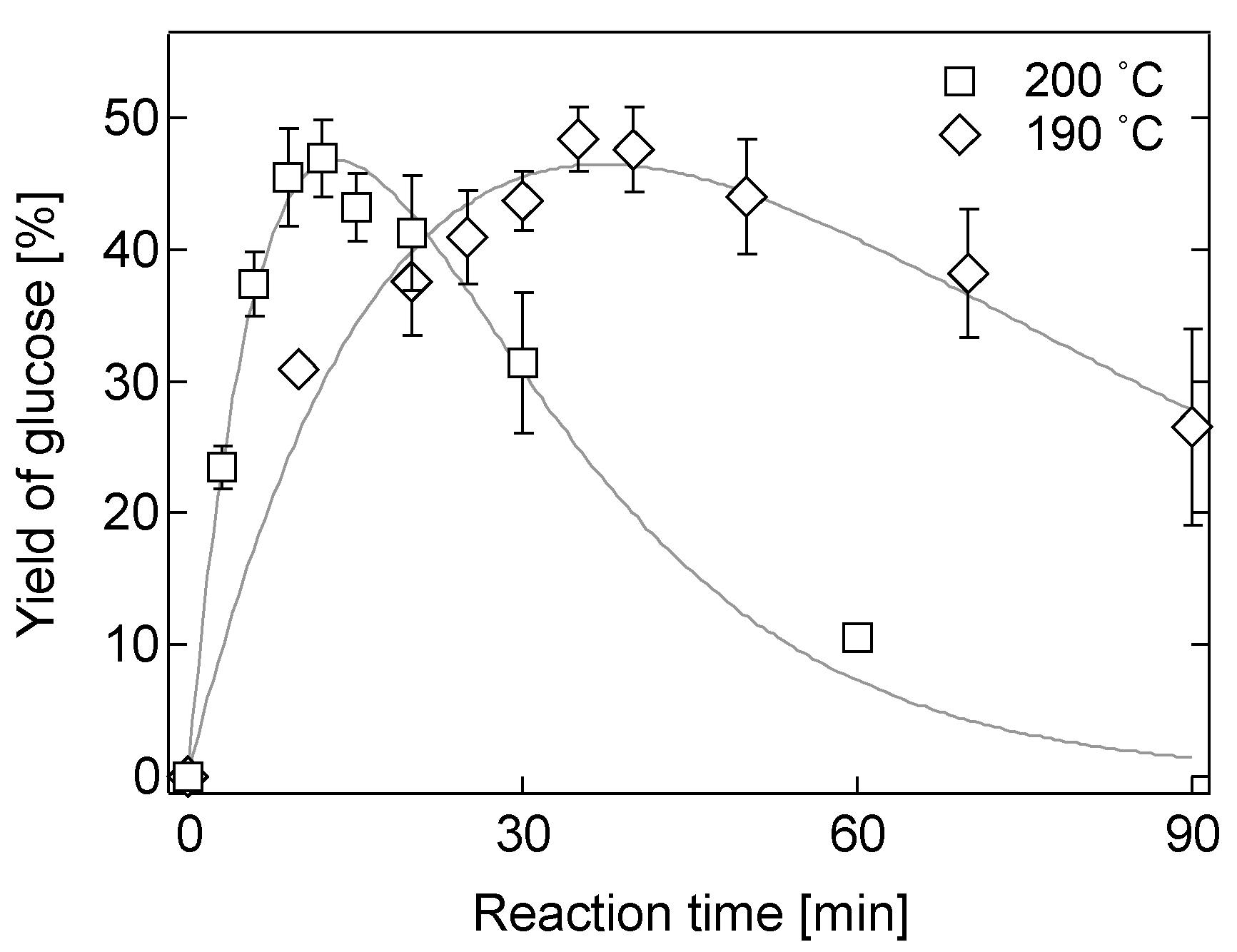
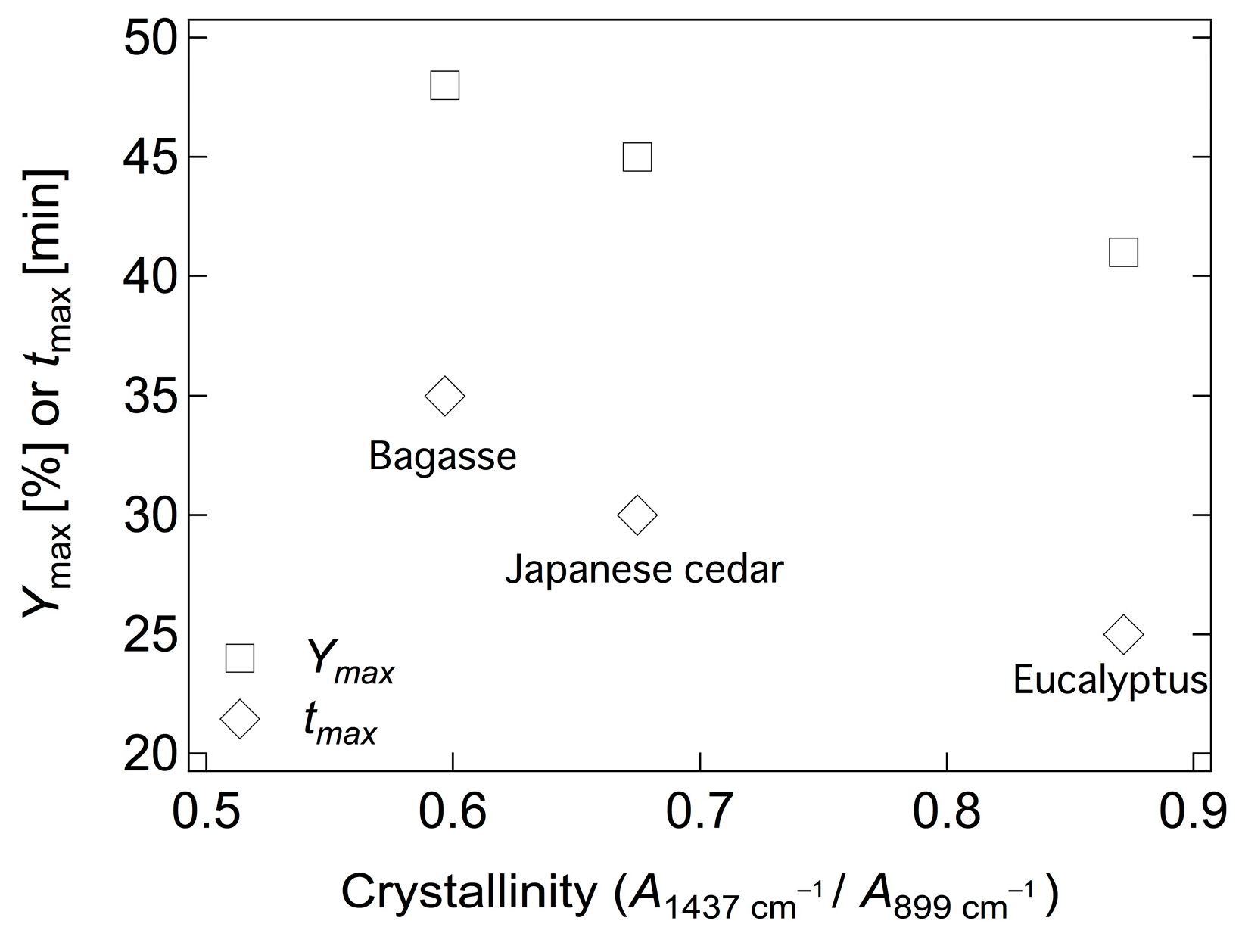
2.3. Hydrolysis of Various Biomass
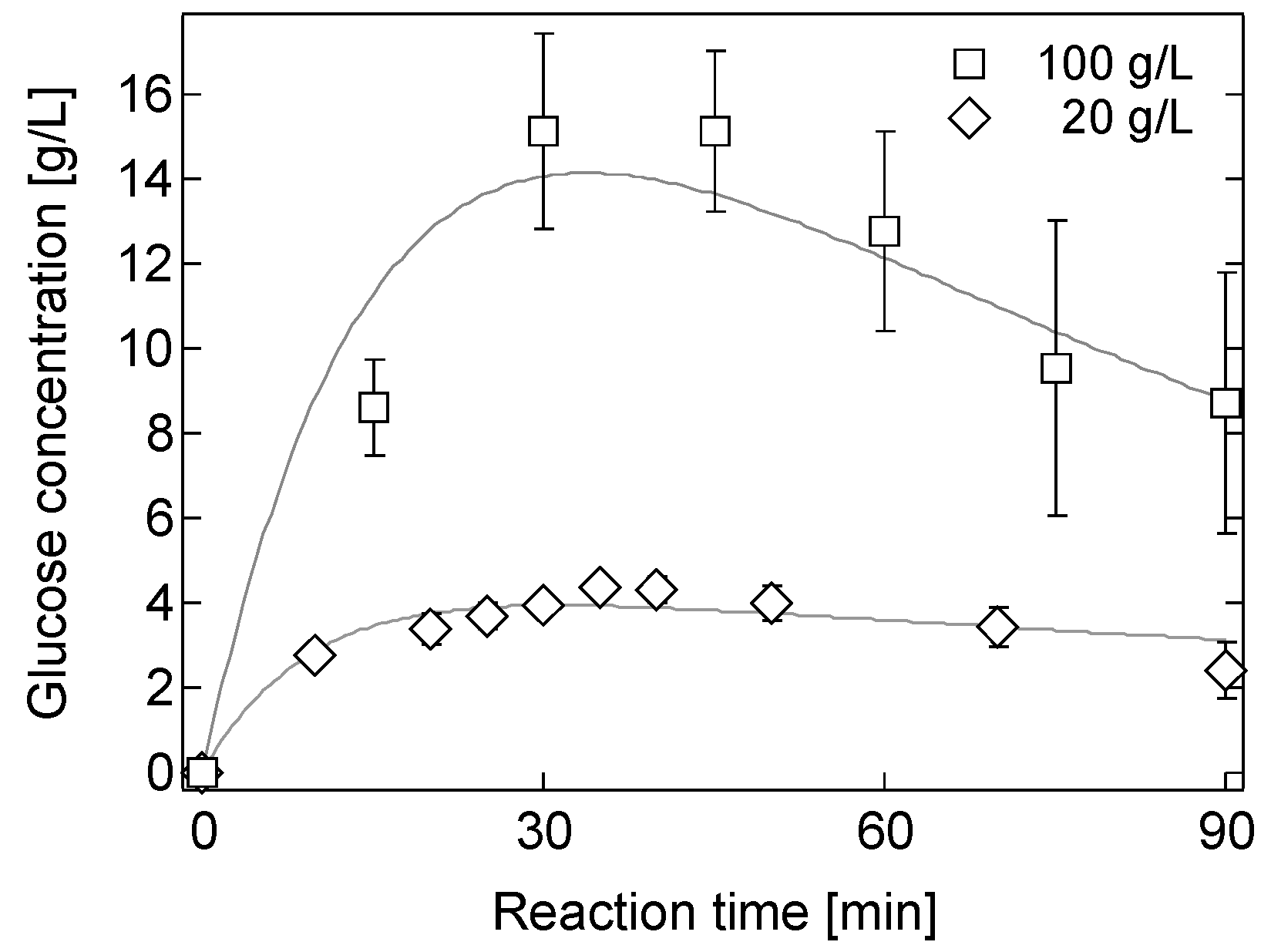
2.4. Effect of Biomass Loading on Hydrolysis
3. Materials and Methods
3.1. Materials
3.2. The Effect of [Sbmim][HSO4] on E. coli Growth
3.3. Microwave-Assisted Hydrolysis of Biomass in an Aqueous [Sbmim][HSO4] Solution
3.4. Analysis of Glucose Yield
3.5. Determination of Reaction Rate Constants
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Song, J.; Fan, H.; Ma, J.; Han, B. Conversion of Glucose and Cellulose into Value-Added Products in Water and Ionic Liquids. Green Chem. 2013, 15, 2619–2635. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G. Top Value Added Chemicals From Biomass Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas; U.S. Department of Energy: Oak Ridge, TN, USA, 2004.
- Amarasekara, A.S.; Wiredu, B. Aryl Sulfonic Acid Catalyzed Hydrolysis of Cellulose in Water. Appl. Catal. A Gen. 2012, 417–418, 259–262. [Google Scholar] [CrossRef]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of Cellose with Ionic Liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef] [PubMed]
- Fukaya, Y.; Hayashi, K.; Wada, M.; Ohno, H. Cellulose Dissolution with Polar Ionic Liquids under Mild Conditions: Required Factors for Anions. Green Chem. 2008, 10, 44–46. [Google Scholar] [CrossRef]
- Fukaya, Y.; Sugimoto, A.; Ohno, H. Superior Solubility of Polysaccharides in Low Viscosity, Polar, and Halogen-Free 1,3-Dialkylimidazolium Formates. Biomacromolecules 2006, 7, 3295–3297. [Google Scholar] [CrossRef] [PubMed]
- Brandt, A.; Gräsvik, J.; Hallett, J.P.; Welton, T. Deconstruction of Lignocellulosic Biomass with Ionic Liquids. Green Chem. 2013, 15, 550–583. [Google Scholar] [CrossRef]
- Armand, M.; Endres, F.; MacFarlane, D.R.; Ohno, H.; Scrosati, B. Ionic-Liquid Materials for the Electrochemical Challenges of the Future. Nat. Mater. 2009, 8, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Kilpeläinen, I.; Xie, H.; King, A.; Granstrom, M.; Heikkinen, S.; Argyropoulos, D.S. Dissolution of Wood in Ionic Liquids. J. Agric. Food Chem. 2007, 55, 9142–9148. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, K.; Kamide, K.; Takahashi, K.; Shimizu, N. Enhanced Enzymatic Saccharification of Kenaf Powder after Ultrasonic Pretreatment in Ionic Liquids at Room Temperature. Bioresour. Technol. 2012, 103, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, K.; Ohta, A.; Omote, S.; Ogino, C.; Takahashi, K.; Shimizu, N. Combined Use of Completely Bio-Derived Cholinium Ionic Liquids and Ultrasound Irradiation for the Pretreatment of Lignocellulosic Material to Enhance Enzymatic Saccharification. Chem. Eng. J. 2013, 215, 811–818. [Google Scholar] [CrossRef]
- Ninomiya, K.; Kohori, A.; Tatsumi, M.; Osawa, K.; Endo, T.; Kakuchi, R.; Ogino, C.; Shimizu, N.; Takahashi, K. Ionic Liquid/Ultrasound Pretreatment and in Situ Enzymatic Saccharification of Bagasse Using Biocompatible Cholinium Ionic Liquid. Bioresour. Technol. 2015, 176, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Dadi, A.P.; Varanasi, S.; Schall, C.A. Enhancement of Cellulose Saccharification Kinetics Using an Ionic Liquid Pretreatment Step. Biotechnol. Bioeng. 2006, 95, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, N.; Matsushita, Y.; Hanaki, M.; Nakashima, K.; Narita, M.; Goto, M.; Takahashi, H. Enzymatic in Situ Saccharification of Cellulose in Aqueous-Ionic Liquid Media. Biotechnol. Lett. 2008, 30, 1037–1040. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Balamurugan, K.; Parthasarathi, R.; Sathitsuksanoh, N.; Zhang, S.; Stavila, V.; Subramanian, V.; Simmons, B.A.; Singh, S. Understanding the Role of Water During Ionic Liquid Pretreatment of Lignocellulose: Co-Solvent or Anti-Solvent? Green Chem. 2014, 16, 3830–3840. [Google Scholar] [CrossRef]
- Cole, A.C.; Jensen, J.L.; Ntai, I.; Tran, K.L.T.; Weaver, K.J.; Forbes, D.C.; Davis, J.H. Novel Brønsted Acidic Ionic Liquids and Their Use as Dual Solvent−Catalysts. J. Am. Chem. Soc. 2002, 124, 5962–5963. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Jiang, T.; Gao, H.; Han, B.; Huang, J.; Sun, D. Mannich Reaction Using Acidic Ionic Liquids as Catalysts and Solventselectronic. Green Chem. 2004, 6, 75–77. [Google Scholar] [CrossRef]
- Joseph, T.; Sahoo, S.; Halligudi, S.B. Brönsted Acidic Ionic Liquids: A Green, Efficient and Reusable Catalyst System and Reaction Medium for Fischer Esterification. J. Mol. Catal. A Chem. 2005, 234, 107–110. [Google Scholar] [CrossRef]
- Han, X.-X.; Du, H.; Hung, C.-T.; Liu, L.-L.; Wu, P.-H.; Ren, D.-H.; Huang, S.-J.; Liu, S.-B. Syntheses of Novel Halogen-Free Brønsted–Lewis Acidic Ionic Liquid Catalysts and Their Applications for Synthesis of Methyl Caprylate. Green Chem. 2015, 17, 499–508. [Google Scholar] [CrossRef]
- Kuroda, K.; Miyamura, K.; Satria, H.; Takada, K.; Ninomiya, K.; Takahashi, K. Hydrolysis of Cellulose Using an Acidic and Hydrophobic Ionic Liquid and Subsequent Separation of Glucose Aqueous Solution from the Ionic Liquid and 5-(Hydroxymethyl)Furfural. ACS Sustain. Chem. Eng. 2016, 4, 3352–3356. [Google Scholar] [CrossRef]
- Amarasekara, A.S.; Wiredu, B. Degradation of Cellulose in Dilute Aqueous Solutions of Acidic Ionic Liquid 1-(1-Propylsulfonic)-3-Methylimidazolium Chloride, and P-Toluenesulfonic Acid at Moderate Temperatures and Pressures. Ind. Eng. Chem. Res. 2011, 50, 12276–12280. [Google Scholar] [CrossRef]
- Kuroda, K.; Inoue, K.; Miyamura, K.; Takada, K.; Ninomiya, K.; Takahashi, K. Enhanced Hydrolysis of Lignocellulosic Biomass Assisted by a Combination of Acidic Ionic Liquids and Microwave Heating. J. Chem. Eng. Jpn. 2016, 49, 809–813. [Google Scholar] [CrossRef]
- Martínez-Palou, R. Ionic Liquid and Microwave-Assisted Organic Synthesis: A “Green” and Synergic Couple. J. Mex. Chem. Soc. 2007, 51, 252–264. [Google Scholar]
- Hoffmann, J.; Nüchter, M.; Ondruschka, B.; Wasserscheid, P. Ionic Liquids and Their Heating Behaviour during Microwave Irradiation—A State of the Art Report and Challenge to Assessment. Green Chem. 2003, 5, 296–299. [Google Scholar] [CrossRef]
- Guerrero-Sanchez, C.; Hoogenboom, R.; Schubert, U.S. Fast and “Green” Living Cationic Ring Opening Polymerization of 2-Ethyl-2-Oxazoline in Ionic Liquids under Microwave Irradiation. Chem. Commun. 2006, 36, 3797–3799. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Liao, Y.; Zhang, Z. Toxicity of Ionic Liquids. CLEAN Soil Air Water 2007, 35, 42–48. [Google Scholar] [CrossRef]
- Lim, G.S.; Zidar, J.; Cheong, D.W.; Jaenicke, S.; Klähn, M. Impact of Ionic Liquids in Aqueous Solution on Bacterial Plasma Membranes Studied with Molecular Dynamics Simulations. J. Phys. Chem. B 2014, 118, 10444–10459. [Google Scholar] [CrossRef] [PubMed]
- Couling, D.J.; Bernot, R.J.; Docherty, K.M.; Dixon, J.K.; Maginn, E.J. Assessing the Factors Responsible for Ionic Liquid Toxicity to Aquatic Organisms via Quantitative Structure-Property Relationship Modeling. Green Chem. 2006, 8, 82–90. [Google Scholar] [CrossRef]
- Pernak, J.; Sobaszkiewicz, K.; Mirska, I. Anti-Microbial Activities of Ionic Liquids. Green Chem. 2003, 5, 52–56. [Google Scholar] [CrossRef]
- Saeman, J.F. Kinetics of Wood Saccharification—Hydrolysis of Cellulose and Decomposition of Sugars in Dilute Acid at High Temperature. Ind. Eng. Chem. 1945, 37, 43–52. [Google Scholar] [CrossRef]
- Bhandari, N.; Macdonald, D.G.; Bakhshi, N.N. Kinetic Studies of Corn Stover Saccharification Using Sulphuric Acid. Biotechnol. Bioeng. 1984, 26, 320–327. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, R.T.; DuPré, E.F.; Mitcham, D. Applications of Infrared Absorption Spectroscopy to Investigations of Cotton and Modified Cottons: Part I: Physical and Crystalline Modifications and Oxidation. Text. Res. J. 1958, 28, 382–392. [Google Scholar] [CrossRef]
- Suliter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; Technical Report; National Renewable Energy Laboratory: Golden, CO, USA, 2008.

| Entry | Temperature (°C) | Concentration (M) | k1 (min−1) | k2 (min−1) | k1/k2 | Yield (%) |
|---|---|---|---|---|---|---|
| 1 | 160 | 0.05 | 1.8 × 10−3 | 0.17 × 10−2 | 1.0 | 37 |
| 2 | 180 | 0.05 | 2.3 × 10−2 | 1.7 × 10−2 | 1.4 | 43 |
| 3 | 190 | 0.05 | 3.4 × 10−2 | 2.1 × 10−2 | 1.7 | 48 |
| 4 | 200 | 0.05 | 9.6 × 10−2 | 5.7 × 10−2 | 1.7 | 47 |
| 5 | 160 | 1.00 | 8.4 × 10−2 | 7.5 × 10−2 | 1.1 | 40 |
| 6 | 190 | 0.10 | 9.8 × 10−2 | 5.9 × 10−2 | 1.7 | 50 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuroda, K.; Inoue, K.; Miyamura, K.; Satria, H.; Takada, K.; Ninomiya, K.; Takahashi, K. Efficient Hydrolysis of Lignocellulose by Acidic Ionic Liquids under Low-Toxic Condition to Microorganisms. Catalysts 2017, 7, 108. https://doi.org/10.3390/catal7040108
Kuroda K, Inoue K, Miyamura K, Satria H, Takada K, Ninomiya K, Takahashi K. Efficient Hydrolysis of Lignocellulose by Acidic Ionic Liquids under Low-Toxic Condition to Microorganisms. Catalysts. 2017; 7(4):108. https://doi.org/10.3390/catal7040108
Chicago/Turabian StyleKuroda, Kosuke, Ken Inoue, Kyohei Miyamura, Heri Satria, Kenji Takada, Kazuaki Ninomiya, and Kenji Takahashi. 2017. "Efficient Hydrolysis of Lignocellulose by Acidic Ionic Liquids under Low-Toxic Condition to Microorganisms" Catalysts 7, no. 4: 108. https://doi.org/10.3390/catal7040108







