1. Introduction
Due to restrictions associated with environmental protection, researchers increasingly use waste in their research as a source of organic compounds, which is beneficial for the environment and has economic justifications. Orange peels are a residue from the process of orange juice production. About 19.8–33 million tons of oranges are produced each year, and 8 to 20 million tons of waste in the form of fruit peels result. Waste orange peels can be used as valuable feed additives for animals; however, during transport orange peels cause considerable corrosion damage to the trailers transporting them. The solution to this problem may be to use orange skins to obtain orange oil, in which the main component is an organic compound with numerous applications—limonene [
1]. Orange oil is possible to obtain from orange peels with the help of hydrodistillation, steam distillation, or microwaves. The amount of limonene in orange oil is above 95%. Limonene is a cyclic terpene that is present in the form of two isomers, which are enantiomers to each other, but in the orange oil obtained from orange peels only R-(+)-limonene is present. The limonene obtained from waste orange peels is used in a wide range of everyday products. Furthermore, it is possible to transform this organic compound into many valuable products, for example in the oxidation and isomerization processes. Such transformations are valuable from the point of view of environmental protection, because we use a renewable raw material obtained from biomass.
During the process of limonene isomerization, γ-terpinene, α-terpinene, and terpinolene can be obtained. Moreover, the dehydrogenation of products of limonene isomerization can also cause the formation of p-cymene. The products of limonene isomerization are valuable compounds widely used as additives for food, cosmetics, or pharmaceuticals.
P-cymene is the only naturally occurring form of cymene (an essential oil of caraway or thyme). This compound is a raw material for the synthesis of cresols. P-cymene is also used as the additive for masking odors in soaps and industrial products. Another application of this monoterpene is its transformation into aromatic monomers, such as terephthalic acid or dimethylstyrene. P-cymene is used as a solvent for dyes and varnishes, as a heat transfer agent, and as an additive in musk fragrances and perfumes. Moreover, p-cymene is applied as a ligand in olefine metathesis [
2,
3,
4]. This compound is industrially produced in the Friedel–Craft reaction from toluene and isopropyl alcohol. In this reaction inorganic acids are used as catalysts, for example hydrochloric acid with AlCl
3. The main disadvantages connected with the application of this catalyst are the corrosion of the apparatus and problems connected with the waste disposal after the reaction and catalyst separation. Many of these problems have been resolved by supporting the acid catalyst on a zeolite material or by application of mesoporous catalysts. The application of the mesoporous catalyst with the Si:Al ratio amounted to 93, allowed to receive p-cymene in 2 h at the temperature of 275 °C, with a selectivity of 75.3% and at the conversion of toluene 70.3% (the molar ratio of isopropanol to toluene amounted to 2:1).
From p-cymene p-cymenesulfonic acid can also be obtained; this has similar properties to the p-toluenesulfonic acid compound, which is used, for example, in Fisher’s esterification reaction. The raw material for p-toluenesulfonic acid synthesis is toxic toluene but a good alternative to this raw material could be p-cymenesulphonic acid, which can be synthesized without the use of any organic solvent. The synthesis of p-cymenesulphonic acid was carried out in two stages. The first stage relied on the obtaining of p-cymene from limonene; the second relied on sulfonation of the received p-cymene. This method allowed us to obtain p-cymenesulfonic acid at a yield of 91% [
5].
α-Terpinene is a natural and fragrant compound that is found in oranges, coriander, and oregano. Thanks to its refreshing fragrance, it is widely used in food products. α-Terpinene also found use as a fragrance compound in cosmetics and in household chemicals. Research carried out on Salmonella showed that this cyclic monoterpe does not exhibit mutagenic activity. α-Terpinene is also a component of tea tree oil and is responsible for this oil’s antioxidant properties, which allow it to be applied topically as an antimicrobial and anti-inflammatory agent, primarily used to treat skin complaints such as acne or mycosis. When α-terpinene is used as the additive for food, medicines, and cosmetics it also provides them with oxidative stability. Studies on α-terpinene demonstrated the effectiveness of this compound in the treatment of parasitic infection of Trypanosoma in horses. The thin plasma layer of α-terpinene is used to encapsulate solar cells, which protects them from atmospheric agents and moisture. Oxidation of α-terpinene, catalyzed by a photocatalyst supported on silica, leads to many valuable products such as ascaridole, isoascaridole, 4-isopropylbenzoic acid, and cuminaldehyde [
6,
7,
8,
9,
10].
γ-Terpinene is a monocyclic compound that demonstrates antimicrobial properties. It is synthesized by certain plant species, including rice, and causes the destruction of the cell membrane of the bacterium that causes disease in this plant as well as other plant species. γ-Terpinene also has the potential to be used in treatment of atherosclerosis. It is known that LDL cholesterol is readily oxidized by free radicals, resulting in atherosclerotic lesions in the blood vessels. γ-Terpinene, thanks to its antioxidant properties, prevents the oxidation of LDL, because it has the ability to catch free radicals. Animal studies showed a significant decrease in total cholesterol of 18.3% and triglycerides of 30.3% in the serum blood of tested animals. Moreover, γ-terpinene is one of the two components in pine oil that exhibit acaricidal properties; thanks to this, pine oil can be used against mites that occur during food storage [
11,
12,
13].
Terpinolene is used as a food additive, for example in baked products, ice cream, soft drinks, and candy. Moreover, terpinolene is a component in cleaning agents and deodorants. It is also used for the synthesis of terpineol, 1-methyl-4-isopropylidene, and cyclohexane-1-ol, and also in polymerization reactions that proceed in aqueous solution [
14]. Terpinolene also exhibits a calming effect. The studies on this compound showed that terpinolene stimulates a different part of the brain than limonene. Terpinolene inhalation affected the action of the nervous system and human psyche. As a consequence, terpinolene reduced tension and increased feelings of relaxation. Therefore, this compound could be used to treat mental disorders such as depression [
15]. Terpinolene is an intermediary compound in the biosynthesis of Hinocitol (4-isopropyltropolone) by cypress cells. This compound has antitumor, antimicrobial, and antifungal properties. Studies on cypress tree cells suggest the possibility of using this compound for the synthesis of tropolone derivatives [
16]. Terpinolene ozonized in the gaseous phase or treated with nitrate radicals is converted into a number of products. In the ozonolysis process it is converted to 4-methylcyclohex-3-en-1-one, 2-hydroxy-4-methylcyclohex-3-en-1-one, glyoxal, methylglyoxal, 3-oxobutanal, and 6-oxo-3-(propane-2-ylidene) heptanal, while in the presence of nitrate radicals it is converted to 2-hydroxy-4-methylcyclohex-3-en-1-one, glyoxal, methylglyoxal, and 4-oxopentanal [
17]. Terpinolene, like γ-terpinene, also has the potential to treat atherosclerosis. This compound, in combination with β-carotene and α-tocopherol, effectively prevents the oxidation of LDL [
18].
The scientific literature does not reveal much about the limonene isomerization process over heterogeneous catalysts.
In 1963 Hunter et al. carried out the isomerization and disproportionation of limonene over a silica gel. The process was performed for 20 min at the temperature of 100 °C, and obtained 20% terpinolene, 18% α-terpinene, 10% γ-terpinene, and small amounts of isoterpinolene, terpinolene, and p-cymene. At these conditions the conversion of limonene amounted to 65%. The studies showed that the isomerization occurred immediately, while disproportion occurred in a small degree. These reactions can be explained by assuming that the carbonium ions are adsorbed onto the surface of the catalyst. Turkevitch and Smith observed that during the conversion of 1-butene to 2-butene hydrogen transfer takes place in the presence of the silica gel. Formation of limonene isomerization products works in the same way. Limonene “pulls out” the proton from the surface of the catalyst, which leads to the formation of an 8-menthenyl carbonium ion. The loss of hydrogen by this carbonium ion leads to α-terpinene and γ-terpinene formation. The hydrogen transfer is reversible, which indicates the presence of small amounts of dipentene during the isomerization of terpinolene, isoterpinolene, α-terpinene, and γ-terpinene over the silica [
19].
Johnson used limonene anatase as the catalyst in the isomerization, prepared by the application of formic acid. This allowed him to obtain terpinolene with a selectivity of 70%, and the conversion of limonene amounted to 55%. This reaction was carried out at a temperature of 100 °C, for a reaction time of 4 h, under a nitrogen atmosphere and in the presence of a buffer (sodium acetate) [
20].
Tanabe et al. used another method for the isomerization of limonene. These studies showed that in the presence of zinc oxide impregnated with aqueous H
2SO
4 and used as the catalyst, terpinolene was obtained with a selectivity of 78% and the conversion of limonene amounted to 65%. The reaction was performed at the relatively low temperature of 60 °C [
14].
Dehydrogenation of limonene to p-cymene was also performed using a Pd/HZSM-5(258) catalyst, which contained silicon oxide and alumina (at the ratio of SiO
2/Al
2O
3 amounted to 258), and palladium in the amount of 1 wt %. The use of this catalyst allowed the researchers to obtain p-cymene with a selectivity of 83% and 100% conversion of limonene. The reaction was performed at a temperature of 260 °C for 2 h, in the presence of n-dodecane and a nitrogen atmosphere. The use of a catalyst with a different ratio of SiO
2/Al
2O
3—Pd/HZSM-5(5), caused the formation of a mixture of isomers (terpinenes and terpinolene) with a selectivity of 53%, and the conversion of limonene amounted to 91% [
21].
Catrinescu et al. also carried out studies on the limonene isomerization process. In these studies betionites derived from Serra de Dentro were used as the catalysts. The examinations showed that nickel-modified betionites were the most active among the tested catalysts. This method produced the following products: 20% terpinolene, 12% α-terpinen, 4% γ-terpinene, and 8% isoterpinolene. The studies were performed at a temperature of 150 °C for 25 min and in the presence of n-dodecane. The obtained results showed that the transformation of limonene to p-cymene takes place via limonene isomers, as shown in
Figure 1 [
22].
Isomerization of limonene was also carried out with ferrierite H-FER (T) as the catalyst (hydrogen forms of ferriarite z Tosoh, in which the Si/Al ratio amounted to 8.9). As a result of isomerization performed at the temperature of 65 °C, a mixture of the following products was obtained: α-terpinene, γ-terpinene, terpinolene, and p-cymene. At this temperature the conversion of limonene achieved 38% after the reaction time of 60 min [
23].
The catalytic conversion of limonene to p-cymene was also carried out by Martin-Luengo et al. This group used sepiolite as the catalyst. Sepiolite is a clay (hydrated magnesium silicate) of natural origin, cheap and easily obtainable in Madrid. In this material the magnesium layer is located between two layers of silicon oxide. Sepiolite consists mainly of magnesium, but some ions of magnesium and silicon are replaced by aluminum ions. In this method of isomerization microwave heating was used and obtained products in a short amount of time. After 5 min of isomerization at a temperature of 210 °C, the amounts of product were as follows: 28% of α-terpinene, 20% of γ-terpinene, and 27% of α-terpinolene. The same catalyst, modified with nickel, obtained p-cymene with a selectivity of 100% and 100% conversion of limonene, after 20 min of reaction [
24]. This reaction was carried out without the use of any solvents. Another achievement of this group was the use in the limonene isomerization of silica–alumina catalysts with different silica content—from 1% (SIRAL 1) to 40% (SIRAL 40). The studies showed that with some catalysts (SIRAL 20 or SIRAL 40) it was possible to obtain p-cymene with a selectivity of 100% and the complete conversion of limonene. The use of a catalyst in which the silica content amounted to 1% caused formation of α-terpinene with a selectivity of 85% and α-terpinolene with a selectivity of 15% after 5 min of reaction, but the conversion of limonene amounted to 15% [
25].
In this work, we present important studies on the isomerization of limonene over one of the latest titanium silicate mesoporous Ti-SBA-15 catalysts. The choice of this heterogeneous catalyst for our research was dictated by the heterogeneous catalyst properties and the advantages of their use, which are broadly described in the scientific literature. Homogeneous catalysts dissolve in reaction and their active catalytic sites are more readily accessible, which allows a reaction to proceed in mild conditions and with good selectivity of the appropriate product. However, these catalysts have limited application due to problems in separating products contaminated by unstable residuals and recycling expensive catalysts. The removal of residual catalytic species from the reaction mixture always requires the use of appropriate techniques, such as chromatography, distillation, or extraction. Heterogeneous catalysts, especially metals containing mesoporous silica catalysts, are more durable and reusable catalysts than homogeneous catalysts. In addition, these catalysts have a large surface area and sufficient inner space in the pores, which allows the reactants to access the catalytic sites and release catalytic products from pores. Mesoporous silica catalysts can be obtained in soft-templating processes, in which self-assembly of organic molecules (surfactants and block copolymers) is used to create pores of controlled sizes. This procedure forms an inorganic network around the templates via a sol-gel process [
26,
27,
28,
29,
30].
Interest in mesoporous materials increased with the discovery of the mesoporous MCM-41 material, which has a large surface area and a uniform and well-defined structure [
31]. In 1998, as a result of the substitution of the usually used surfactants with block copolymers, highly ordered mesoporous materials labeled with the acronym SBA were obtained. The SBA-15 material is similar to the MCM-41 material because it has a hexagonal structure. However, the undoubted advantage of this material is the presence, apart from the hexagonally arranged mesopores, of significantly smaller pores that account for more than 70% of the surface area of this material. These micropores are located in the walls of this type of material and are a combination of ordered main pores [
32]. The advantage of SBA-15 is not only the unique construction of this material and better stability of its structure but also the possibility of a safe application of this material because it does not exhibit toxicity. Also, the synthesis of this material is ecologically friendly in comparison to the MCM-41 material. Our earlier studies related to MCM-41 and SBA-15 materials but containing titanium in the structure (Ti-MCM-41 and Ti-SBA-15) have shown that these materials can be very active catalysts in oxidation (also epoxidation) and isomerization processes. During these studies, the Ti-SBA-15 material showed better stability and better resistance to titanium leaching; thus, for the studies presented in this work, the Ti-SBA-15 catalyst was chosen [
33,
34].
The aim of this work was a preliminary study of the possibility of performing the isomerization of limonene over the Ti-SBA-15 catalyst and establishing the ways of transformation of limonene over this catalyst. These studies have not been described in the literature data but, taking into account the properties of the Ti-SBA-15 catalyst, this material can have good catalytic properties in this process. The main parameters that have an influence on the course of isomerization are: the reaction time, temperature, and content of the catalyst. The isomerization was performed without any solvent, so the proposed isomerization method is environmentally friendly. The choice of these parameters allowed us to establish the best conditions for performing limonene isomerization in search of the most desirable products: terpinolene, α-terpinene, γ-terpinene, and p-cymene. Research on this process should be developed in the future, taking into account our preliminary studies presented in this work and the numerous applications of the products of limonene isomerization.
2. Results and Discussion
The performed research allowed us to obtain terpinolene, α-terpinene, γ-terpinene, and p-cymene by the process of limonene isomerization and over the Ti-SBA-15 catalyst. The reactions that proceeded during the studied process are presented in
Figure 2.
The conversion of limonene was monitored during the process. It was carried out with appropriate values of process parameters such as reaction time, temperature, and catalyst content (
Table 1). During the studies changes in the yields of the appropriate products were also observed (
Table 2).
Because the isomerization of limonene did not occur when the content of the catalyst amounted to 5 wt % at the temperatures of 140 and 150 °C,
Table 1 and
Table 2 do not present results for these conditions.
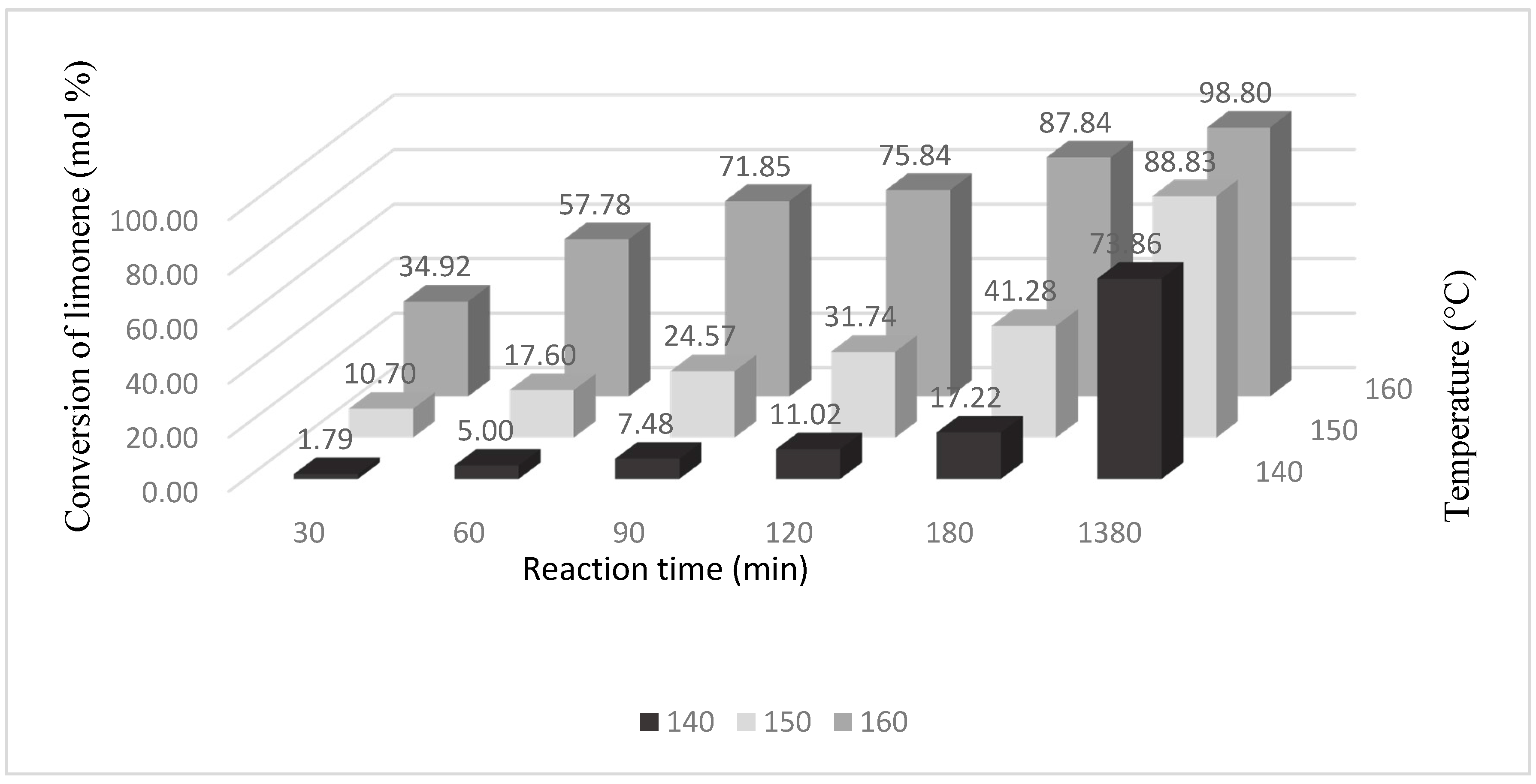
Table 1 shows that for the catalyst content of 5 wt % and temperature of 160 °C the conversion of limonene rose during prolongation of the reaction time from 3.13 mol % (reaction time: 30 min) to 86.31 mol % (1380 min). For a catalyst content of 10 wt % an increase in the conversion of limonene with the increase of temperature and prolongation of the reaction time was also observed. The highest values of limonene conversion were observed for a temperature of 160 °C: at this temperature the conversion of limonene rose from 9.25 mol % to 96.41 mol %. The highest values of limonene conversion were observed for a catalyst content of 15 wt %. At the highest studied temperature (160 °C), the values of limonene conversion changed from 34.92 mol % to 98.80 mol %. These results show that an increase in the amount of the catalyst (at the same time as an increase in the number of active sites with Ti) caused an increase in the conversion of limonene because more limonene molecules undergo transformations in active centers of Ti. At the same time, large pores of the Ti-SBA-15 catalyst facilitate the access of organic particles to active centers of Ti and, moreover, the increase in temperature accelerates the diffusion of these molecules into the pores.
Table 2 presents the changes in values of yields of main products of limonene isomerization depending on the reaction time, temperature, and catalyst content. Also, for a better interpretation the obtained results, in
Figure 3,
Figure 4,
Figure 5,
Figure 6,
Figure 7 and
Figure 8 the changes in values of yields of products and conversion of limonene depending on the appropriate parameters are presented.
Table 2 shows that all the tested parameters had an influence on the yields of the main products. In all performed syntheses, the main products were: α-terpinene, γ-terpinene, terpinolene, and p-cymene. An increase in the amount of the catalyst caused an increase in the yields of α-terpinene, γ-terpinene, and terpinolene in a shorter time, and faster transformation of these limonene isomers to p-cymene. These results are similar to the results presented in the literature data, taking into account the products formed during the isomerization of limonene and a possible follow-up reaction in which p-cymene is formed. This allows us to assume that this reaction follows the mechanism outlined in the introduction to this article on the basis of the literature data [
22].
Figure 3 shows that the yields of isomers of limonene in the reaction mixture (for the isomerization performed at a temperature of 160 °C and the amount of the catalyst 15 wt %) decrease significantly for a reaction in the range of 180–1380 min, and simultaneously the yield of p-cymene increases. This demonstrates the validity of the aforementioned reaction mechanism, according to which the first limonene isomers are formed and are subsequently dehydrogenated to p-cymene. Reducing the yields of the isomerization products and simultaneously increasing the p-cymene yield may be attributed to the fact that isomerization products remain in the pores of the catalyst and do not diffuse outside the pores of the catalyst. In this way, the limonene isomerization products facilitate access to the active centers of the Ti-SBA-15 catalyst and undergo further transformation, at the same time blocking the access of limonene molecules to active centers.
The best results were obtained for the catalyst Ti-SBA-15 content of 15 wt % and at the temperature of 160 °C. Under these conditions, the conversion of limonene was close to 90 mol % after a reaction time of 180 min and after a reaction time of 1380 min almost all limonene underwent transformation. At a lower process temperature (150 °C), limonene conversion after the reaction time of 1380 min was below 90 mol % (88.83 mol %), the same as for 140 °C, at which the conversion amounted to 73.86 mol % (
Figure 4). The results show that at the highest content of the catalyst the number of active centers in the catalyst is enough for transformation not only of limonene molecules but also of the products of limonene isomerization. Simultaneously, the increase in temperature at the highest content of the catalyst accelerates these reactions. Probably, acceleration of the temperature influences the limonene diffusion rate and also the rate of binding of organic molecules to the active centers, and the durability of formed active derivatives.
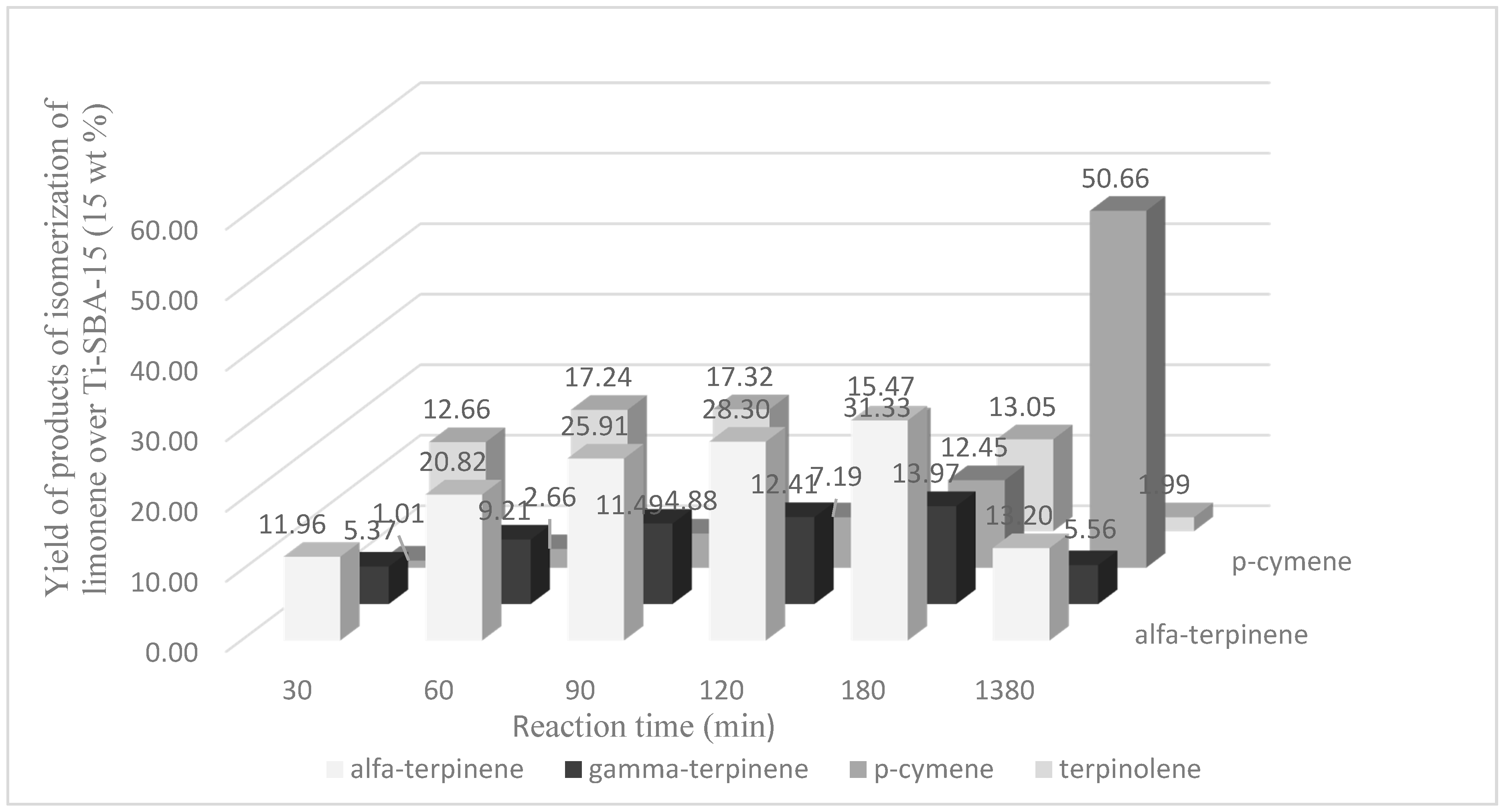
The yield of terpinolene after a reaction time of 60 min (catalyst content 15 wt %, temperature 160 °C) achieved a value of 17.24 mol % and limonene conversion of 57.78 mol %; after the reaction time of 90 min the value of the yield of this compound was also high and amounted to 17.32 mol % and a limonene conversion of 71.85 mol % (
Figure 5).
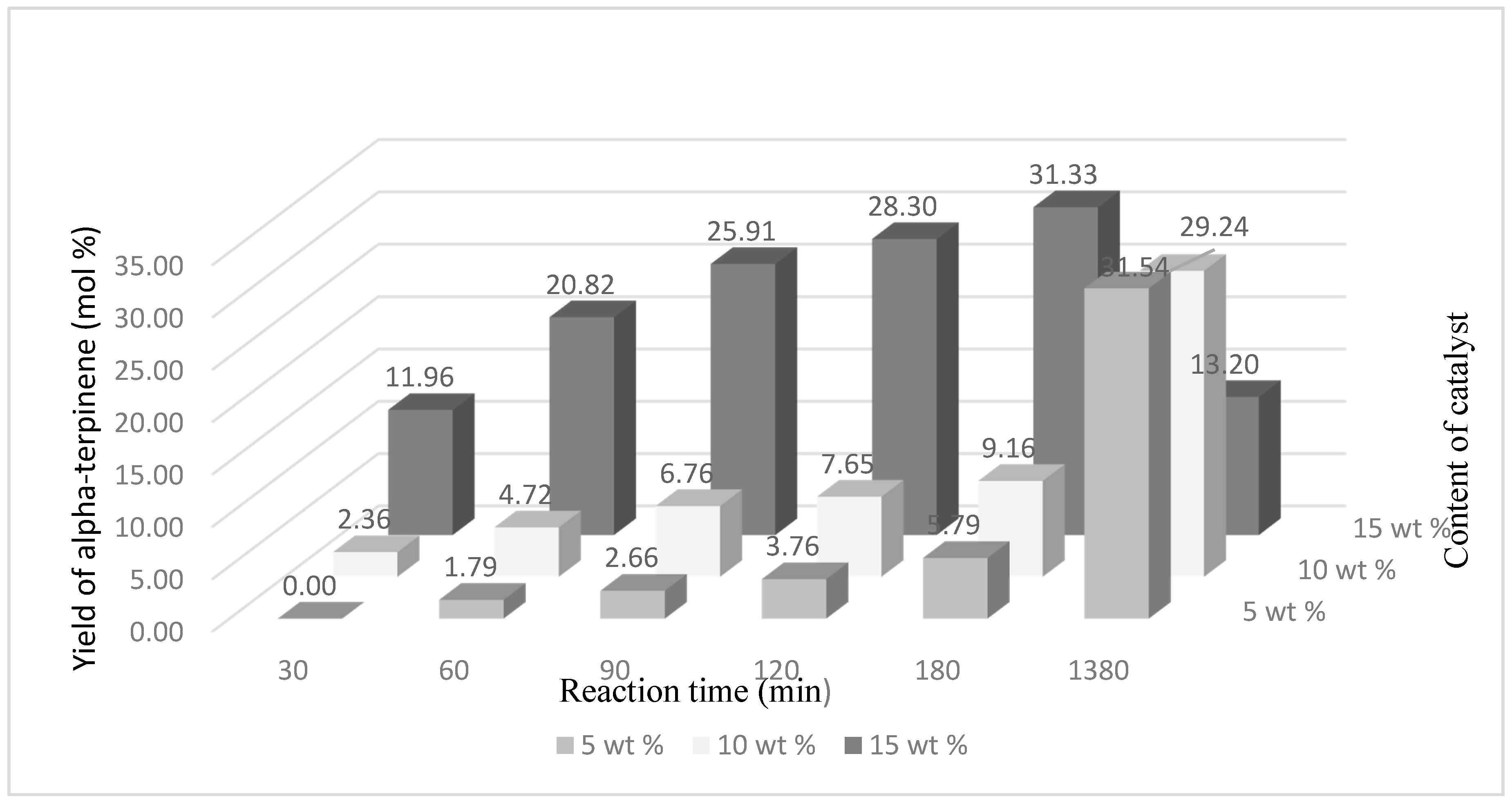
The highest yield of α-terpinene (31.33 mol %) was obtained for the amount of the catalyst of 15 wt % (temperature 160 °C) and after the reaction time amounted to 180 min (
Figure 6). A similar yield of this compound was also obtained after 1380 min but for smaller amounts of the catalyst—5 wt % and 10 wt %, respectively: 31.54 mol % and 29.24 mol %.
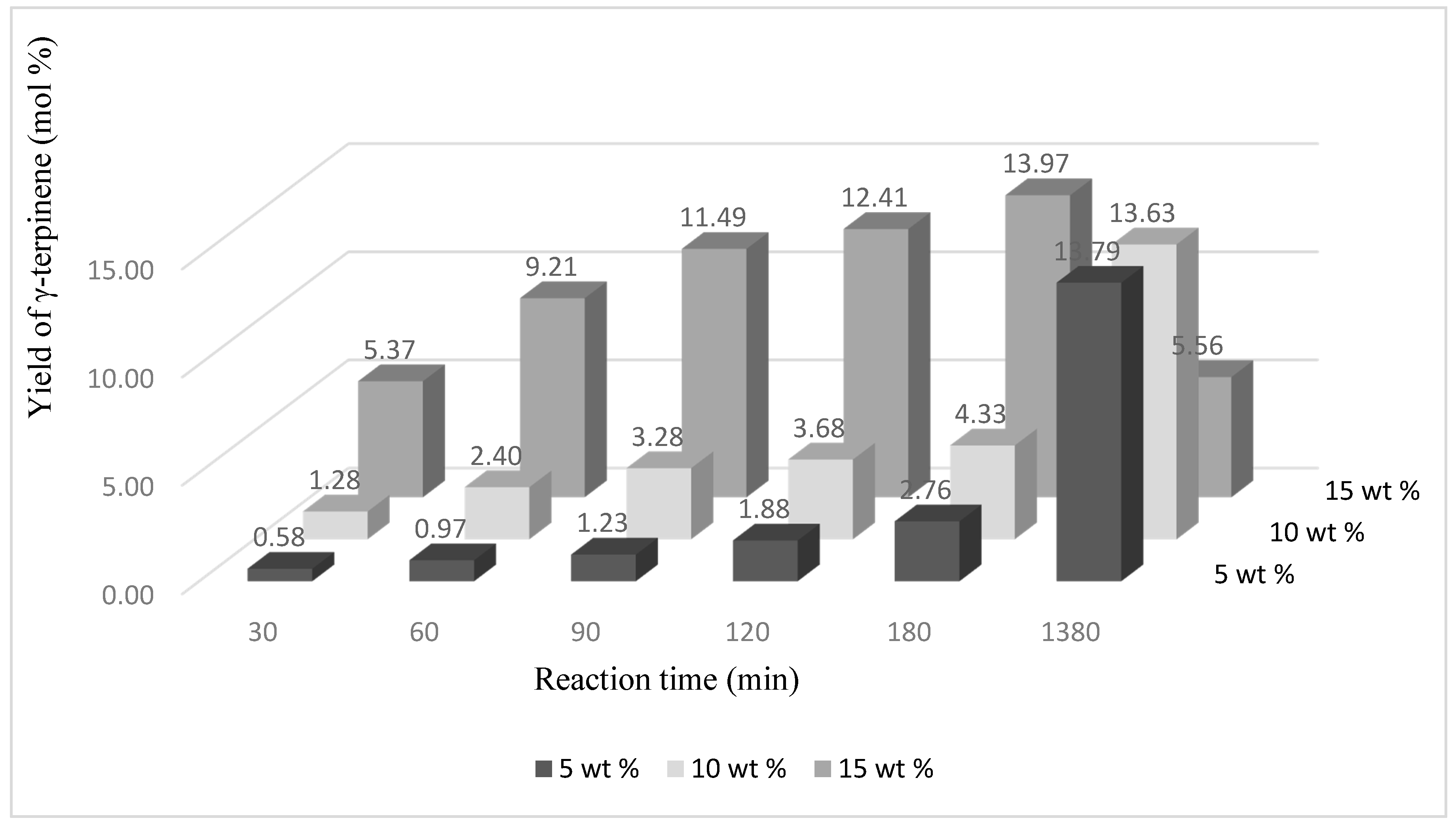
After the reaction time of 180 min the yield of γ-terpinene was also the highest, amounting to 13.97 mol % (
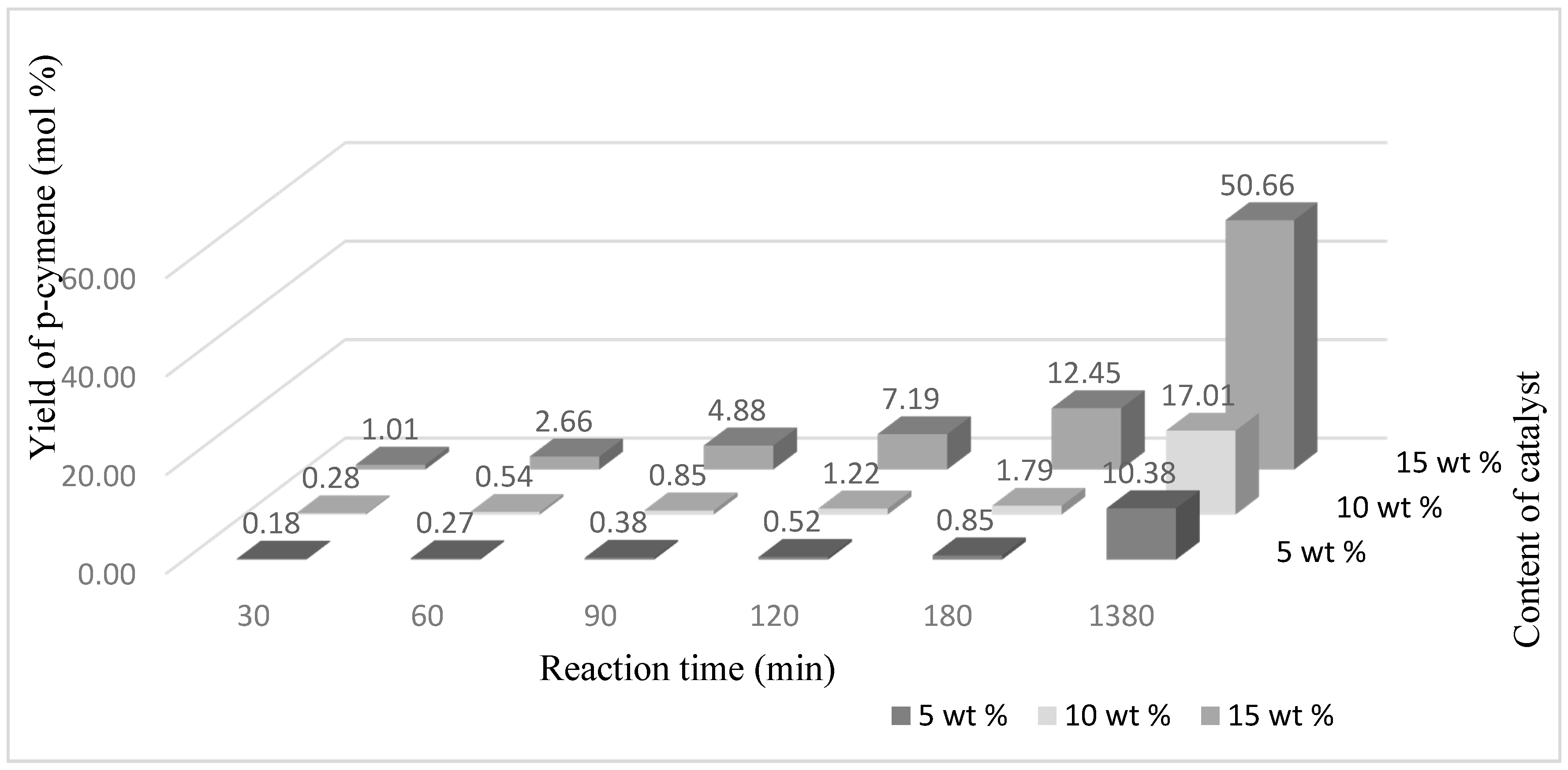
Figure 7). A very similar yield value of this compound is obtained for the catalyst content 5 wt % and 10 wt % but for a reaction time of 1380 min. Longer reaction times (for catalyst content 15 wt %) promoted formation of p-cymene. The highest yield of this compound was obtained after 1380 min and amounted to 50.66 mol % (
Figure 8).
A comparison of the yields of products depending on the conditions in which the performed were obtained shows that of all the isomers of limonene the highest yields of α-terpinene were obtained. γ-Terpinene and terpinolene yields were usually lower than α-terpinene. This indicates that α-terpinene was the preferred product for a reaction time of 180 min, and prolongation of the reaction time caused a transformation of the products of limonene isomerization to p-cymene.
3. Experimental
3.1. Raw Materials
For the synthesis of Ti-SBA-15 catalyst and the isomerization of limonene, the following raw materials were used: R-(+)-limonene (>93%, Sigma, St. Louis, MO, USA), hydrochloric acid (HCl, 37%, Hartim, Tyrol Austria), Pluronic P123 (Aldrich, MW = 5800, St. Louis, MO, USA) as a template, tetraethyl o-silicate (TEOS, 98%, Aldrich, St. Louis, MO, USA) as a silica source, and tetraisopropyl o-titanate (TiPOT, >98%, Merc, Schuchardt OHG Hohennbrunn) as a titanium source.
3.2. The Synthesis of the Ti-SBA-15 Catalyst
The Ti-SBA-15 catalyst was synthesized by the direct method described by Berube et al. [
35]. In this synthesis of the Ti-SBA-15, a Pluronic P123 triblock copolymer was used as the structure-directing agent, whereas the source of silica was o-tetraethyl silicate (TEOS). Ti-SBA-15 was obtained by the dissolving of the triblock copolymer in 37% hydrochloric acid at a temperature of 35 °C (with constant stirring). A silica source and tertiary isopropyl o-titanate were then added (the Si/Ti molar ratio amounted to 40). The receiving mixture was stirred for 24 h, maintaining a temperature of 35 °C. After this time, the reaction mixture was transferred to an autoclave, where it was kept for another 24 h at a temperature of 35 °C. The resulting precipitate was filtered off and then washed with deionized water and methanol on a filter. After drying at a temperature of 100 °C for 24 h, the product was calcined for 5 h at 550 °C. As a result, Ti-SBA-15 catalyst containing 2.5 wt % of titanium was obtained. A detailed description of this catalyst was presented in our previous work [
36]. Additionally, for the obtained Ti-SBA-15 catalyst, ICP-AES analysis was performed and textural properties were also determined. For ICP-AES analysis, samples were dissolved by applying a four step procedure utilizing H
2SO
4, HF, HNO
3, and H
3PO
4 in sequence. The mixtures were treated by acids in a microwave oven (Milestone MLS 1200 Mega, Milestone, Sorisole, Italy) with a power of 150–650 W (20 min for each step). ICP-AES analysis showed that the content of Ti in the catalysts amounted to 2.53 wt %. Textural properties were determined on the basis of nitrogen sorption at −196 °C (QUADRASORB evoTM Gas Sorption Surface Area and Pore Size Analyzer, Quantachrome Instruments, Boynton Beach, FL, USA). Before sorption measurements, all samples were outgassed at 250 °C for at least 20 h. The specific surface area (S
BET) was calculated on the basis of the Brunauer–Emmett–Teller (S
BET) equation and the multi-point method. The relative pressure range was selected on the basis of the linear section of the BET plot, i.e., in the relative pressure range (0.05–0.2). Pore volume distribution, micropore volume (V
micro), and mean pore diameter (d
m) were calculated by the DTF method. The total pore volume (V
tot) was obtained from the nitrogen volume, adsorbed at a relative pressure of 0.98. The studies on textural properties of the Ti-SBA-15 catalyst showed that this catalyst is characterized by the following textural properties: S
BET = 731 m
2/g, V
tot = 0.585 cm
3/g, V
micro = 0.091 cm
3/g, and d
m = 5.32 nm.
3.3. The Method of the Isomerization of Limonene over the Ti-SBA-15 Catalyst
The studies on the influence of reaction time, temperature, and Ti-SBA-15 catalyst content on the course of limonene isomerization were carried out in a glass reactor with a capacity of 25 cm3. The reactor was equipped with a reflux condenser and a magnetic stirrer with a heating function. In the glass reactor were introduced: 5.00 g of limonene and 0.25 g, 0.5 g, or 0.75 g of the catalyst. The reactor was placed in the paw and then immersed in an oil bath before stirring was started (500 rpm). Limonene isomerization was tested at the temperatures of 160 °C, 150 °C, and 140 °C. Samples of the reaction mixture for analysis were taken at the following intervals of time: 30 min, 60 min, 90 min, 120 min, 180 min, and 1380 min. To separate the catalyst from the sample, the reaction mixture was centrifuged in a centrifuge.
3.4. The Identification Products by the Gas Chromatography Method
The qualitative and quantitative analyses of the post-reaction mixtures were made by a gas chromatography method (GC), for which the FOCUS apparatus from Thermo (Waltham, MA, USA) was used. This apparatus is equipped with a flame-ionization detector (FID), a capillary column ZEBRON ZB-WAXplus type (0.32 mm × 30 m × 0.5 μm, filled with polyethylene glycol), and an autosampler. The parameters of the analyses were as follows: helium pressure 60 kPa, temperature of the sample chamber 240 °C, detector temperature 250 °C, ad thermostat temperature raised according to the following program: isothermally to 60 °C for 2 min, increase of temperature at a rate of 10 °C/min to 240 °C, isothermally to 240 °C for 4 min, and finally cooling to 60 °C. The samples for GC analysis were prepared in this way: first, the catalyst was separated from the post-reaction mixture with the help of a centrifuge; next, 0.250 g of the obtained solution was diluted with 0.750 g of acetone. The quantitative analyses were made with help of the external standard method.