Sol-Gel-Assisted Microwave-Derived Synthesis of Anatase Ag/TiO2/GO Nanohybrids toward Efficient Visible Light Phenol Degradation
Abstract
:1. Introduction
2. Results and Discussion
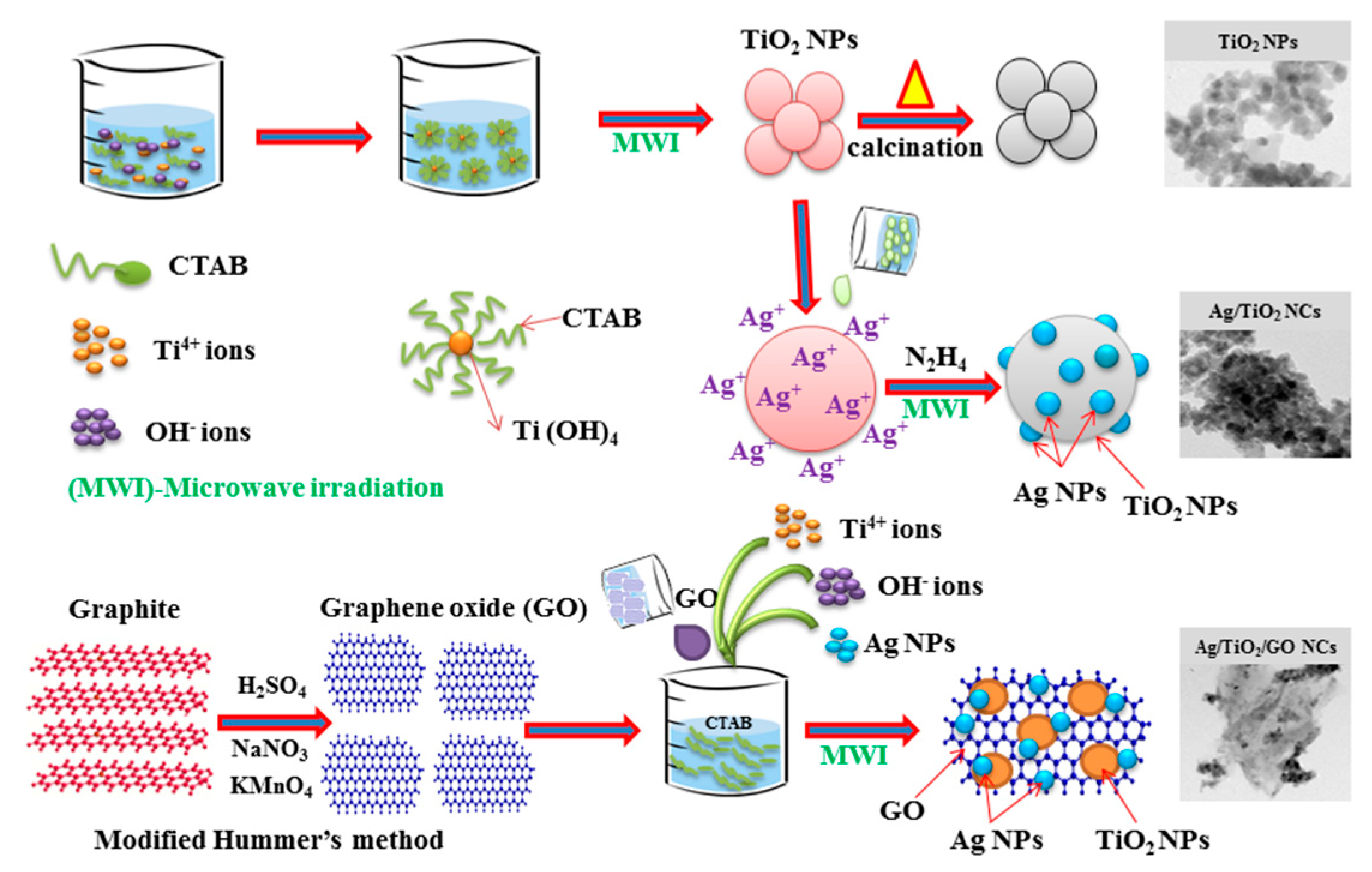
2.1. Nucleation Mechanism
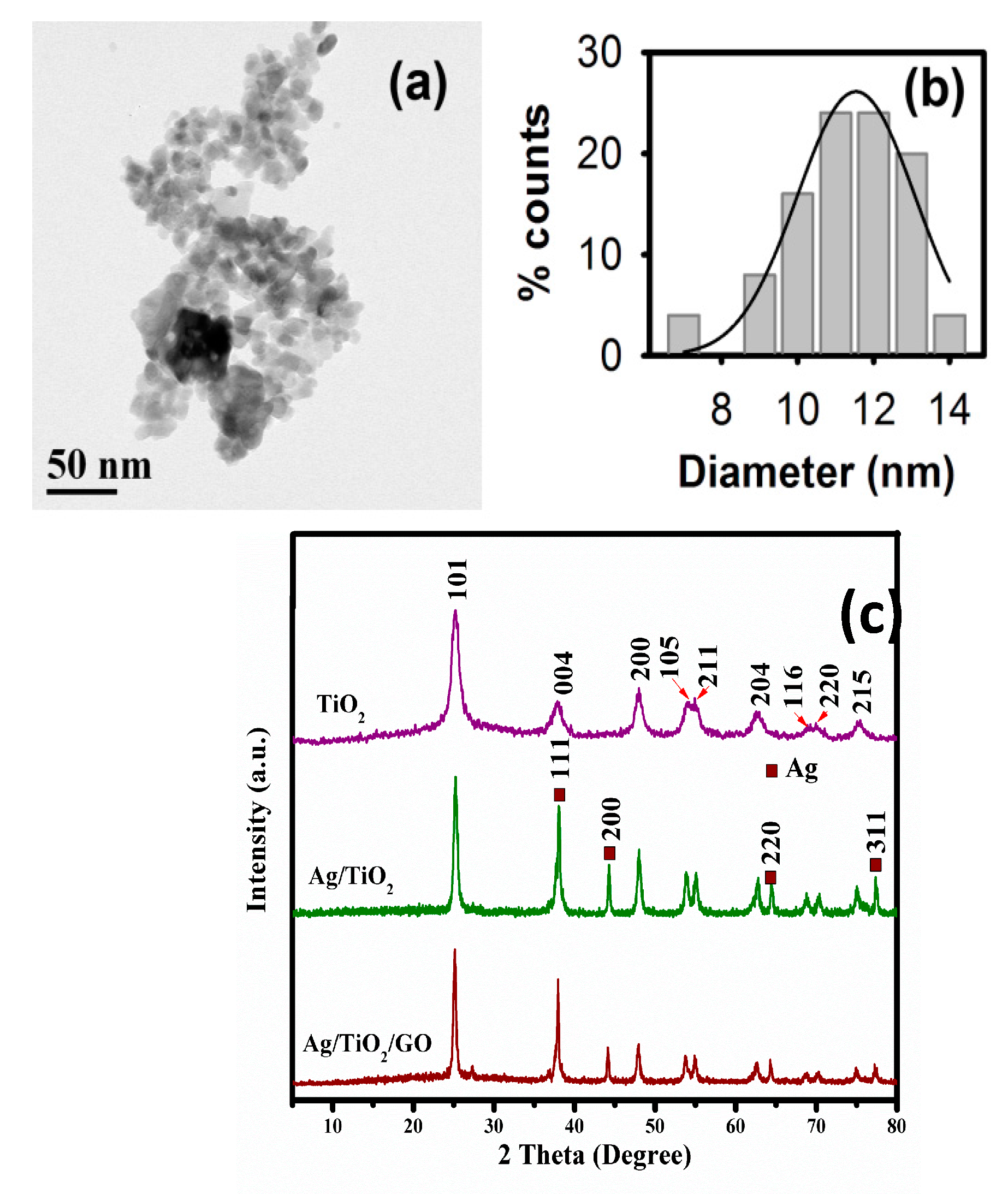
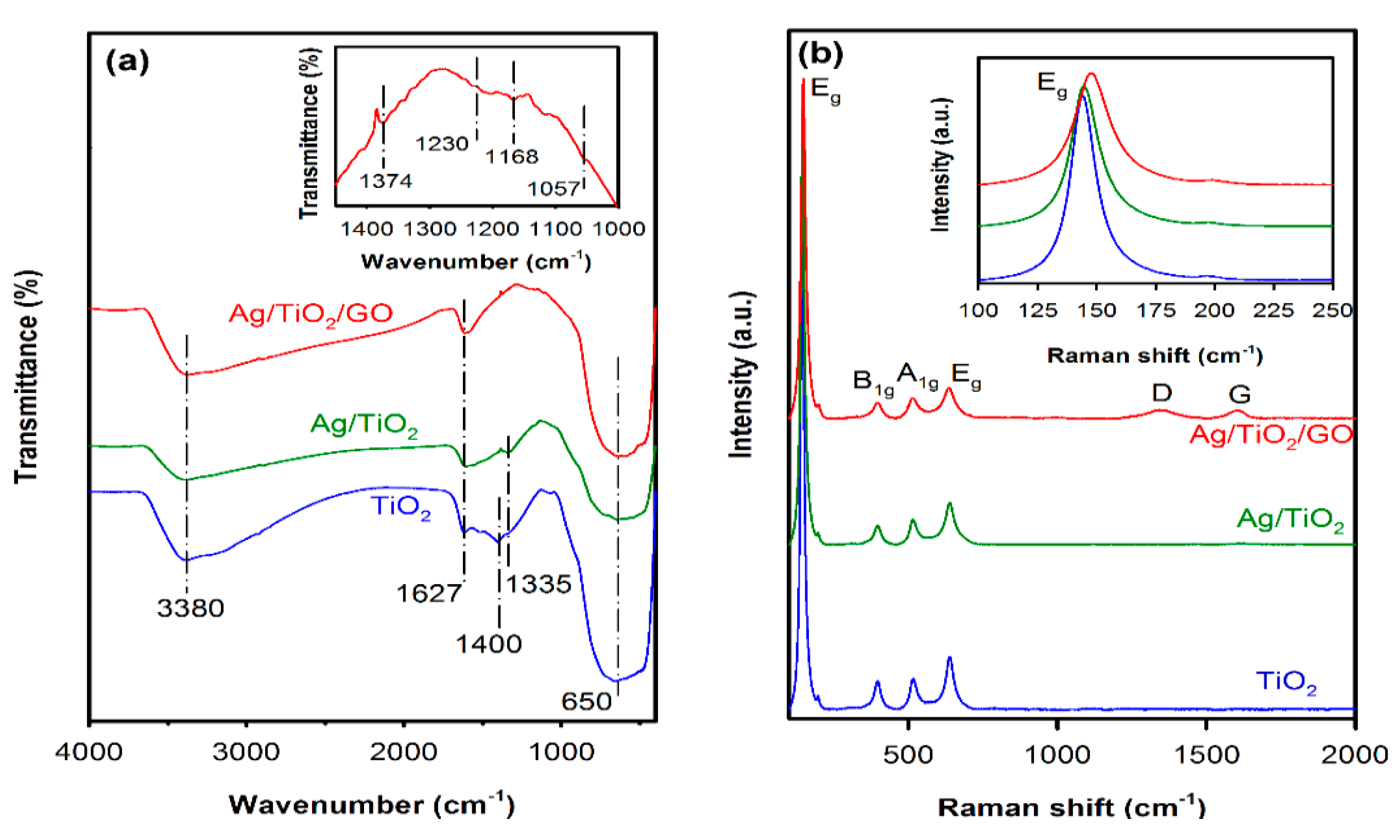
2.2. Structural and Morphological Analysis
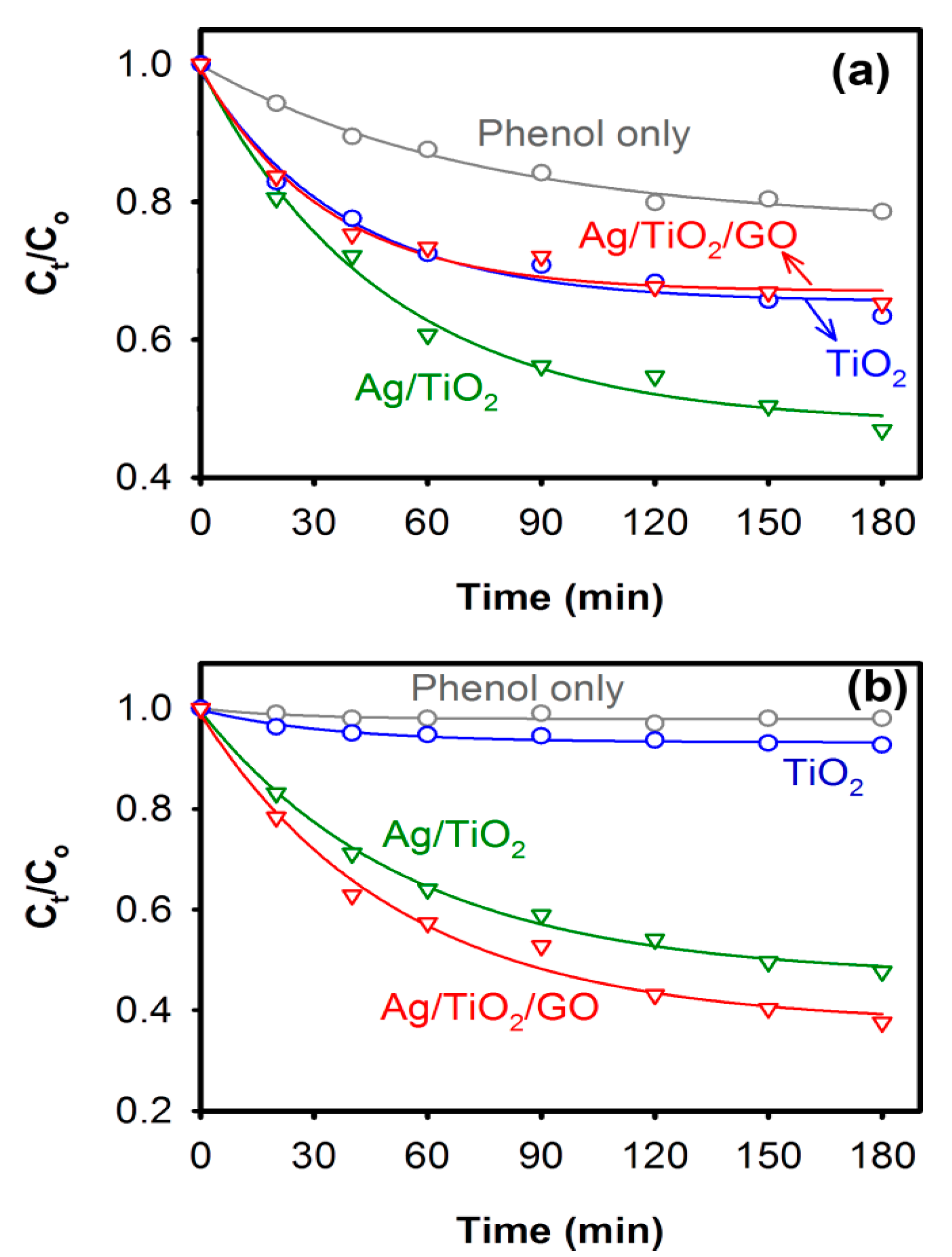
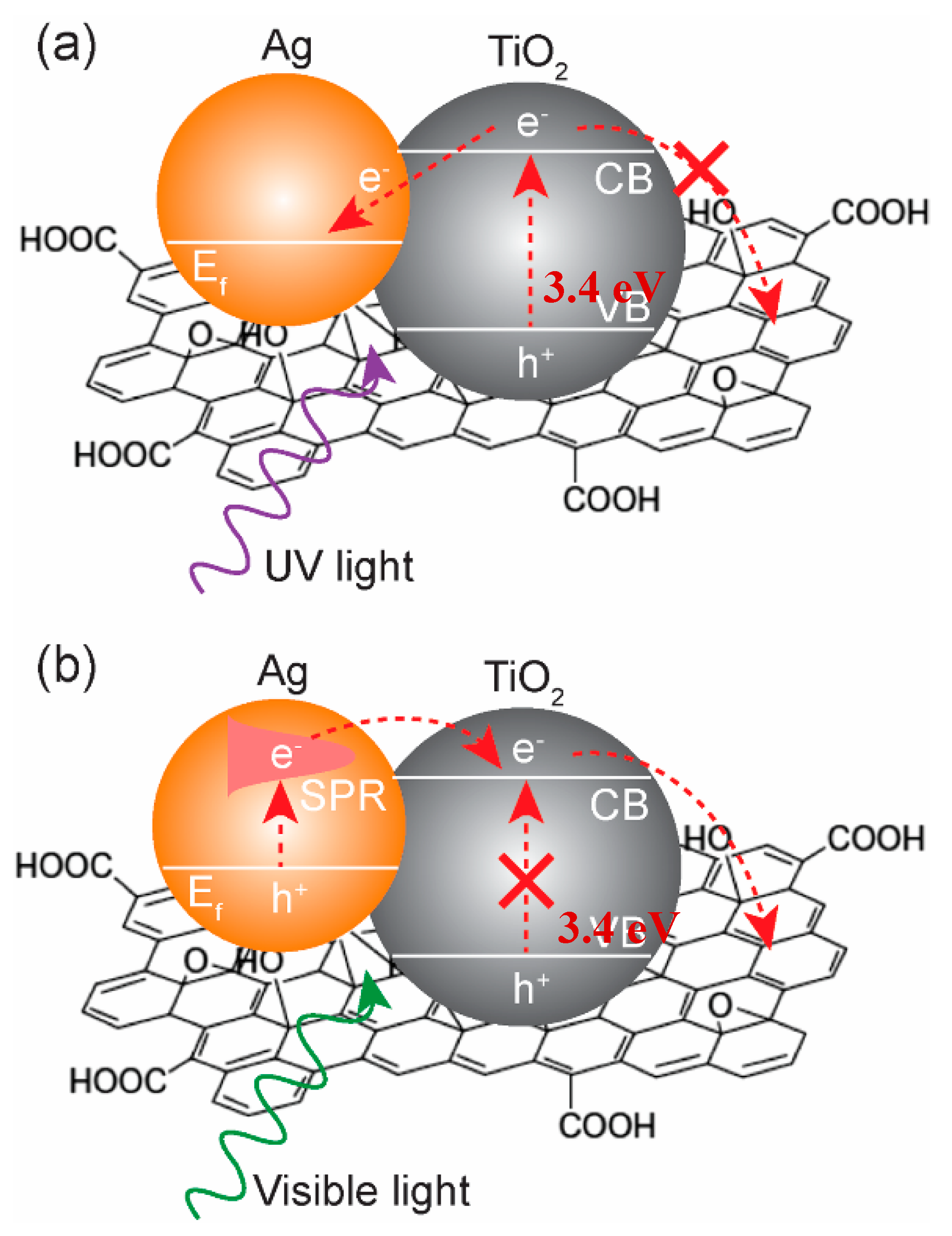
2.3. Photocatalytic Properties
3. Experimental
3.1. Preparation of TiO2 NPs
3.2. Preparation of the Ag/TiO2 Nanocomposite
3.3. Preparation of Ag/TiO2/GO Nanocomposites
3.4. Characterization Techniques
3.5. Photocatalytic Tests
Analytical Method for Degradation Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Hajkova, P.; Spatenka, P.; Horsky, J.; Horska, I.; Kolouch, A. Photocatalytic effect of TiO2 films on viruses and bacteria. Plasma Process. Polym. 2007, 4, S397. [Google Scholar] [CrossRef]
- Puma, G.L.; Bono, A.; Krishnaiah, D.; Collin, J.G. Preparation of titanium dioxide photocatalyst loaded onto activated carbon support using chemical vapor deposition: A review paper. J. Hazard. Mater. 2008, 157, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Agrios, H.A.G.; Gray, K.A.; Rajh, T.; Thurnauer, M.C. Anatase TiO2 nano-needle and heterostructures for Photocatalysis, Electrochemical and Photoelectrochemical applications. J. Phys. Chem. B 2003, 107, 4545. [Google Scholar]
- Macwan, D.P.; Dave, P.N.; Chaturvedi, S. A review on nano-TiO2 sol–gel type syntheses and its applications. J. Mater. Sci. 2011, 46, 3669–3686. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.M.; Tripathi, M. A review on the synthesis of TiO2 nanoparticles by solution route. Cent. Eur. J. Chem. 2012, 10, 279–294. [Google Scholar] [CrossRef]
- Corradi, B.; Bondioli, F.; Focher, B.; Ferrari, A.M.; Grippo, C.; Mariani, E.; Villa, C. Conventional and Microwave-Hydrothermal Synthesis of TiO2 Nanopowders. J. Am. Ceram. Soc. 2005, 88, 2639–2641. [Google Scholar] [CrossRef]
- Huang, C.-H.; Yang, Y.-T.; Doong, R.-A. Microwave-assisted hydrothermal synthesis of mesoporous anatase TiO2 via sol–gel process for dye-sensitized solar cells. Microporous Mesoporous Mater. 2011, 142, 473–480. [Google Scholar] [CrossRef]
- Wang, H.-E.; Zheng, L.-X.; Liu, C.-P.; Liu, Y.-K.; Luan, C.-Y.; Cheng, H.; Li, Y.Y.; Martinu, L.; Zapien, J.A.; Bello, I. Rapid microwave synthesis of porous TiO2 spheres and their applications in dye-sensitized solar cells. J. Phys. Chem. C 2011, 115, 10419–10425. [Google Scholar] [CrossRef]
- Cui, L.; Hui, K.N.; Hui, K.S.; Lee, S.K.; Zhou, W.; Wan, Z.P.; Thuc, C.-N.H. Facile microwave-assisted hydrothermal synthesis of TiO2 nanotubes. Mater. Lett. 2012, 75, 175–178. [Google Scholar] [CrossRef]
- Etacheri, V.; di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-light activation of TiO2 photocatalysts: advances in theory and experiments. J. Photochem. Photobiol. C Photochem. Rev. 2015, 25, 1–29. [Google Scholar] [CrossRef]
- Truong, Q.D.; Le, T.H.; Liu, J.Y.; Chung, C.C.; Ling, Y.C. Synthesis of TiO2 nanoparticles using novel titanium oxalate complex towards visible light-driven photocatalytic reduction of CO2 to CH3OH. Appl. Catal. A Gen. 2012, 437, 28–35. [Google Scholar] [CrossRef]
- Truong, Q.D.; Liu, J.Y.; Chung, C.C.; Ling, Y.C. Design and fabrication of semiconductor photocatalyst for photocatalytic reduction of CO2 to solar fuel. Catal. Commun. 2012, 19, 8589. [Google Scholar] [CrossRef]
- Saoud, K.; Alsoubaihi, R.; Bensalah, N.; Bora, T.; Bertino, M.; Dutta, J. Synthesis of supported silver nano-spheres on zinc oxide nanorods for visible light photocatalytic applications. Mater. Res. Bull. 2015, 63, 134–140. [Google Scholar] [CrossRef]
- Bora, T.; Myint, M.T.Z.; Al-Harthi, S.H. Dutta, Role of surface defects on visible light enabled plasmonic photocatalysis in Au–ZnO nanocatalysts. J. RSC Adv. 2015, 5, 96670. [Google Scholar] [CrossRef]
- Bora, T.; Zoepfl, D.; Dutta, J. Importance of plasmonic heating on visible light driven photocatalysis of gold nanoparticle decorated zinc oxide nanorods. Sci. Rep. 2016, 6, 26913. [Google Scholar] [CrossRef] [PubMed]
- Mathpal, M.C.; Tripathi, A.K.; Kumar, P.; R, B.; Singh, M.K.; Chung, J.S.; Hur, S.H.; Agarwal, A. Polymorphic transformations and optical properties of graphene-based Ag-doped titania nanostructures. Phys. Chem. Chem. Phys. 2014, 16, 23874. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.S.A.S.; Zhang, K.; Park, A.R.; Kim, K.S.; Park, N.-G.; Park, J.H.; Yoo, P.J. Single-step solvothermal synthesis of mesoporous Ag–TiO2–reduced graphene oxide ternary composites with enhanced photocatalytic activity. Nanoscale 2013, 5, 5093–5101. [Google Scholar] [CrossRef] [PubMed]
- Garg, B.; Bisht, T.; Ling, Y. Graphene-based nanomaterials as heterogeneous acid catalysts: A comprehensive perspective. Molecules 2014, 19, 14582–14614. [Google Scholar] [CrossRef] [PubMed]
- Umrao, S.; Abraham, S.; Theil, F.; Pandey, S.; Ciobota, V.; Shukla, P.K.; Rupp, C.J.; Chakraborty, S.; Ahuja, R.; Popp, J. A possible mechanism for the emergence of an additional band gap due to a Ti–O–C bond in the TiO2–graphene hybrid system for enhanced photodegradation of methylene blue under visible light. RSC Adv. 2014, 4, 59890. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, C. TiO2/graphene composite from thermal reaction of graphene oxide and its photocatalytic activity in visible light. J. Mater. Sci. 2010, 46, 2622–2626. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, H.-Y.; Zhao, J.-C.; Zheng, Z.-F.; Gao, X.-P. Visible-Light-Driven Oxidation of Organic Contaminants in Air with Gold Nanoparticle Catalysts on Oxide Supports. Angew. Chem. Int. Ed. 2008, 47, 5353–5356. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.L.; Liu, R.S.; Tsai, D.P. Plasmonic photocatalysis. Rep. Prog. Phys. 2013, 76, 046401. [Google Scholar] [CrossRef] [PubMed]
- Yokomizo, Y.; Krishnamurthy, S.; Kamat, P.V. Photoinduced electron charge and discharge of graphene–ZnO nanoparticle assembly. Catal. Today 2013, 199, 36–41. [Google Scholar] [CrossRef]
- Lightcap, V.; Murphy, S.; Schumer, T.; Kamat, P.V. Electron hopping through single-to-few-layer graphene oxide films. Side-selective photocatalytic deposition of metal nanoparticles. J. Phys. Chem. Lett. 2012, 3, 1453. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Makhal, A.; Bora, T.; Baruah, S.; Dutta, J.; Pal, S.K. Photoselective excited state dynamics in ZnO–Au nanocomposites and their implications in photocatalysis and dye-sensitized solar cells. Phys. Chem. Chem. Phys. 2011, 13, 12488. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsharaeh, E.H.; Bora, T.; Soliman, A.; Ahmed, F.; Bharath, G.; Ghoniem, M.G.; Abu-Salah, K.M.; Dutta, J. Sol-Gel-Assisted Microwave-Derived Synthesis of Anatase Ag/TiO2/GO Nanohybrids toward Efficient Visible Light Phenol Degradation. Catalysts 2017, 7, 133. https://doi.org/10.3390/catal7050133
Alsharaeh EH, Bora T, Soliman A, Ahmed F, Bharath G, Ghoniem MG, Abu-Salah KM, Dutta J. Sol-Gel-Assisted Microwave-Derived Synthesis of Anatase Ag/TiO2/GO Nanohybrids toward Efficient Visible Light Phenol Degradation. Catalysts. 2017; 7(5):133. https://doi.org/10.3390/catal7050133
Chicago/Turabian StyleAlsharaeh, E. H., T. Bora, A. Soliman, Faheem Ahmed, G. Bharath, M. G. Ghoniem, Khalid M. Abu-Salah, and J. Dutta. 2017. "Sol-Gel-Assisted Microwave-Derived Synthesis of Anatase Ag/TiO2/GO Nanohybrids toward Efficient Visible Light Phenol Degradation" Catalysts 7, no. 5: 133. https://doi.org/10.3390/catal7050133






