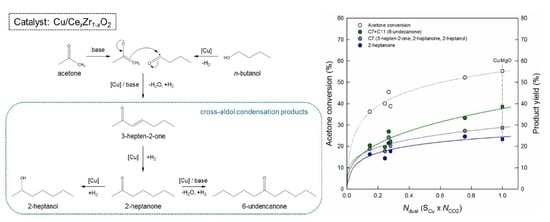
Cross-Aldol Condensation of Acetone and n-Butanol into Aliphatic Ketones over Supported Cu Catalysts on Ceria-Zirconia
Abstract
:1. Introduction
2. Results
2.1. Catalyst Characterization Results
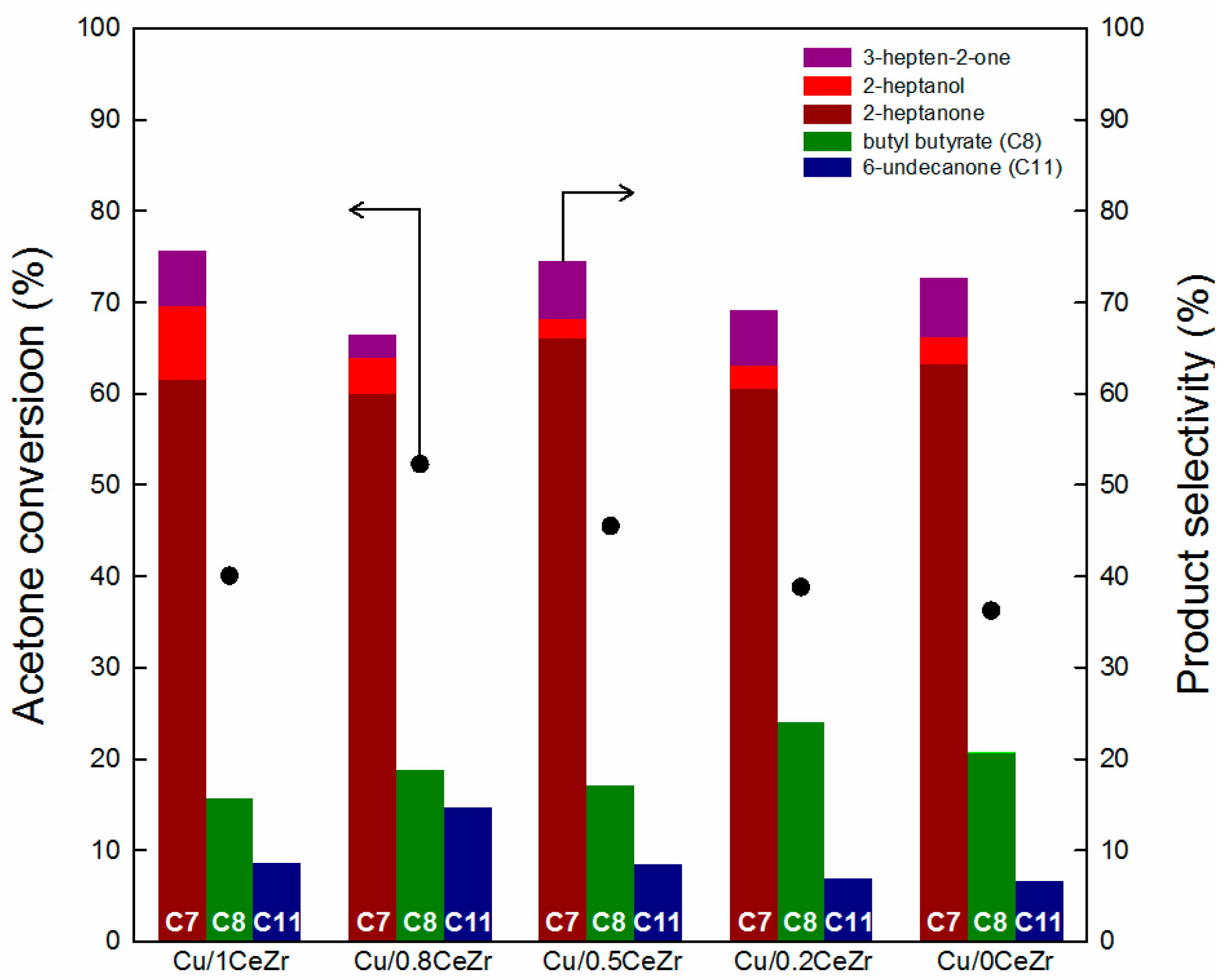
2.2. Catalytic Performance in the Cross-Aldol Condensation of Acetone and n-Butanol
3. Discussion
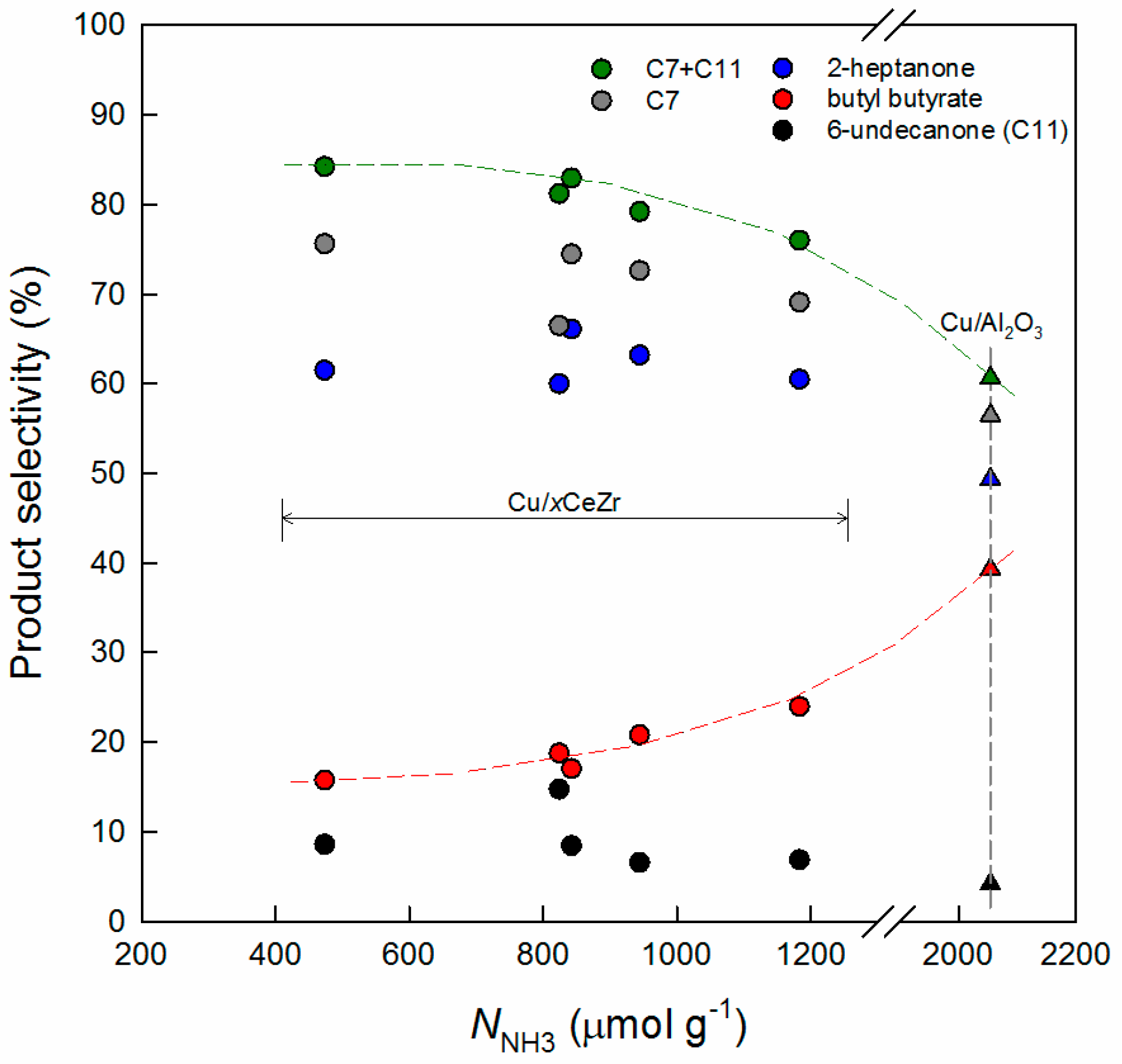
3.1. Key Properties of Cu/xCeZr Catalysts in the Cross-Aldol Condensation of Acetone and n-Butanol
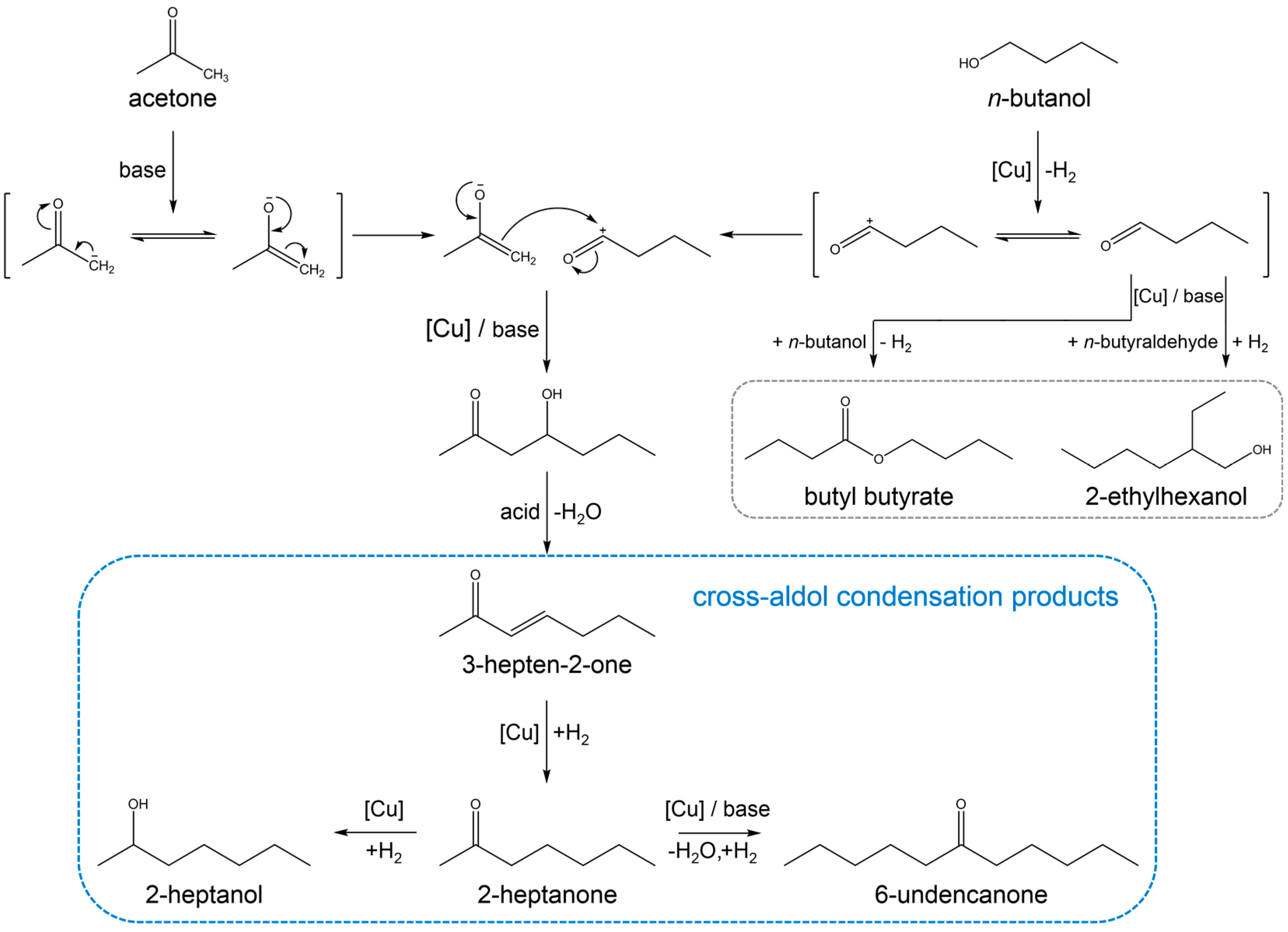
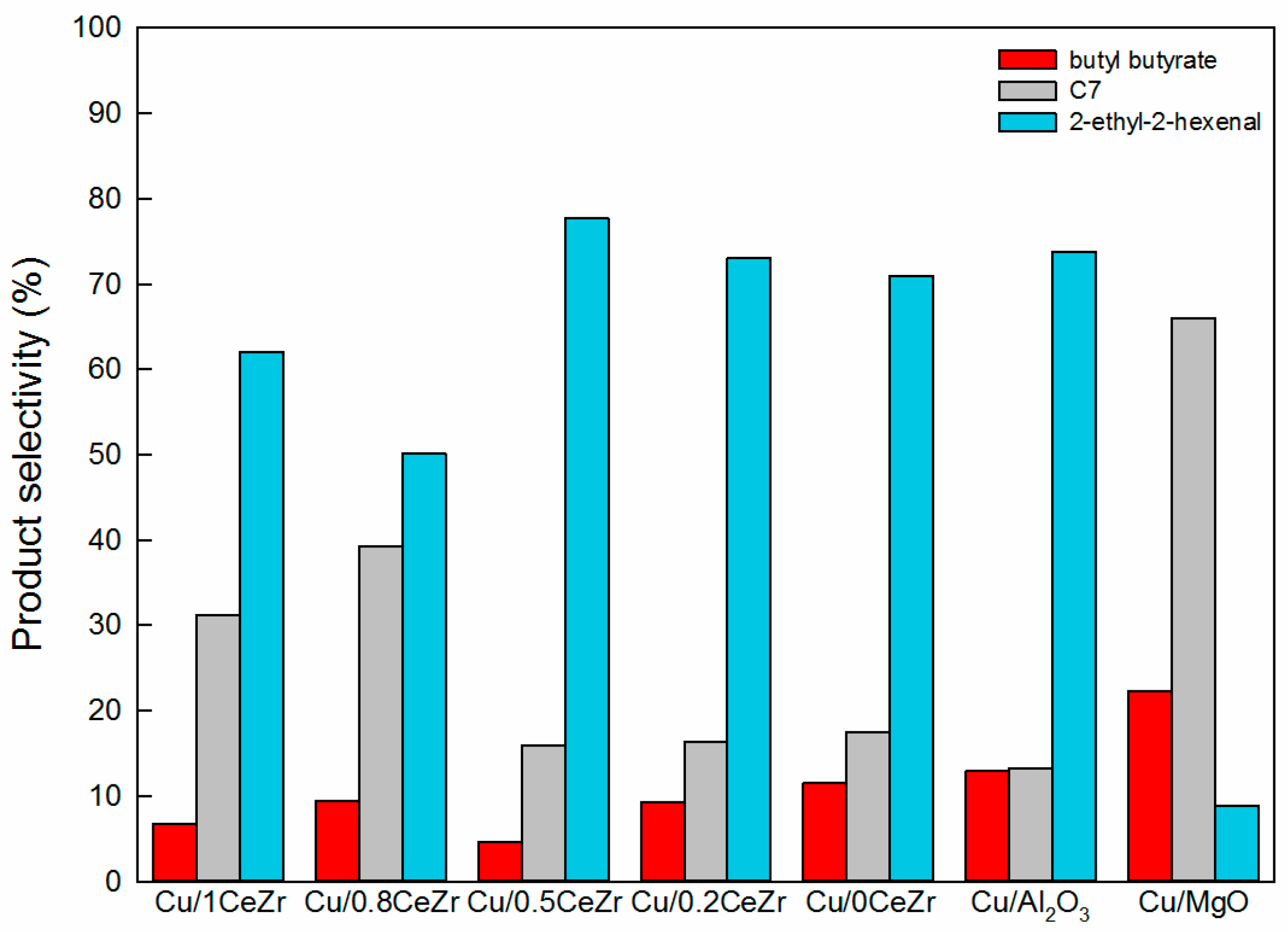
3.2. Reaction Pathway in the Cross-Aldol Condensation of Acetone and n-Butanol over Cu/xCeZr
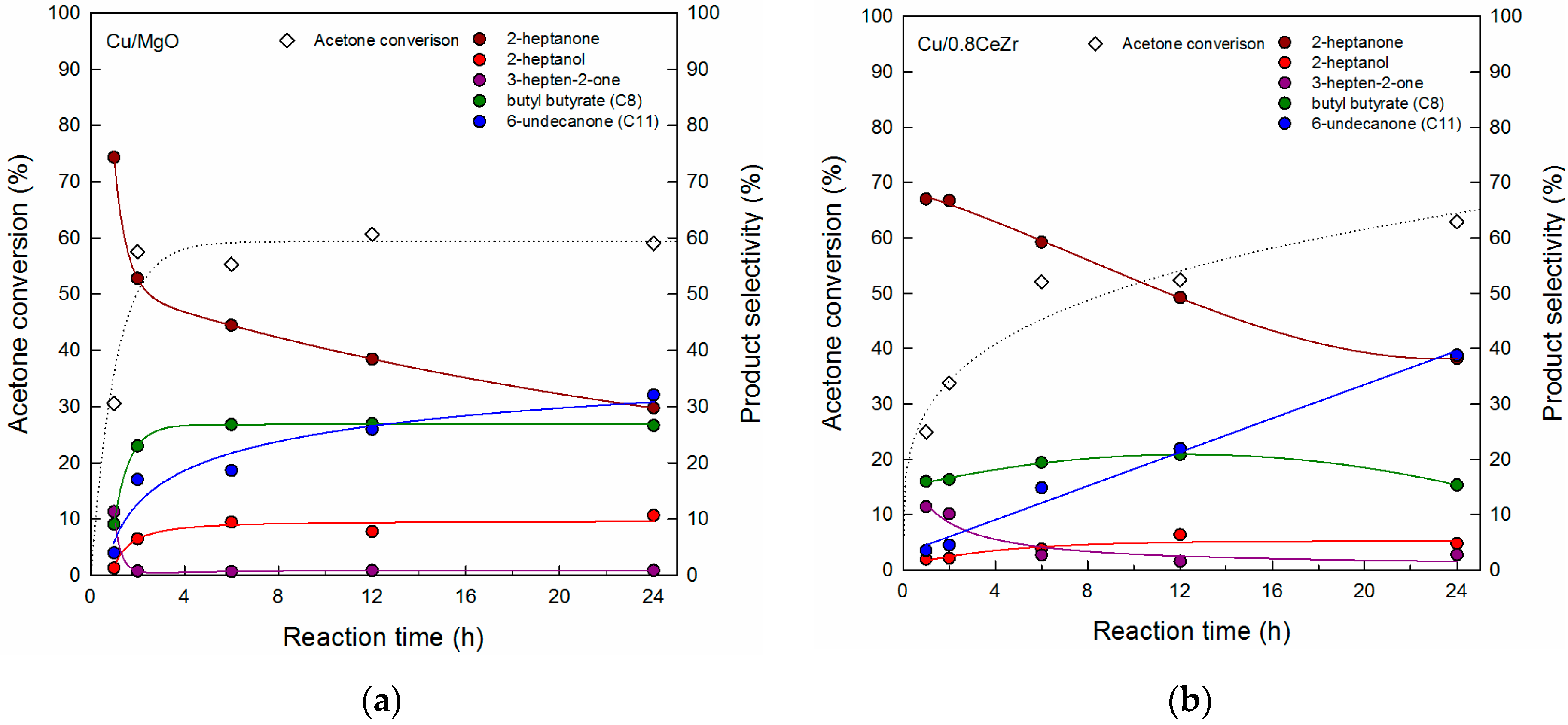
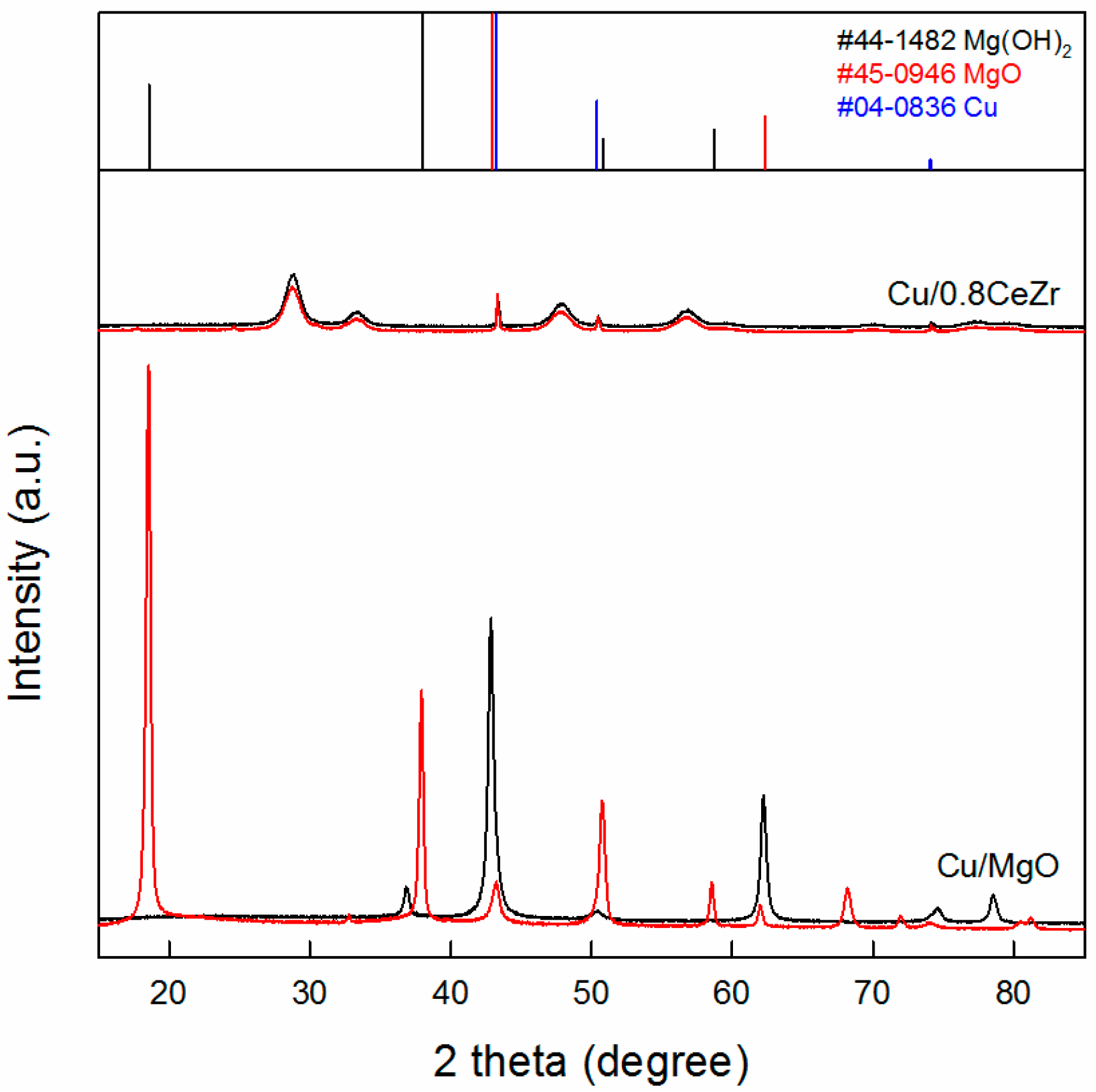
3.3. Comparison of Cu/0.8CeZr and Cu/MgO Catalysts
4. Experimental
4.1. Catalyst Preparation
4.2. Catalyst Characterization
4.3. Activity Test
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- McKendry, P. Energy production from biomass (part 2): Conversion technologies. Bioresour. Technol. 2002, 83, 47–54. [Google Scholar] [CrossRef]
- Eggeman, T.; Verser, D. The importance of utility systems in today’s biorefineries and a vision for tomorrow. Appl. Biochem. Biotechnol. 2006, 129–132, 361–381. [Google Scholar] [CrossRef]
- Granda, C.B.; Zhu, L.; Holtzapple, M.T. Sustainable liquid biofuels and their environmental impact. Environ. Prog. 2007, 26, 233–250. [Google Scholar] [CrossRef]
- Breitkreuz, K.; Menne, A.; Kraft, A. New process for sustainable fuels and chemicals from bio-based alcohols and acetone. Biofuels Bioprod. Biorefin. 2014, 8, 504–515. [Google Scholar] [CrossRef]
- Anbarasan, P.; Baer, Z.C.; Sreekumar, S.; Gross, E.; Binder, J.B.; Blanch, H.W.; Clark, D.S.; Toste, F.D. Integration of chemical catalysis with extractive fermentation to produce fuels. Nature 2012, 491, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Sreekumar, S.; Baer, Z.C.; Gross, E.; Padmanaban, S.; Goulas, K.; Gunbas, G.; Alayoglu, S.; Blanch, H.W.; Clark, D.S.; Toste, F.D. Chemocatalytic upgrading of tailored fermentation products toward biodiesel. ChemSusChem 2014, 7, 2445–2448. [Google Scholar] [CrossRef] [PubMed]
- Goulas, K.A.; Sreekumar, S.; Song, Y.; Kharidehal, P.; Gunbas, G.; Dietrich, P.J.; Johnson, G.R.; Wang, Y.C.; Grippo, A.M.; Grabow, L.C.; et al. Synergistic effects in bimetallic palladium-copper catalysts improve selectivity in oxygenate coupling reactions. J. Am. Chem. Soc. 2016, 138, 6805–6812. [Google Scholar] [CrossRef] [PubMed]
- Goulas, K.A.; Gunbas, G.; Dietrich, P.J.; Sreekumar, S.; Grippo, A.; Chen, J.P.; Gokhale, A.A.; Toste, F.D. ABE condensation over monometallic catalysts: Catalyst characterization and kinetics. ChemCatChem 2017, 9, 677–684. [Google Scholar] [CrossRef]
- Gangadharan, A.; Shen, M.; Sooknoi, T.; Resasco, D.E.; Mallinson, R.G. Condensation reactions of propanal over CexZr1-xO2 mixed oxide catalysts. Appl. Catal. A 2010, 385, 80–91. [Google Scholar] [CrossRef]
- Di Cosimo, J.I.; Torres, G.; Apesteguia, C.R. One-step MIBK synthesis: A new process from 2-propanol. J. Catal. 2002, 208, 114–123. [Google Scholar] [CrossRef]
- Hakim, S.H.; Shanks, B.H.; Dumesic, J.A. Catalytic upgrading of the light fraction of a simulated bio-oil over CeZrOx catalyst. Appl. Catal. B 2013, 142–143, 368–376. [Google Scholar] [CrossRef]
- Postole, G.; Chowdhury, B.; Kramakar, B.; Pinki, K.; Banerji, J.; Auroux, A. Knoevenagel condensation reaction over acid—Base bifunctional nanocrystalline CexZr1−xO2 solid solutions. J. Catal. 2010, 269, 110–121. [Google Scholar] [CrossRef]
- Gaertner, C.A.; Serrano-Ruiz, J.C.; Braden, D.J.; Dumesic, J.A. Catalytic coupling of carboxylic acids by ketonization as a processing step in biomass conversion. J. Catal. 2009, 266, 71–78. [Google Scholar] [CrossRef]
- Gaertner, C.A.; Serrano-Ruiz, J.C.; Braden, D.J.; Dumesic, J.A. Ketonization reactions of carboxylic acids and esters over ceria-zirconia as biomass-upgrading processes. Ind. Eng. Chem. Res. 2010, 49, 6027–6033. [Google Scholar] [CrossRef]
- Kunkes, E.L.; Gürbüz, E.I.; Dumesic, J.A. Vapour-phase C–C coupling reactions of biomass-derived oxygenates over Pd/CeZrOx catalysts. J. Catal. 2009, 266, 236–249. [Google Scholar] [CrossRef]
- Gürbüz, E.I.; Kunkes, E.L.; Dumesic, J.A. Integration of C–C coupling reactions of biomass-derived oxygenates to fuel-grade compounds. Appl. Catal. B 2010, 94, 134–141. [Google Scholar] [CrossRef]
- Kunkes, E.L.; Simonetti, D.A.; West, R.M.; Serrano-Ruiz, J.C.; Gärtner, C.A.; Dumesic, J.A. Catalytic conversion of biomass to monofunctional hydrocarbons and targeted liquid-fuel classes. Science 2008, 322, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Faba, L.; Díaz, E.; Ordóñez, S. One-pot aldol condensation and hydrodeoxygenation of biomass-derived carbonyl compounds for biodiesel synthesis. ChemSusChem 2014, 7, 2816–2820. [Google Scholar] [CrossRef] [PubMed]
- Requies, J.; Güemez, M.B.; Maireles, P.; Iriondo, A.; Barrio, V.L.; Cambra, J.F.; Arias, P.L. Zirconia supported Cu systems as catalysts for n-butanol conversion to butyraldehyde. Appl. Catal. A 2012, 423–424, 185–191. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, C.H.; Hanson, J.C.; Robinson, R.D.; Herman, I.P.; Chan, S.-W. Phases in ceria-zirconia binary oxide (1−x)CeO2−xZrO2 nanoparticles: The effect of particle size. J. Am. Ceram. Soc. 2006, 89, 1028–1036. [Google Scholar] [CrossRef]
- Reddy, B.M.; Bharail, P.; Saikia, P.; Park, S.-E.; van den Berg, M.W.E.; Muhler, M.; Grünert, W. Structural characterization and catalytic activity of nanosized CexM1-xO2 (M = Zr and Hf) mixed oxides. J. Phys. Chem. C 2008, 112, 11729–11737. [Google Scholar] [CrossRef]
- Liu, L.; Yao, Z.; Liu, B.; Dong, L. Correlation of structural characteristics with catalytic performance of CuO/CexZr1-xO2 catalysts for NO reduction by CO. J. Catal. 2010, 275, 45–60. [Google Scholar] [CrossRef]
- Zou, Z.-Q.; Meng, M.; Guo, L.-H.; Zha, Y.-Q. Synthesis and characterization of CuO/Ce1-xTixO2 catalysts used for low-temperature CO oxidation. J. Hazard. Mater. 2009, 163, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Moretti, E.; Lenarda, M.; Riello, P.; Storaro, L.; Talon, A.; Frattini, R.; Reyes-Carmona, A.; Jiménez-López, A.; Rodríguez-Castellón, E. Influence of synthesis parameters on the performance of CeO2-CuO and CeO2-ZrO2-CuO systems in the catalytic oxidation of CO in excess of hydrogen. Appl. Catal. B 2013, 129, 556–565. [Google Scholar] [CrossRef]
- Das, D.; Llorca, J.; Dominguez, M.; Colussi, S.; Trovarelli, A.; Gayen, A. Methanol steam reforming behavior of copper impregnated over CeO2-ZrO2 derived from a surfactant assisted coprecipitation route. Int. J. Hydrog. Energy 2015, 40, 10463–10479. [Google Scholar] [CrossRef] [Green Version]
- Di Cosimo, J.I.; Apesteguía, C.R.; Ginés, M.J.L.; Iglesia, E. Structural requirements and reaction pathways in condensation reactions of alcohols on MgyAlOx catalysts. J. Catal. 2000, 190, 261–275. [Google Scholar] [CrossRef]
- Díez, V.K.; Di Cosimo, J.I.; Apesteguía, C.R. Study of the citral/acetone reaction on MgyAlOx oxides: Effect of the chemical composition on catalyst activity, selectivity and stability. Appl. Catal. A 2008, 345, 143–151. [Google Scholar] [CrossRef]
- Subbaramaiah, V.; Srivastava, V.C.; Mall, I.D. Optimization of reaction parameters and kinetic modeling of catalytic wet peroxidation of picoline by Cu/SBA-15. Ind. Eng. Chem. Res. 2013, 52, 9021–9029. [Google Scholar] [CrossRef]
- Sagar, G.V.; Rao, P.V.R.; Srikanth, C.S.; Chary, K.V.R. Dispersion and reactivity of copper catalysts supported on Al2O3-ZrO2. J. Phys. Chem. B 2006, 110, 13881–13888. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Saleh, R.Y.; Ozkan, U.S. Reaction network of aldehyde hydrogenation over sulfided Ni–Mo/Al2O3 catalysts. J. Catal. 2005, 231, 20–32. [Google Scholar] [CrossRef]
- Wawrzetz, A.; Peng, B.; Hrabar, A.; Jentys, A.; Lemonidou, A.A.; Lercher, J.A. Towards understanding the bifunctional hydrodeoxygenation and aqueous phase reforming of glycerol. J. Catal. 2010, 269, 411–420. [Google Scholar] [CrossRef]
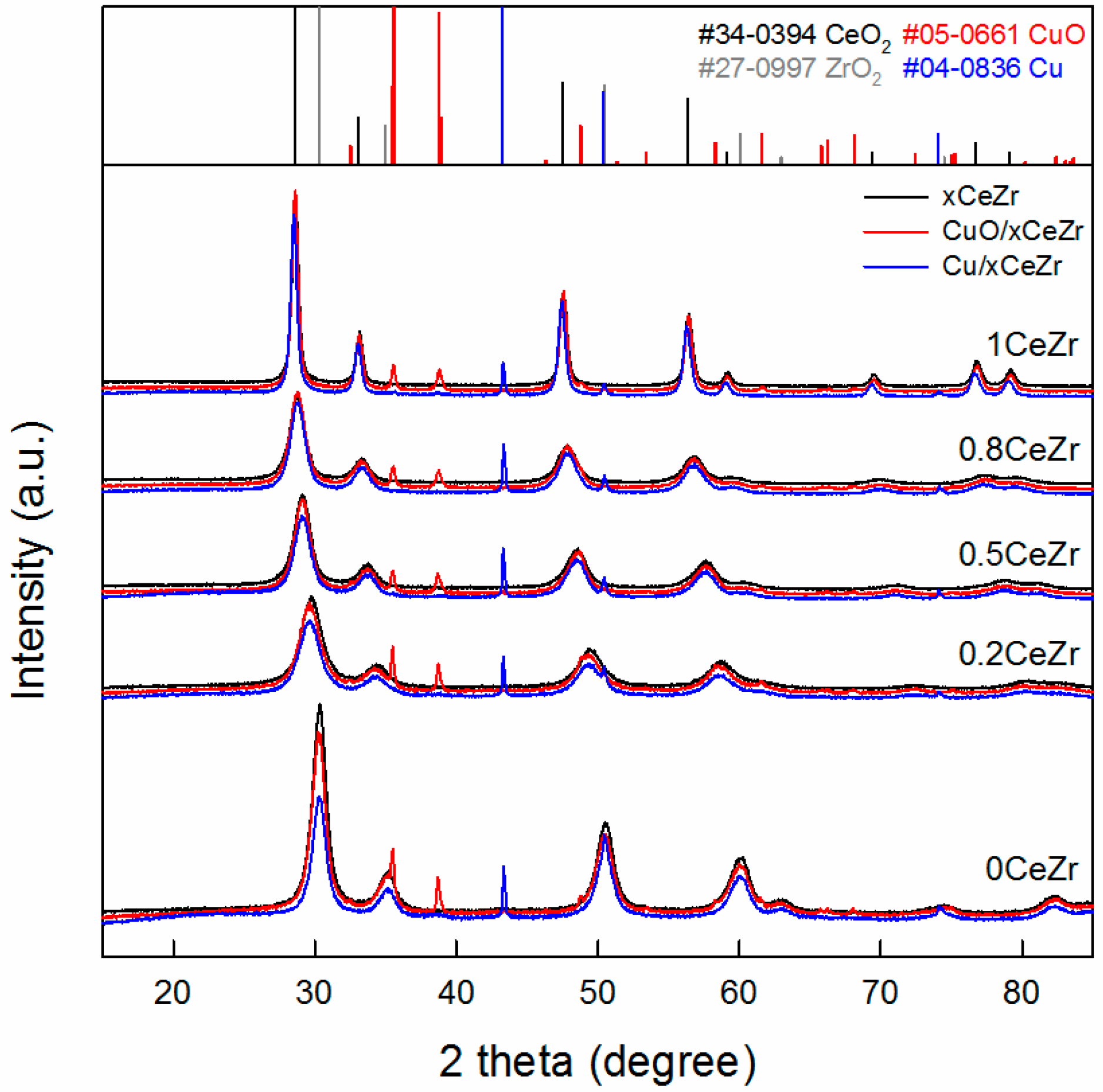
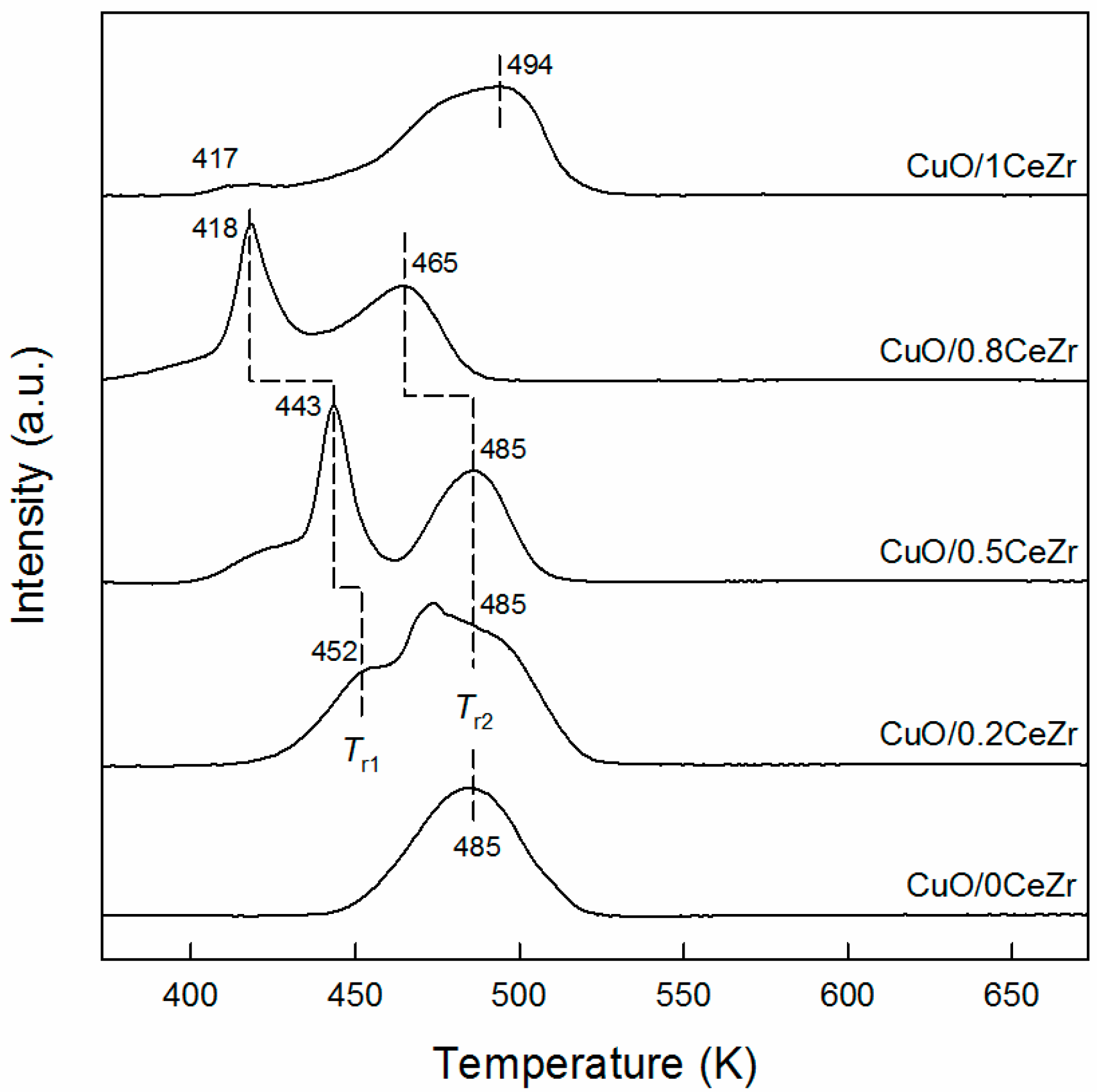



| xCeZr | CuO/xCeZr | Cu/xCeZr | ||||||
|---|---|---|---|---|---|---|---|---|
| SBET (m2 g−1) | SBET (m2 g−1) | DCuO 1 (nm) | SCu 2 (m2 gCu−1) | DCu 2 (nm) | dCu 2 (%) | NCO2 3 (μmol g−1) | NNH3 3 (μmol g−1) | |
| 1CeZr | 43 | 62 | 53 | 15.1 | 45 | 7.4 | 321.7 | 473.4 |
| 0.8CeZr | 104 | 84 | 35 | 24.3 | 35 | 11.9 | 619.0 | 823.7 |
| 0.5CeZr | 104 | 82 | 51 | 10.7 | 63 | 5.2 | 497.6 | 842.3 |
| 0.2CeZr | 98 | 66 | 72 | 9.0 | 75 | 4.4 | 618.7 | 1183.2 |
| 0CeZr | 102 | 57 | 78 | 5.2 | 131 | 2.5 | 560.4 | 944.2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Park, J.; Kannapu, H.P.R.; Suh, Y.-W. Cross-Aldol Condensation of Acetone and n-Butanol into Aliphatic Ketones over Supported Cu Catalysts on Ceria-Zirconia. Catalysts 2017, 7, 249. https://doi.org/10.3390/catal7090249
Kim M, Park J, Kannapu HPR, Suh Y-W. Cross-Aldol Condensation of Acetone and n-Butanol into Aliphatic Ketones over Supported Cu Catalysts on Ceria-Zirconia. Catalysts. 2017; 7(9):249. https://doi.org/10.3390/catal7090249
Chicago/Turabian StyleKim, Minseok, Jongha Park, Hari Prasad Reddy Kannapu, and Young-Woong Suh. 2017. "Cross-Aldol Condensation of Acetone and n-Butanol into Aliphatic Ketones over Supported Cu Catalysts on Ceria-Zirconia" Catalysts 7, no. 9: 249. https://doi.org/10.3390/catal7090249