S- and N-Doped Graphene Nanomaterials for the Oxygen Reduction Reaction
Abstract
:1. Introduction
2. Results and Discussion
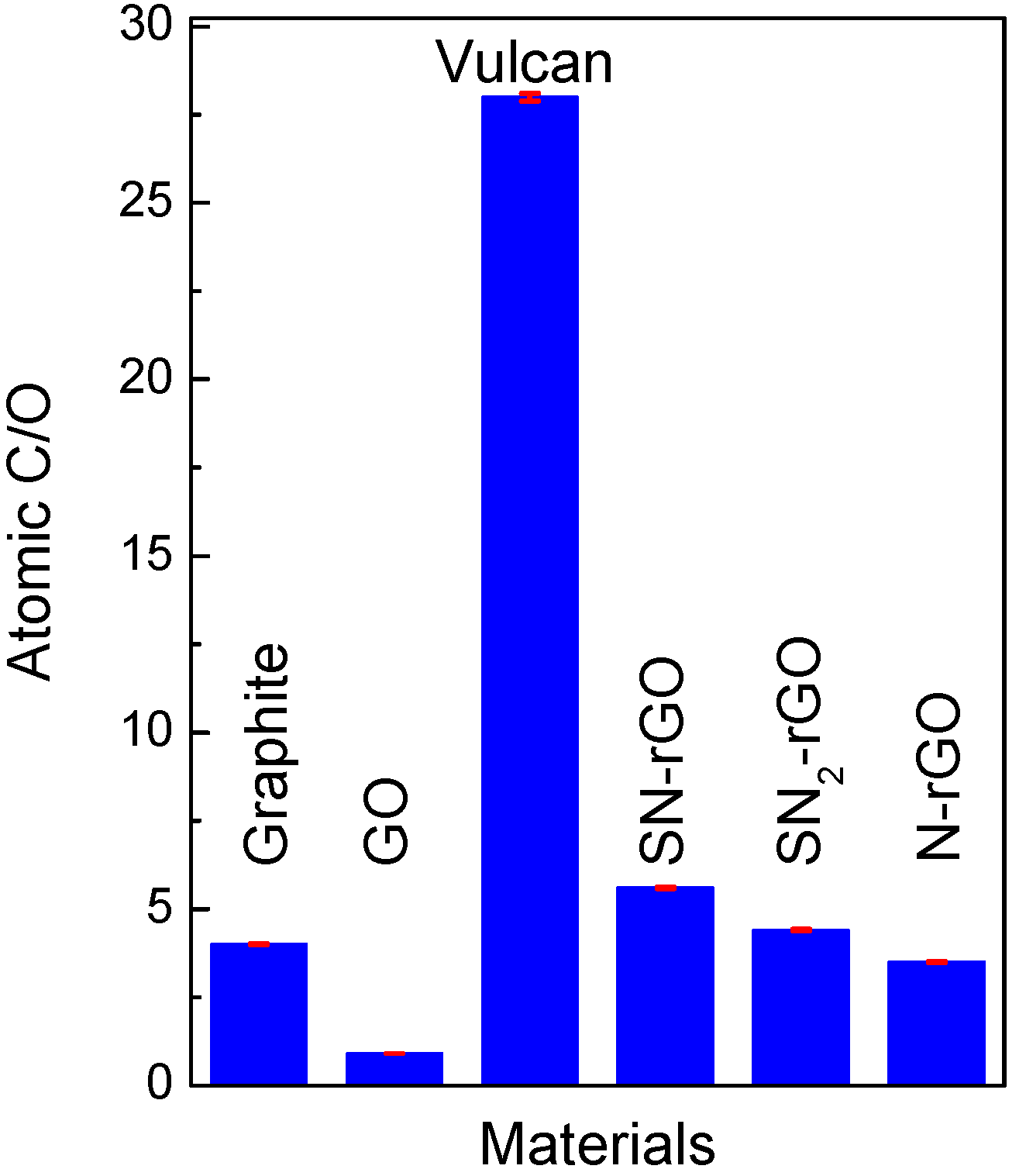
2.1. Physicochemical Characterization
2.2. Electrochemical Characterization
- (A)
- Associative mechanism:O2 → O2(ads)O2 + H2O + e− → OOH(ads) + OH−OOH(ads) + e− → O(ads) + OH−OOH(ads) + e− → OOH−O(ads) + H2O + e− → OH(ads) + OH−OH(ads) + e− → OH−
- (B)
- Dissociative mechanism:½O2 → O(ads)O(ads) + H2O + e− → OH(ads) + OH−OH(ads) + e− → OH−
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Graphene Oxide
3.3. Synthesis of Reduced Graphene Oxide Materials
3.4. Physicochemical Characterization
3.5. Electrochemical Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gottesfeld, S. Electrocatalysis of Oxygen Reduction in Polymer Electrolyte Fuel Cells: A Brief History and a Critical Examination of Present Theory and Diagnostics. In Book Fuel Cell Catalysis a Surface Science Approach; Marc, T., Koper, M., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 1–30. [Google Scholar]
- Wang, X.; Sun, G.; Routh, P.; Kim, D.-H.; Huang, W.; Chen, P. Heteroatom-doped graphene materials: Syntheses, properties and applications. Chem. Soc. Rev. 2014, 43, 7067–7098. [Google Scholar] [CrossRef] [PubMed]
- Le, T.X.H.; Esmilaire, R.; Drobek, M.; Bechelany, M.; Vallicari, C.; Nguyen, D.-L.; Julbe, A.; Tingrya, S.; Cretin, M. Design of a novel fuel cell-Fenton system: A smart approach to zero energy depollution. J. Mater. Chem. A 2016, 4, 17686–17693. [Google Scholar] [CrossRef]
- Le, T.X.H.; Esmilaire, R.; Drobek, M.; Bechelany, M.; Vallicari, C.; Cerneaux, S.; Julbe, A.; Cretin, M. Nitrogen-Doped Graphitized Carbon Electrodes for Biorefractory Pollutants Removal. J. Phys. Chem. C 2017, 121, 15188–15197. [Google Scholar] [CrossRef]
- Pei, S.-F.; Cheng, H.-M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-W.; Su, D. Heterogeneous nanocarbon materials for oxygen reduction reaction. Energy Environ. Sci. 2014, 7, 576–591. [Google Scholar] [CrossRef]
- Park, M.; Lee, T.; Kim, B.S. Covalent functionalization based heteroatom doped graphene nanosheet as a metal-free electrocatalyst for oxygen reduction reaction. Nanoscale 2013, 5, 12255–12260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheng, Z.H.; Gao, H.L.; Bao, W.J.; Wang, F.B.; Xia, X.H. Synthesis of boron doped graphene for oxygen reduction reaction in fuel cells. J. Mater. Chem. 2012, 22, 390–395. [Google Scholar] [CrossRef]
- Zhu, C.; Dong, S. Recent progress in graphene-based nanomaterials as advanced electrocatalysts towards oxygen reduction reaction. Nanoscale 2013, 5, 1753–1767. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Nie, H.; Chen, X.; Chen, X.; Huang, S. Recent progress in doped carbon nanomaterials as effective cathode catalysts for fuel cell oxygen reduction reaction. J. Power Sources 2013, 236, 238–249. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, Y.; Zhuang, X.; Li, S.; Zhang, F.; Feng, X. Low-temperature synthesis of nitrogen/sulfur CO-doped three-dimensional graphene frameworks as efficient metal-free electrocatalyst for oxygen reduction reaction. Carbon 2013, 62, 296–301. [Google Scholar] [CrossRef]
- Kicinski, W.; Szala, M.; Bystrzejewski, M. Sulfur-doped porous carbons: Synthesis and applications. Sulfur-doped porous carbons: Synthesis and applications. Carbon 2014, 68, 1–32. [Google Scholar] [CrossRef]
- Saner, B.; Dinç, F.; Yürüm, Y. Utilization of multiple graphene nanosheets in fuel cells: 2. The effect of oxidation process on the characteristics of graphene nanosheets. Fuel 2011, 90, 2609–2616. [Google Scholar] [CrossRef]
- Chen, C.-H.; Chung, T.-Y.; Shen, C.-C.; Yu, M.-S.; Tsao, C.-S.; Shi, G.-N.; Huang, C.-C.; Ger, M.-D.; Lee, W.-L. Hydrogen storage performance in palladium-doped graphene/carbon composites. Int. J. Hydrogen Energy 2013, 38, 3681–3688. [Google Scholar] [CrossRef]
- Botas, C.; Álvarez, P.; Blanco, C.; Santamaría, R.; Granda, M.; Gutiérrez, M.D.; Rodríguez-Reinoso, F.; Menéndez, R. Critical temperatures in the synthesis of graphene-like materials by thermal exfoliation—Reduction of graphite oxide. Carbon 2013, 52, 476–485. [Google Scholar] [CrossRef] [Green Version]
- Jin, H.; Huang, H.; He, Y.; Feng, Y.-X.; Wang, S.; Dai, L.; Wang, J. Graphene Quantum Dots Supported by Graphene Nanoribbons with Ultrahigh Electrocatalytic Performance for Oxygen Reduction. J. Am. Chem. Soc. 2015, 137, 7588–7591. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yan, L.; Bangal, P.R. Chemical Reduction of Graphene Oxide to Graphene by Sulfur-Containing Compounds. J. Phys. Chem. C 2010, 114, 19885–19890. [Google Scholar] [CrossRef]
- Poh, H.-L.; Simek, P.; Sofer, Z.; Pumera, M. Sulfur-Doped Graphene via Thermal Exfoliation of Graphite Oxide in H2S, SO2, or CS2 Gas. ACS Nano 2013, 7, 5262–5272. [Google Scholar] [CrossRef] [PubMed]
- Brownson, D.A.C.; Kampouris, D.K.; Banks, C.E. Graphene electrochemistry: Fundamental concepts through to prominent applications. Chem. Soc. Rev. 2012, 41, 6944–6976. [Google Scholar] [CrossRef] [PubMed]
- Cançado, L.G.; Takai, K.; Enoki, T.; Endo, M.; Kim, Y.A.; Mizusaki, H.; Coelho, L.N.; Magalhães-Paniago, R.; Pimenta, M.A. General equation for the determination of the crystallite size La of nanographite by Raman spectroscopy. Appl. Phys. Lett. 2006, 88, 163106. [Google Scholar] [CrossRef]
- Zickler, G.A.; Smarsly, B.; Gierlinger, N.; Peterlik, H.; Paris, O. A reconsideration of the relationship between the crystallite size La of carbons determined by X-ray diffraction and Raman spectroscopy. Carbon 2006, 44, 3239–3246. [Google Scholar] [CrossRef]
- Koskinen, P.; Malola, S.; Häkkinen, H. Evidence for graphene edges beyond zigzag and armchair. Phys. Rev. B 2009, 80, 073401. [Google Scholar] [CrossRef]
- Shin, D.; Jeong, B.; Choun, M.; Ocon, J.D.; Lee, J. Diagnosis of the measurement inconsistencies of carbon-based electrocatalysts for the oxygen reduction reaction in alkaline media. RSC Adv. 2015, 5, 1571–1580. [Google Scholar] [CrossRef]
- Ambrosi, A.; Chua, C.K.C.; Latiff, N.M.; Loo, A.; Wong, C.H.; Eng, A.Y.S.; Bonanni, A.; Pumera, M. Graphene and its electrochemistry—An update. Chem. Soc. Rev. 2016, 45, 2458–2493. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Nabae, Y.; Hayakawa, T.; Kakimoto, M.-A. Highly Selective Two-Electron Oxygen Reduction Catalyzed by Mesoporous Nitrogen-Doped Carbon. ACS Catal. 2014, 4, 3749–3754. [Google Scholar] [CrossRef]
- Ikeda, T.; Hou, Z.; Chai, G.-L.; Terakura, K. Possible Oxygen Reduction Reactions for Graphene Edges from First Principles. J. Phys. Chem. C 2014, 118, 17616–17625. [Google Scholar] [CrossRef]
- Fellinger, T.-P.; Hasché, F.; Strasser, P.; Antonietti, M. Mesoporous Nitrogen-Doped Carbon for the Electrocatalytic Synthesis of Hydrogen Peroxide. J. Am. Chem. Soc. 2012, 134, 4072–4075. [Google Scholar] [CrossRef] [PubMed]
- Chai, G.; Hou, Z.; Ikeda, T.; Terakura, K. Two-Electron Oxygen Reduction on Carbon Materials Catalysts: Mechanisms and Active Sites. J. Phys. Chem. C 2017, 121, 14524–14533. [Google Scholar] [CrossRef]
- Bag, S.; Mondal, B.; Das, A.K.; Raj, C.R. Nitrogen and Sulfur Dual-Doped Reduced Graphene Oxide: Synergistic Effect of Dopants towards Oxygen Reduction Reaction. Electrochim. Acta 2015, 163, 16–23. [Google Scholar] [CrossRef]
- Yu, L.; Pan, X.; Cao, X.; Hu, P.; Bao, X. Oxygen reduction reaction mechanism on nitrogen-doped graphene: A density functional theory study. J. Catal. 2011, 282, 183–190. [Google Scholar] [CrossRef]
- Florez-Montano, J.; Calderon-Cardenas, A.; Lizcano-Valbuena, W.; Rodríguez, J.L.; Pastor, E. Ni@Pt nanodisks with low Pt content supported on reduced graphene oxide for methanol electrooxidation in alkaline media. Int. J. Hydrogen Energy 2016, 41, 19799–19809. [Google Scholar] [CrossRef]
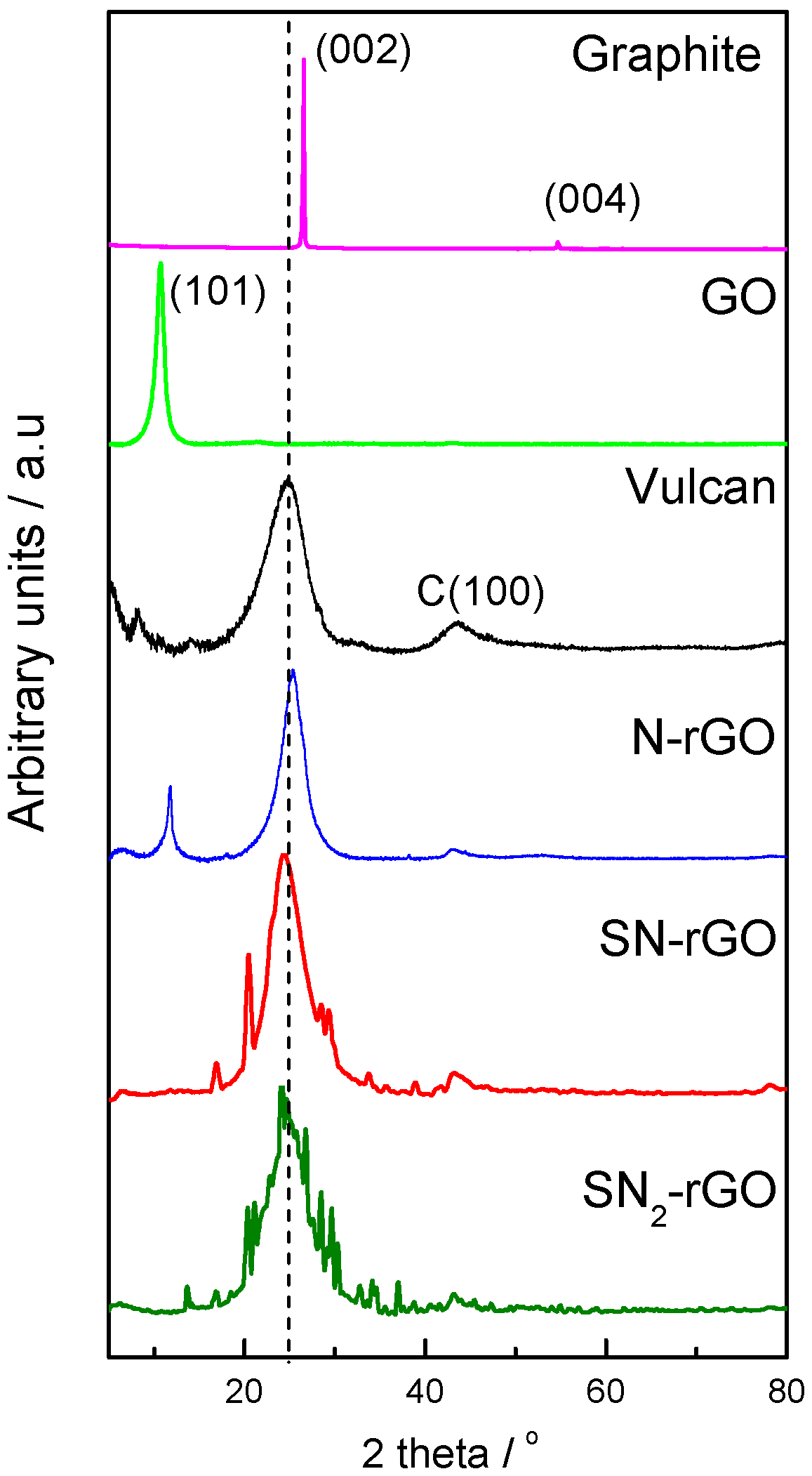
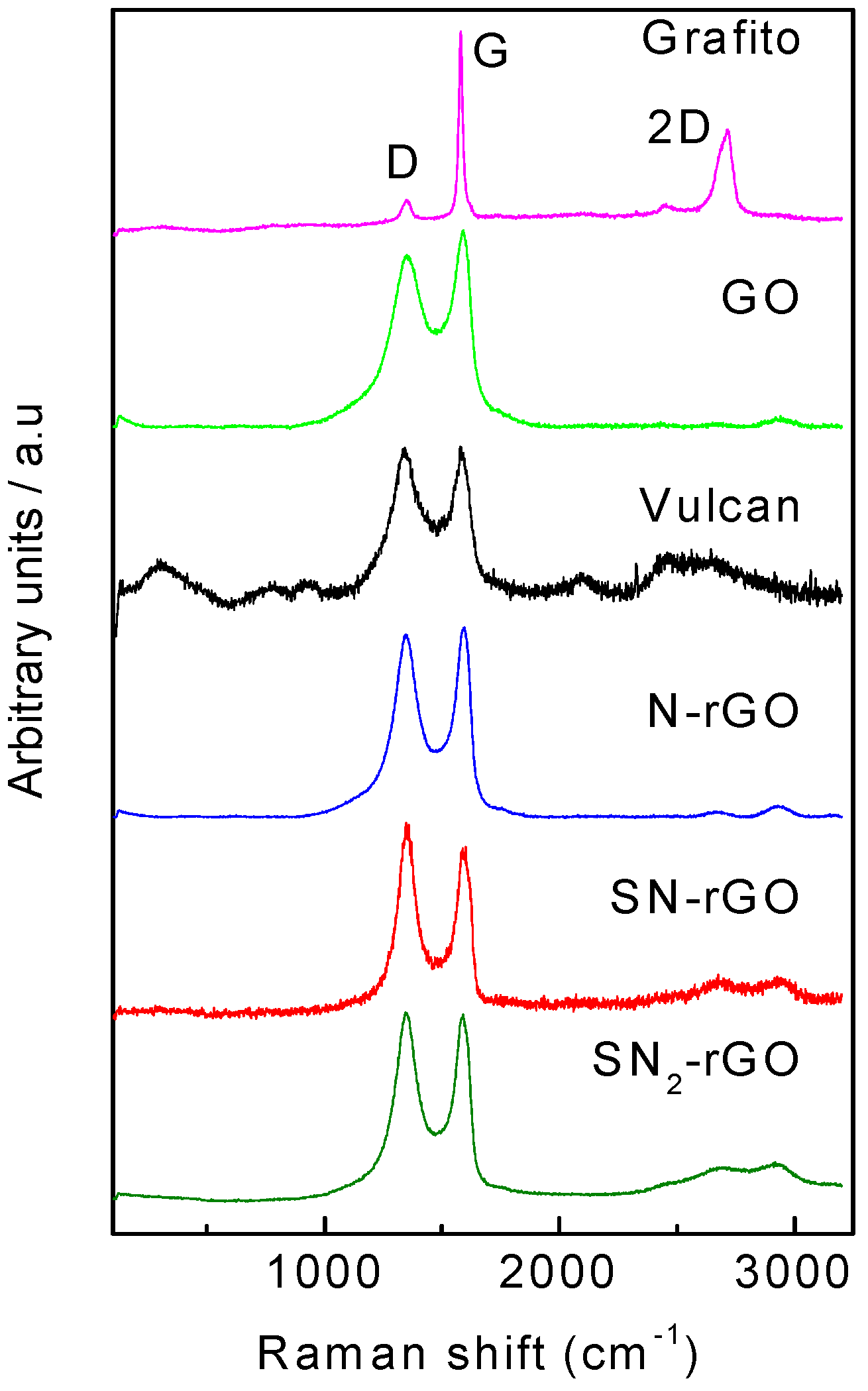
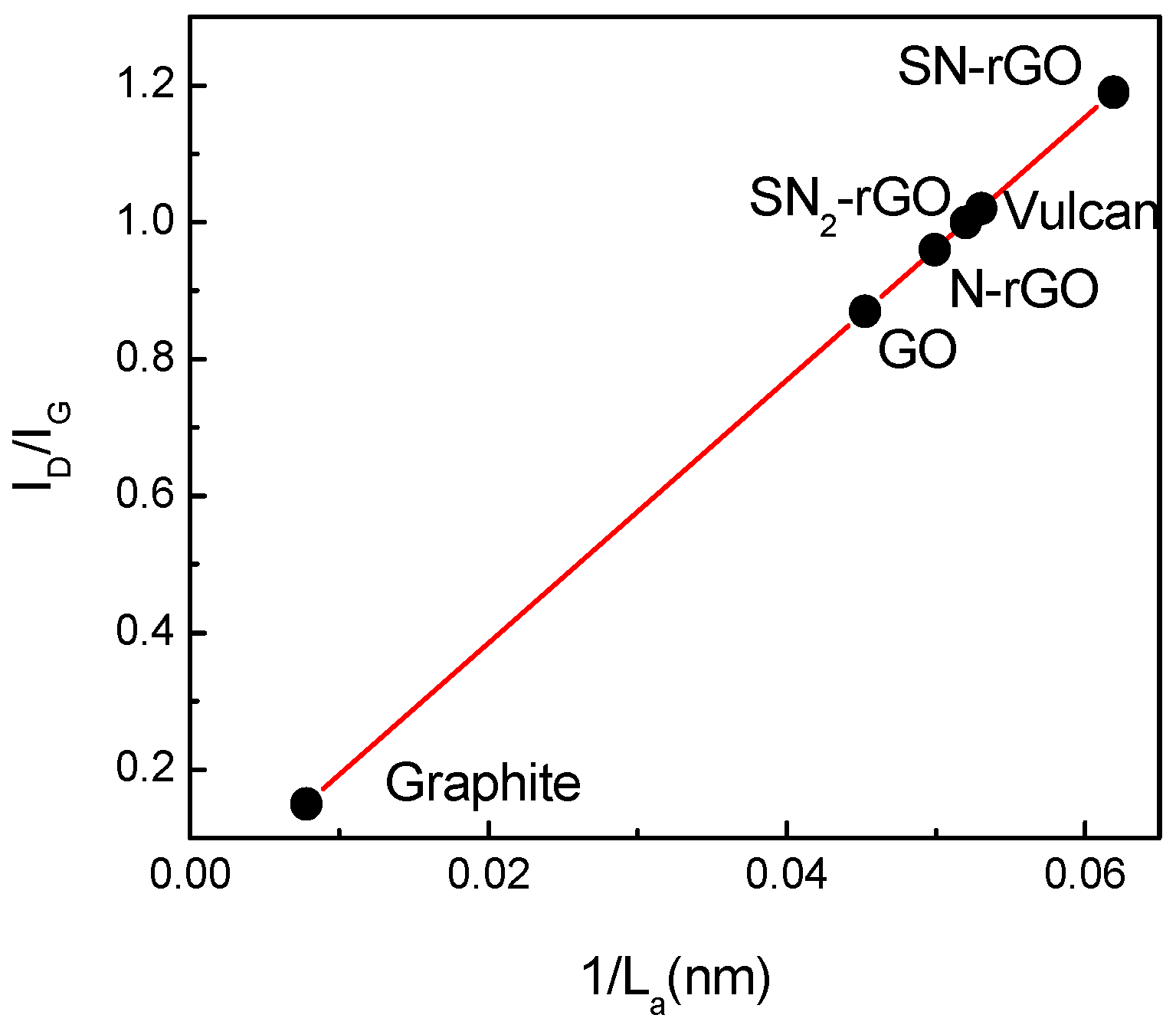
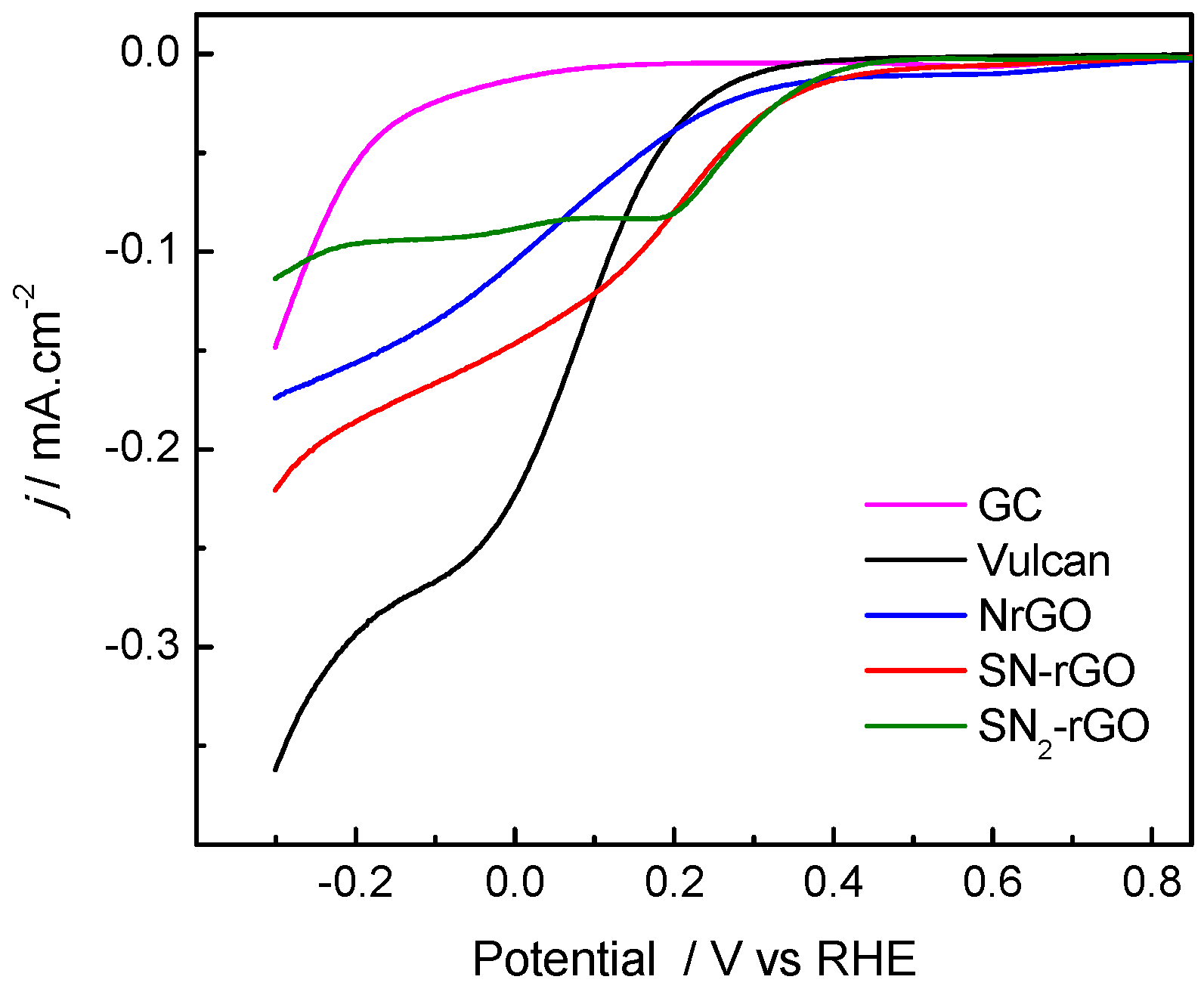
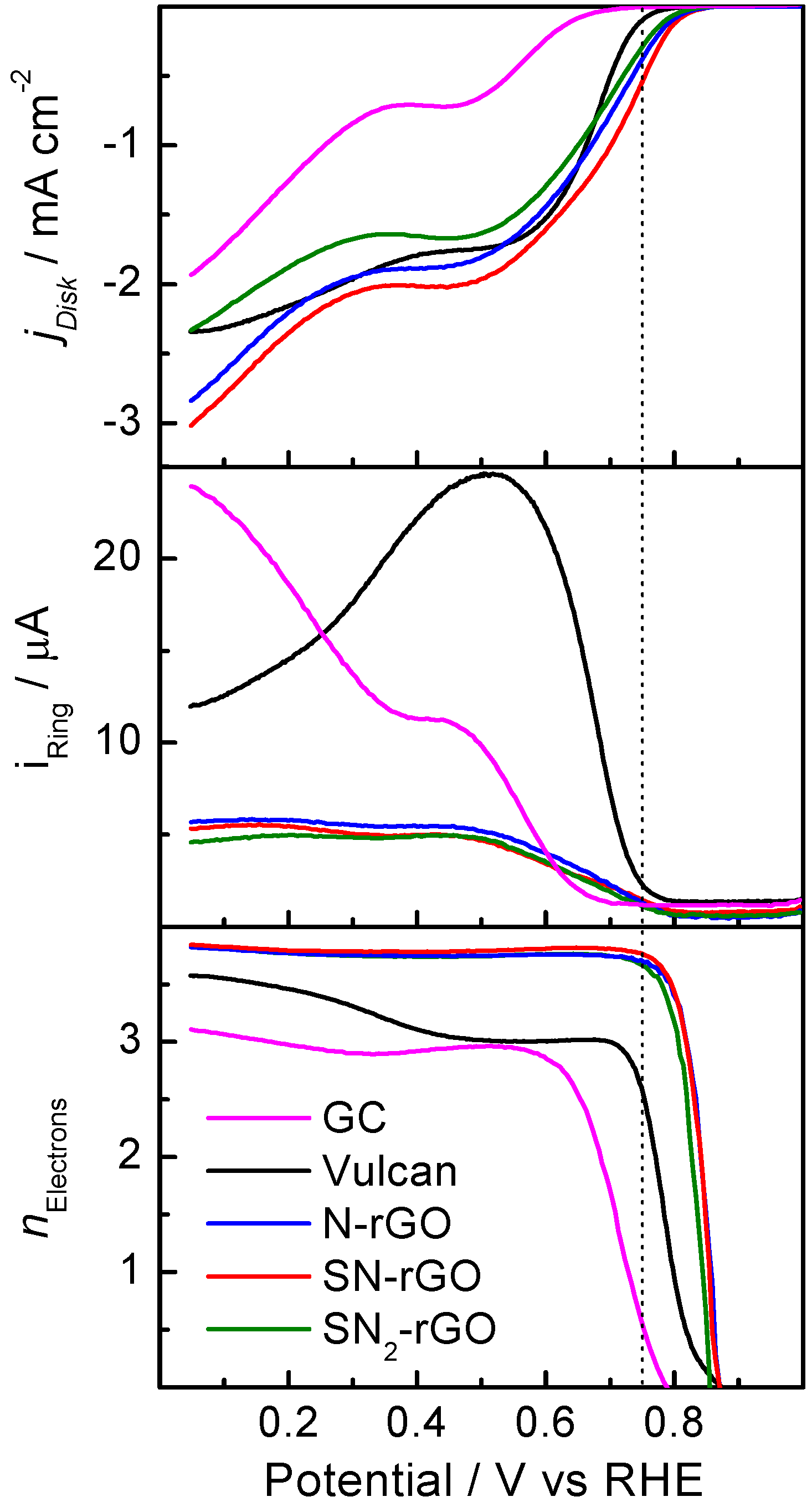
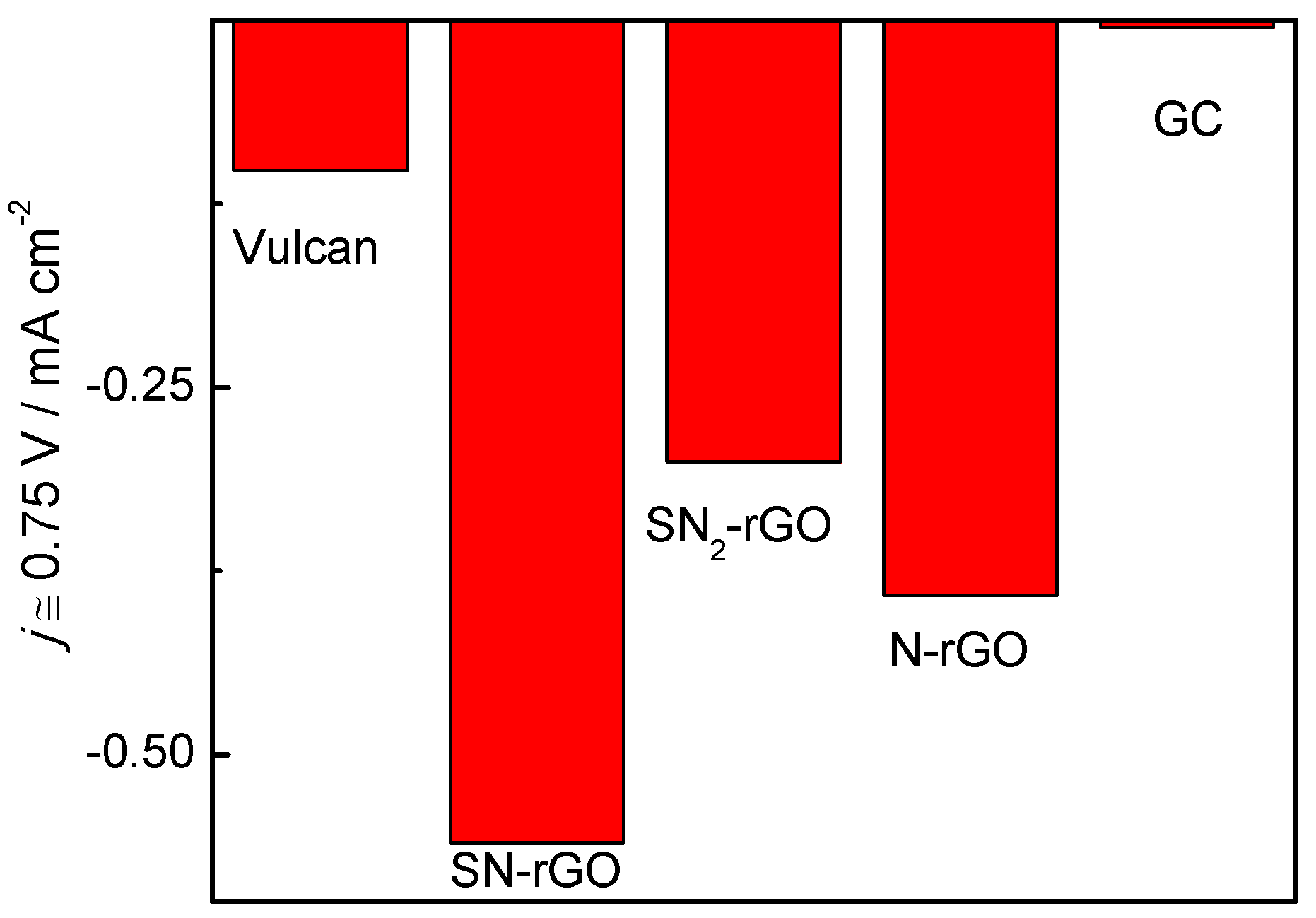
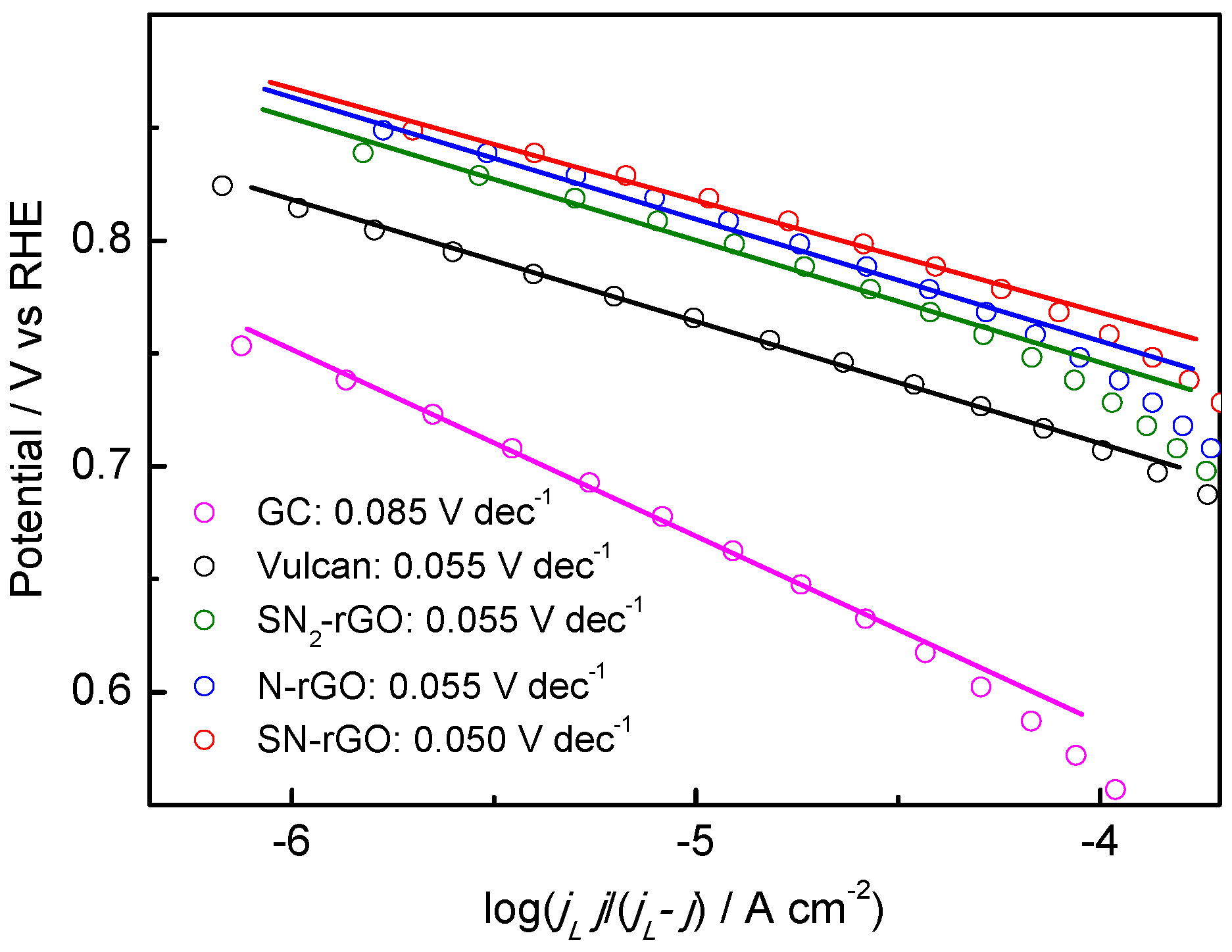
| Graphene Materials | Interplanar Spacing (d)/nm | ID/IG | Crystallite Size (nm) | Number of Layer (nl) |
|---|---|---|---|---|
| Graphite | 0.34 | 0.15 | 47.18 | 140 |
| Graphene oxide | 0.84 | 0.87 | 8.24 | 11 |
| Vulcan | 0.37 | 1.02 | 1.65 | 4, 5 |
| SN-rGO | 0.36 | 1.19 | 1.88 | 5, 6 |
| SN2-rGO | 0.36 | 1 | 1.76 | 5, 6 |
| N-rGO | 0.35 | 0.96 | 2.9 | 9 |
| Material | Elemental Composition (wt %); Experimental Error ~0.04% | |||
|---|---|---|---|---|
| C | O | S | N | |
| Graphite | 80.0 | 20.0 | - | - |
| Graphene oxide | 48.0 | 52.0 | - | - |
| Vulcan | 96.5 | 3.5 | ~1 | ~1 |
| SN-rGO | 76.4 | 13.6 | 7.3 | 2.7 |
| SN2-rGO | 62.8 | 14.4 | 13.6 | 9.2 |
| N-rGO | 73.0 | 21.0 | - | 6 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera, L.M.; Fajardo, S.; Arévalo, M.D.C.; García, G.; Pastor, E. S- and N-Doped Graphene Nanomaterials for the Oxygen Reduction Reaction. Catalysts 2017, 7, 278. https://doi.org/10.3390/catal7090278
Rivera LM, Fajardo S, Arévalo MDC, García G, Pastor E. S- and N-Doped Graphene Nanomaterials for the Oxygen Reduction Reaction. Catalysts. 2017; 7(9):278. https://doi.org/10.3390/catal7090278
Chicago/Turabian StyleRivera, Luis Miguel, Sergio Fajardo, María Del Carmen Arévalo, Gonzalo García, and Elena Pastor. 2017. "S- and N-Doped Graphene Nanomaterials for the Oxygen Reduction Reaction" Catalysts 7, no. 9: 278. https://doi.org/10.3390/catal7090278






