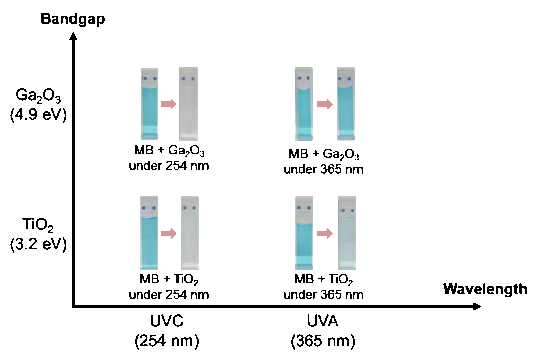A Zero-Power, Low-Cost Ultraviolet-C Colorimetric Sensor Using a Gallium Oxide and Reduced Graphene Oxide Hybrid via Photoelectrochemical Reactions
Abstract
:1. Introduction
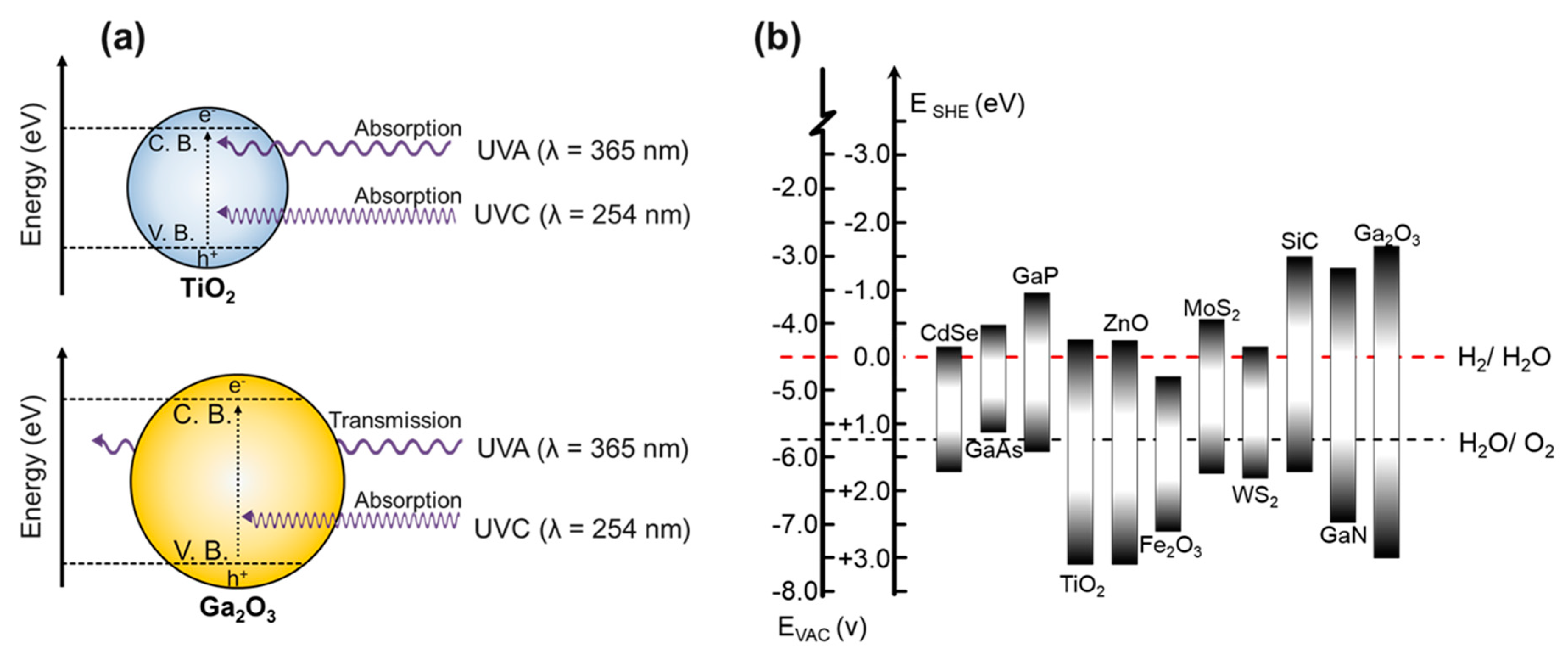
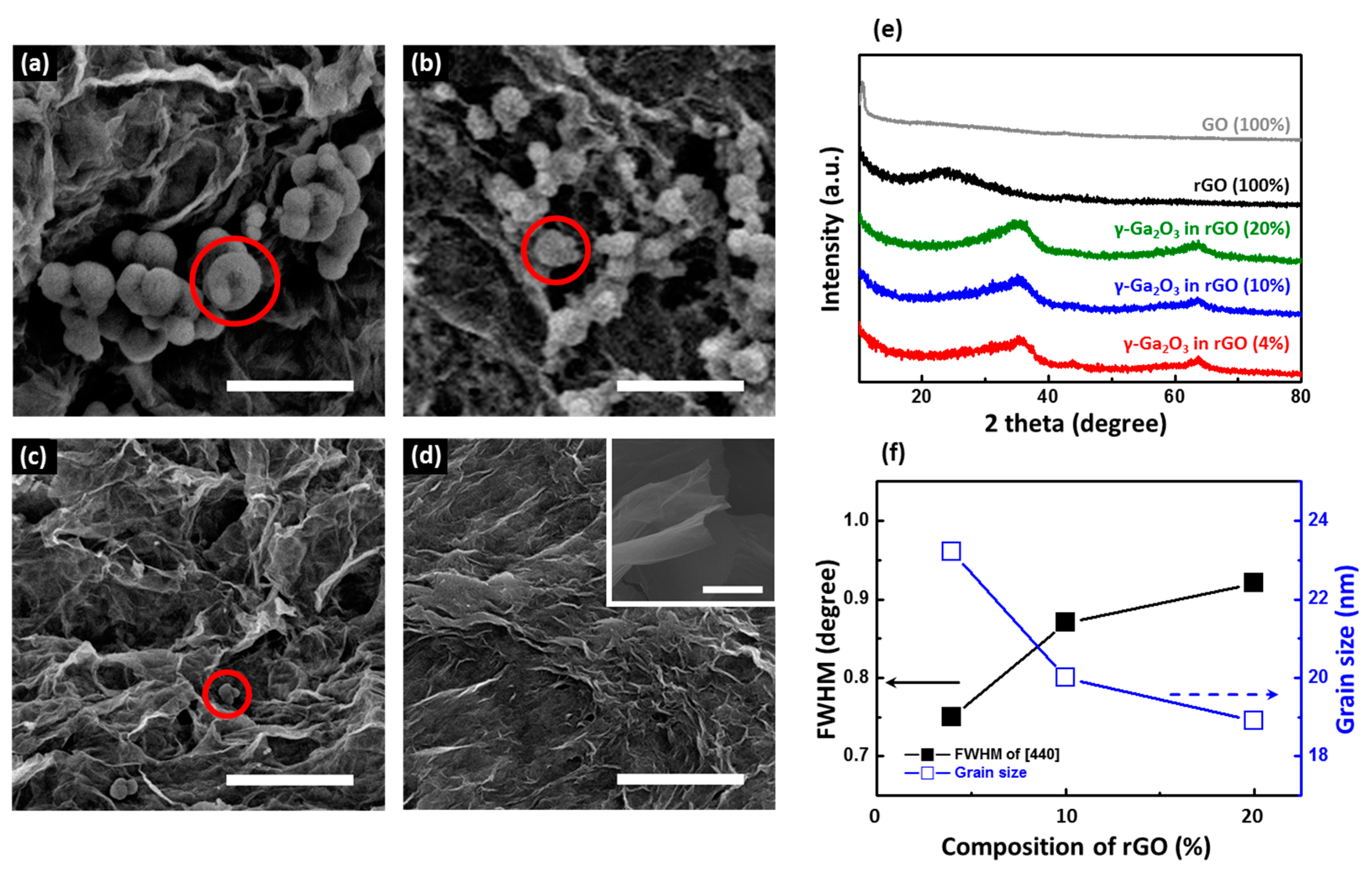
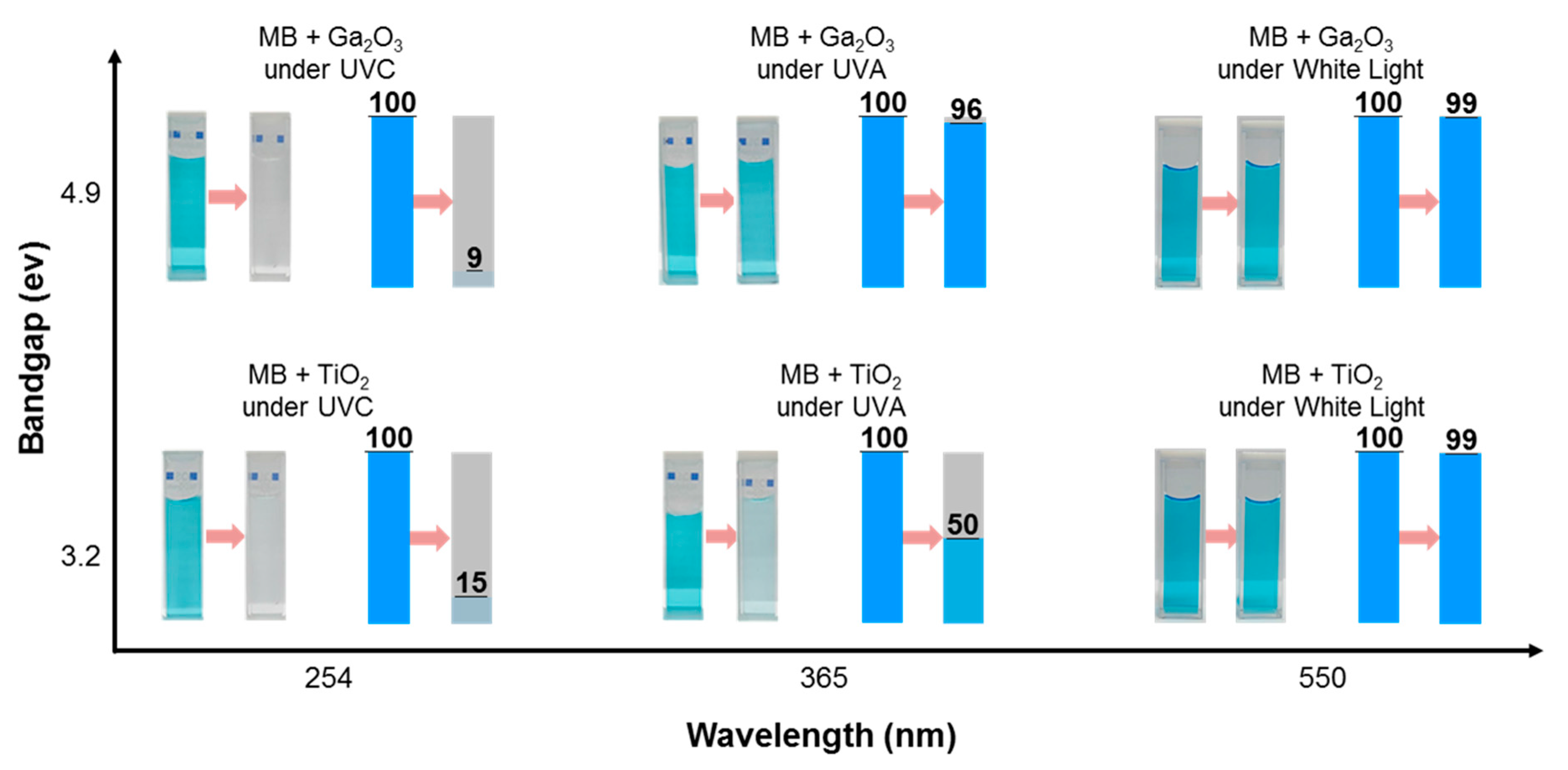
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mondal, S.; Helidelberger, C. Transformation of C3H/10T1/2 CL8 mouse embryo fibroblasts by ultraviolet irradiation and a phorbol ester. Nature 1976, 260, 710–711. [Google Scholar] [CrossRef] [PubMed]
- Brash, D.E.; Rudolph, J.A.; Simon, J.A.; Lin, A.; McKenna, G.J.; Baden, H.P.; Halperin, A.J.; Ponten, J. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc. Natl. Acad. Sci. USA 1991, 88, 10124–10128. [Google Scholar] [CrossRef] [PubMed]
- Lipiec, E.; Sekine, R.; Bielecki, J.; Kwiatek, W.M.; Wood, B.R. Molecular Characterization of DNA Double Strand Breaks with Tip-Enhanced Raman Scattering. Angew. Chem. Int. Ed. 2014, 53, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Thomson, E.E.; Carra, R.; Nicolelis, M.A.L. Perceiving invisible light through a somatosensory cortical prosthesis. Nat. Commun. 2013, 4, 1482. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.C.; Rpezelin, B.B.; Baker, K.S.; Bidigare, R.R.; Boucher, R.R.; Coley, T.; Karents, D.; Macintyre, S.; Matlick, H.A.; Menzies, D.; et al. Ozone depletion: Ultraviolet radiation and phytoplankton biology in Antarctic waters. Science 1992, 255, 952. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Barick, K.C.; Bahadur, D. Functional Oxide Nanomaterials and Nanocomposites for the Removal of Heavy Metals and Dyes. Nanomater. Nanotechnol. 2013, 3, 20. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Sosnin, E.A.; Stoffels, E.; Erofeev, M.V.; Kieft, I.E.; Kunts, S.E. The effects of UV irradiation and gas plasma treatment on living mammalian cells and bacteria: A comparative approach. IEEE Trans. Plasma Sci. 2004, 32, 1544–1550. [Google Scholar] [CrossRef]
- Guo, D.; Wu, Z.; Li, P.; An, Y.; Liu, H.; Guo, X.; Yan, H.; Wang, G.; Sun, C.; Li, L.; et al. Fabrication of β-Ga2O3 thin films and solar-blind photodetectors by laser MBE technology. Opt. Mat. Exp. 2014, 4, 1067–1076. [Google Scholar] [CrossRef]
- Morkoc, H.; Strite, S.; Gao, G.B.; Lin, M.E.; Sverdlov, B.; Burnsless, M. Large-band-gap SiC, III-V nitride, and II-VI ZnSe-based semiconductor device technologies. J. Appl. Phys. 1994, 76, 1363–1398. [Google Scholar] [CrossRef]
- Suzuki, R.; Nakagomi, S.; Kokubun, Y. Solar-blind photodiodes composed of a Au Schottky contact and a β-Ga2O3 single crystal with a high resistivity cap layer. Appl. Phys. Lett. 2011, 98, 131114. [Google Scholar] [CrossRef]
- Kumar, S.; Rubio, E.; Noor, M.; Martinez, G.; Manandha, S.; Shutthanandan, V.; Thevuthasan, S.; Ramana, C. Structure, morphology, and optical properties of amorphous and nanocrystalline gallium oxide thin films. J. Phys. Chem. C 2013, 117, 4194–4200. [Google Scholar] [CrossRef]
- Kokubun, Y.; Miura, K.; Endo, F.; Nakagomi, S. Sol-gel prepared β-Ga2O3 thin films for ultraviolet photodetectors. Appl. Phys. Lett. 2007, 90, 031912. [Google Scholar] [CrossRef]
- Li, S.S.; Tu, K.H.; Lin, C.C.; Chen, C.W.; Chhowalla, M. Solution-processable graphene oxide as an efficient hole transport layer in polymer solar cells. ACS Nano 2010, 4, 3169–3174. [Google Scholar] [CrossRef] [PubMed]
- Gratzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Guo, Y.; Dong, H.; Zhou, X. Tailoring the electronic structure of β-Ga2O3 by non-metal doping from hybrid density functional theory calculations. Phys. Chem. Chem. Phys. 2015, 17, 5817–5825. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Han, K.I.; Lee, I.G.; Park, W.K.; Yoon, Y.; Yoo, C.S.; Yang, W.S.; Hwang, W.S. A Gallium Oxide-Graphene Oxide Hybrid Composite for Enhanced Photocatalytic Reaction. Nanomaterials 2016, 6, 127. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Kim, S.; Yang, W.; Kim, H.; Shin, M.; Kim, J.; Lee, I.; Cho, B.; Hwang, W. Material characteristics and equivalent circuit models of stacked graphene oxide for capacitive humidity sensors. AIP Adv. 2016, 6, 035203. [Google Scholar] [CrossRef]
- Park, W.; Kim, H.; Kim, T.; Kim, Y.; Yoo, S.; Kim, S.; Yoon, D.; Yang, W. Facile synthesis of graphene oxide in a Couette–Taylor flow reactor. Carbon 2015, 83, 217–223. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Han, K.I.; Lee, I.G.; Yoon, Y.; Park, W.K.; Hong, S.W.; Yang, W.S.; Hwang, W.S. A Zero-Power, Low-Cost Ultraviolet-C Colorimetric Sensor Using a Gallium Oxide and Reduced Graphene Oxide Hybrid via Photoelectrochemical Reactions. Catalysts 2017, 7, 248. https://doi.org/10.3390/catal7090248
Kim S, Han KI, Lee IG, Yoon Y, Park WK, Hong SW, Yang WS, Hwang WS. A Zero-Power, Low-Cost Ultraviolet-C Colorimetric Sensor Using a Gallium Oxide and Reduced Graphene Oxide Hybrid via Photoelectrochemical Reactions. Catalysts. 2017; 7(9):248. https://doi.org/10.3390/catal7090248
Chicago/Turabian StyleKim, Seungdu, Kook In Han, In Gyu Lee, Yeojoon Yoon, Won Kyu Park, Suck Won Hong, Woo Seok Yang, and Wan Sik Hwang. 2017. "A Zero-Power, Low-Cost Ultraviolet-C Colorimetric Sensor Using a Gallium Oxide and Reduced Graphene Oxide Hybrid via Photoelectrochemical Reactions" Catalysts 7, no. 9: 248. https://doi.org/10.3390/catal7090248