Direct Hydroxylation of Benzene to Phenol over TS-1 Catalysts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalytic Activity Test
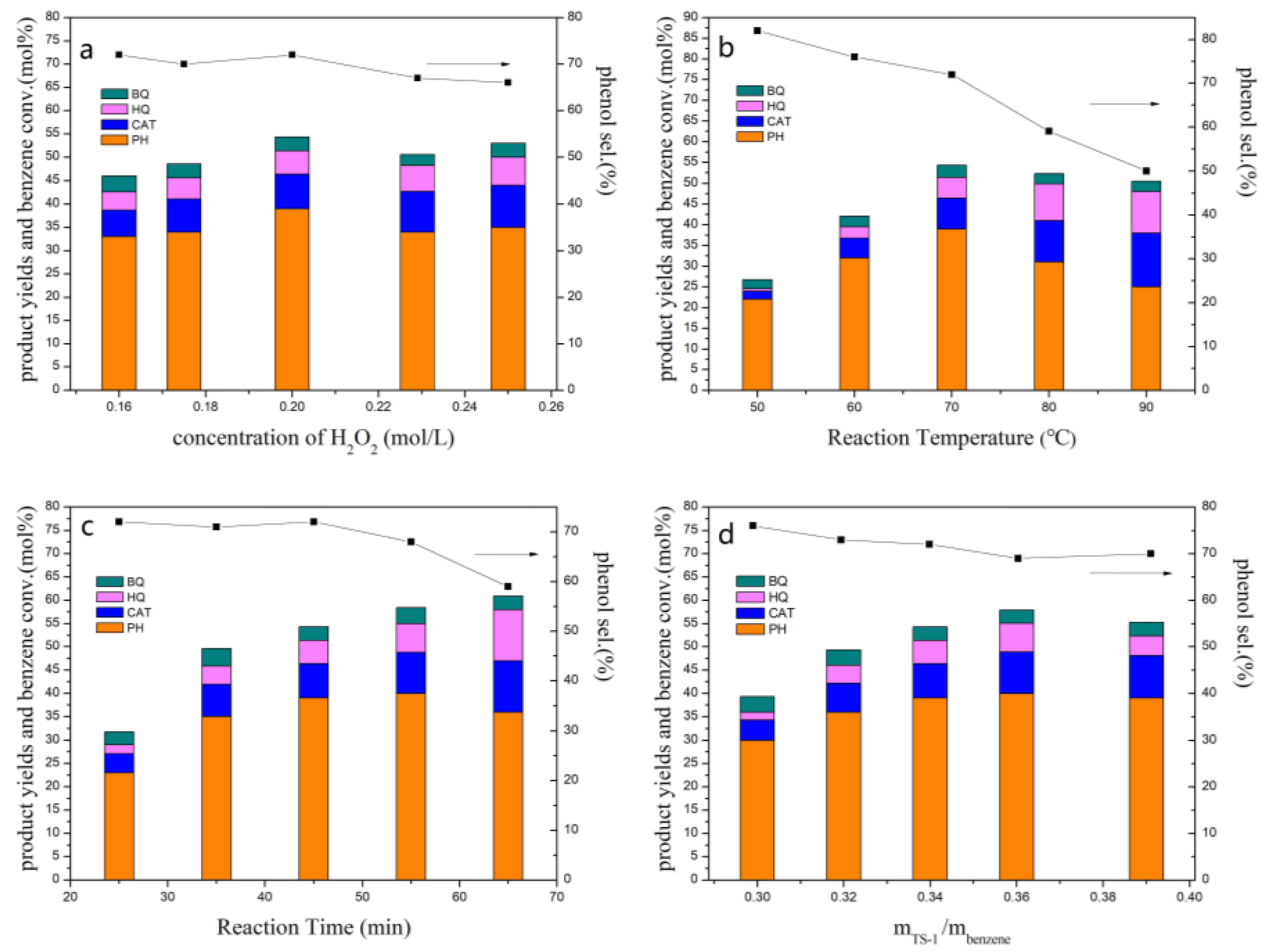
2.1.1. Optimization of Reaction Conditions
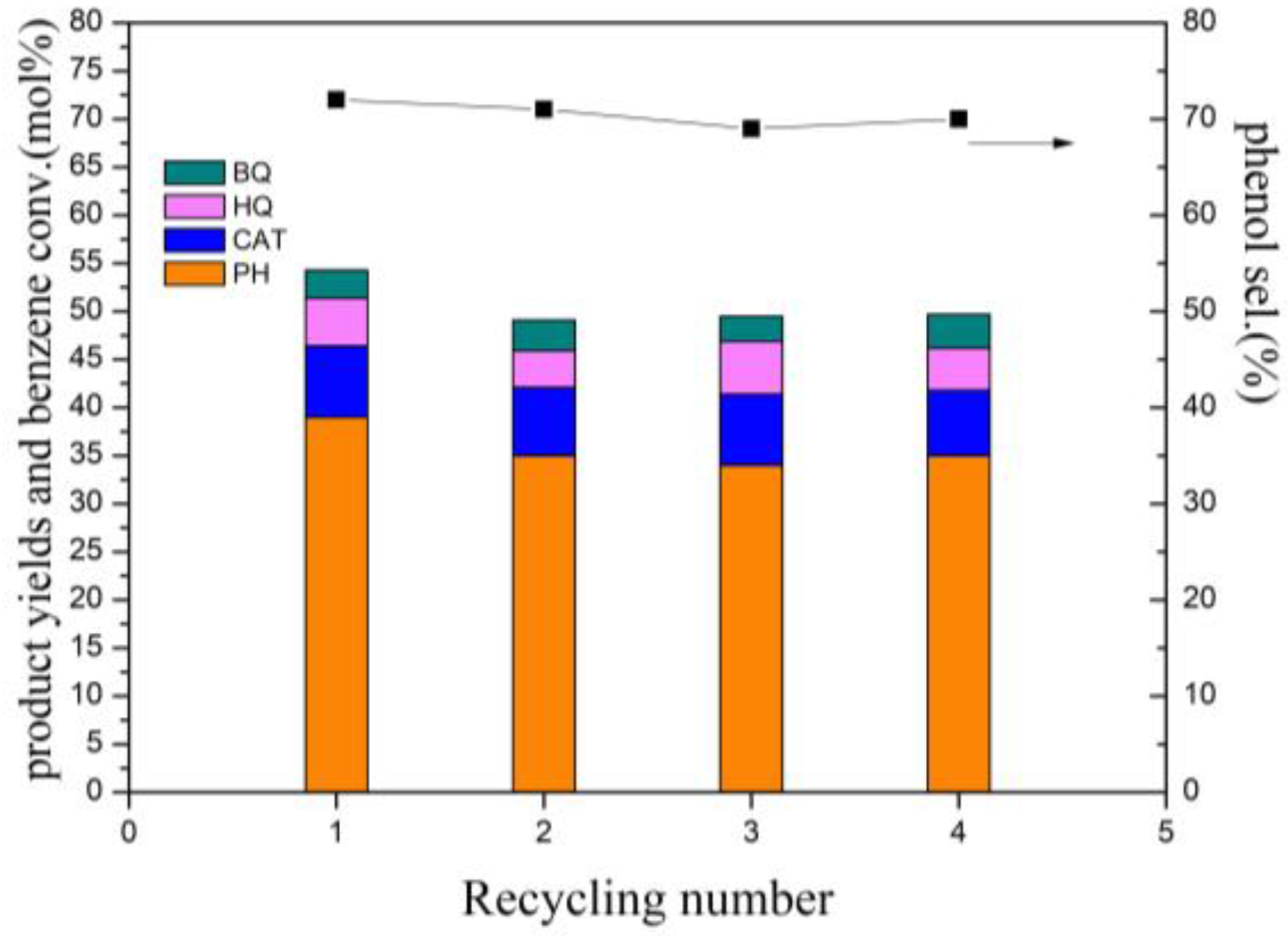
2.1.2. Recycling of the Catalyst
2.2. Characterization of the Catalyst
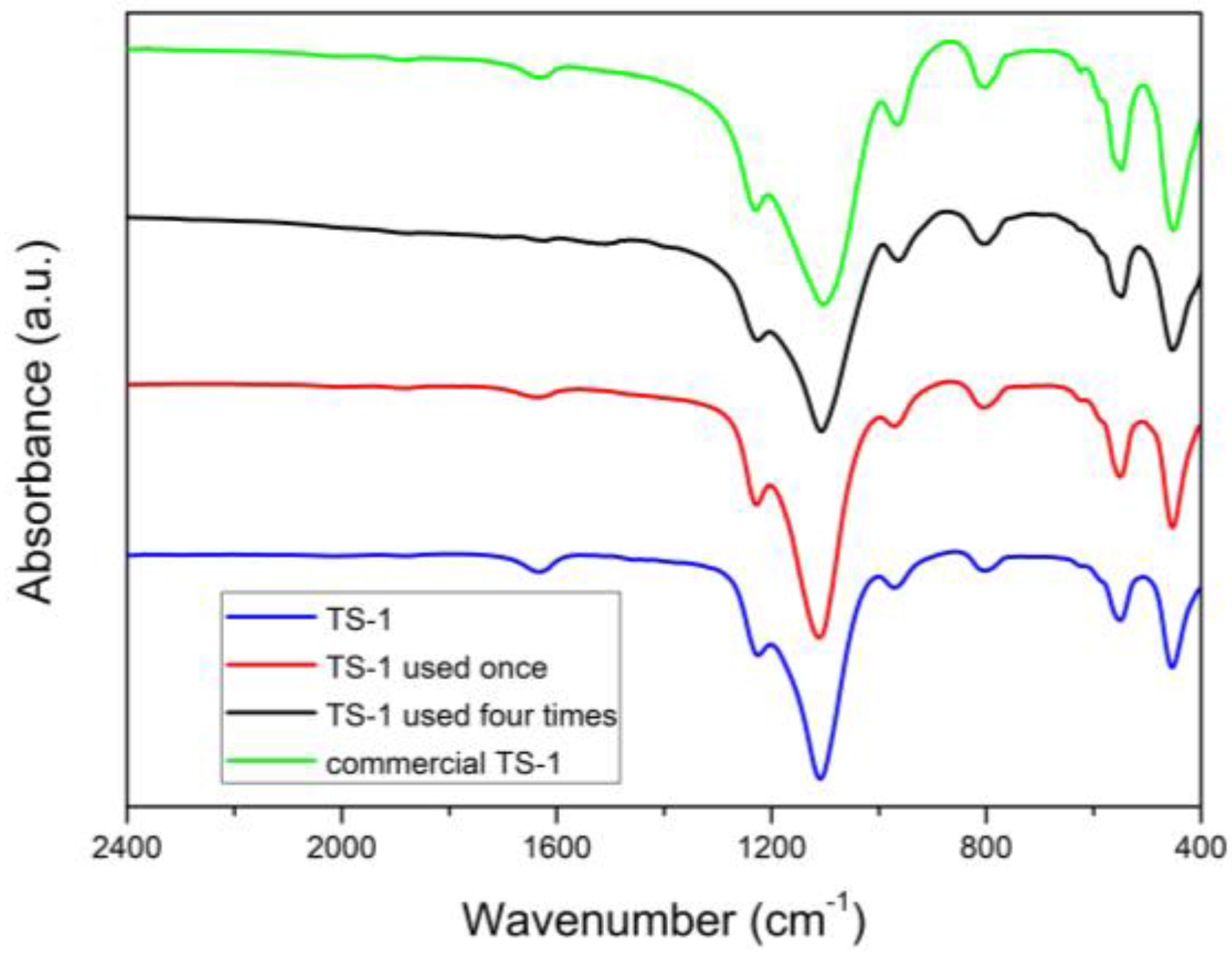
2.2.1. FT-IR Spectroscopy
2.2.2. Diffuse Reflectance UV-Vis
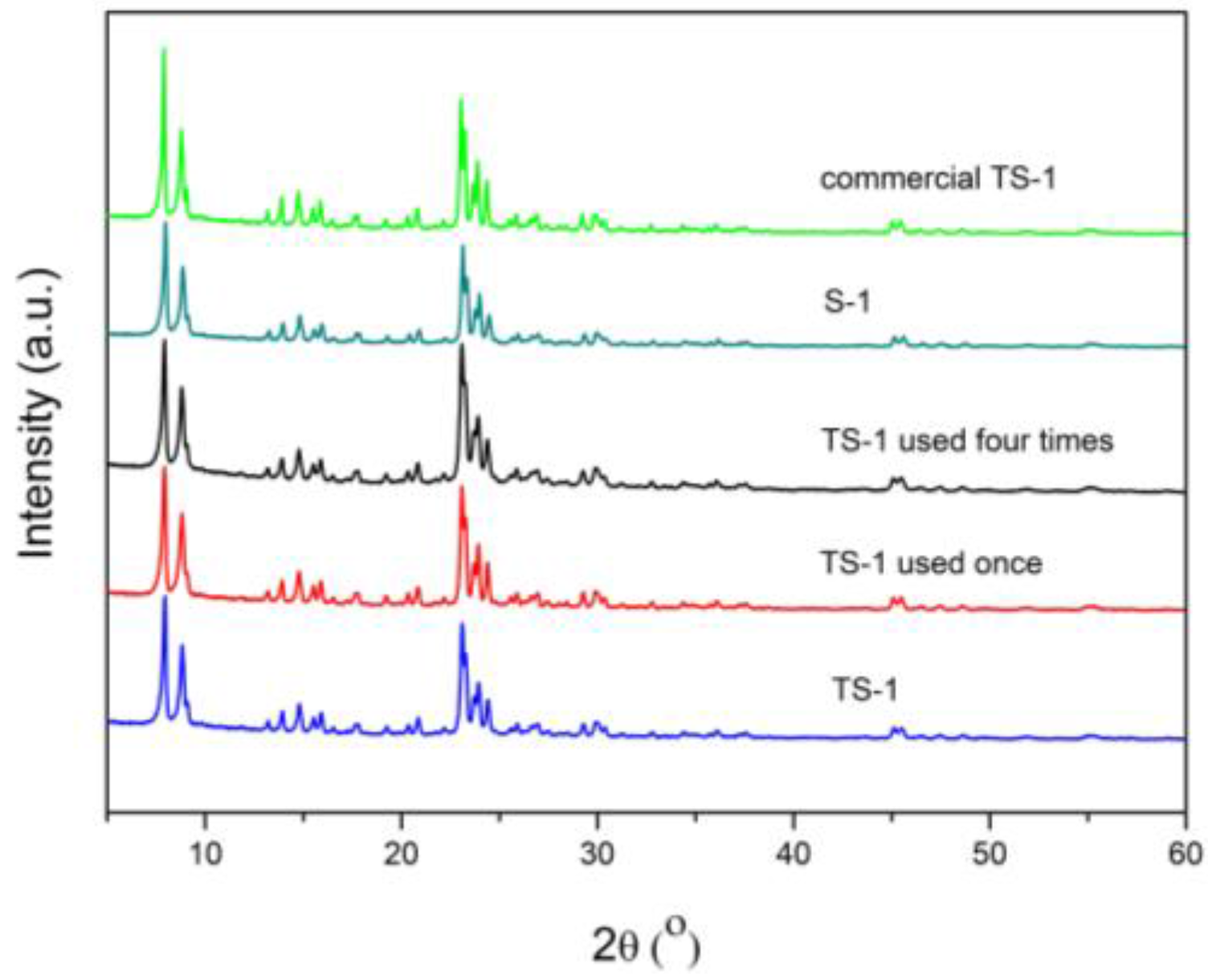
2.2.3. X-ray Diffraction
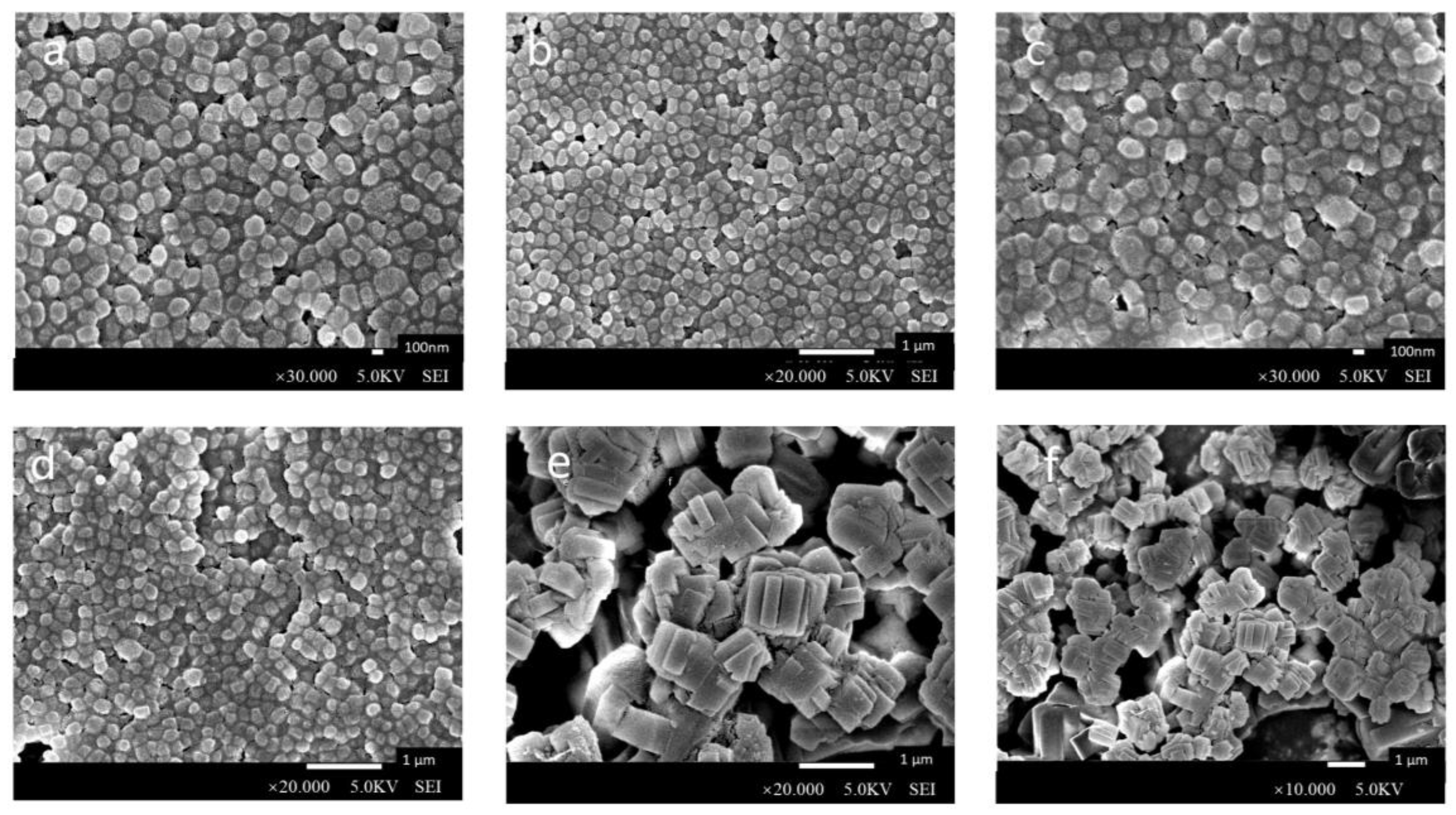
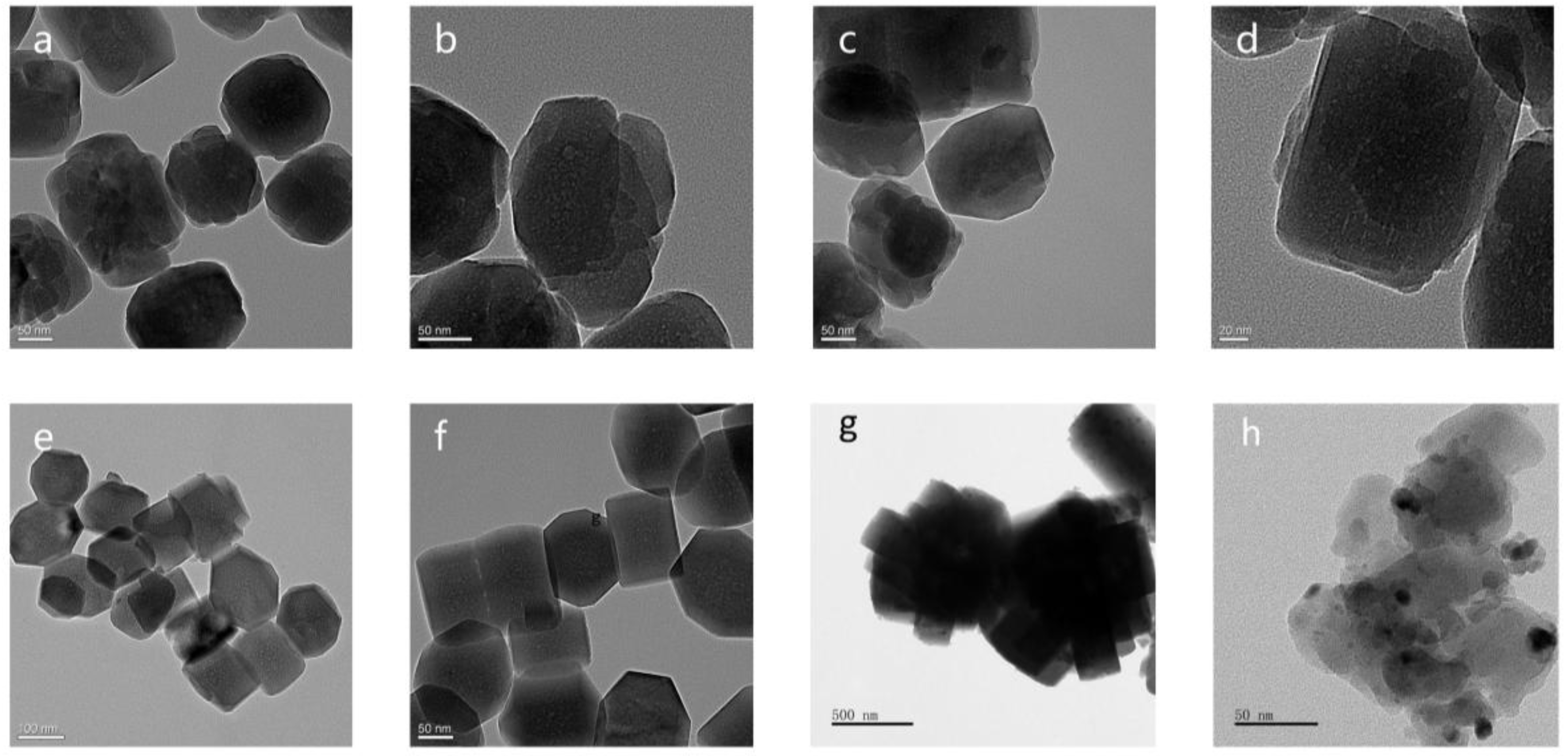
2.2.4. Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM)
2.2.5. Inductively Coupled Plasma (ICP) and X-ray Photoelectron Spectroscopy (XPS)
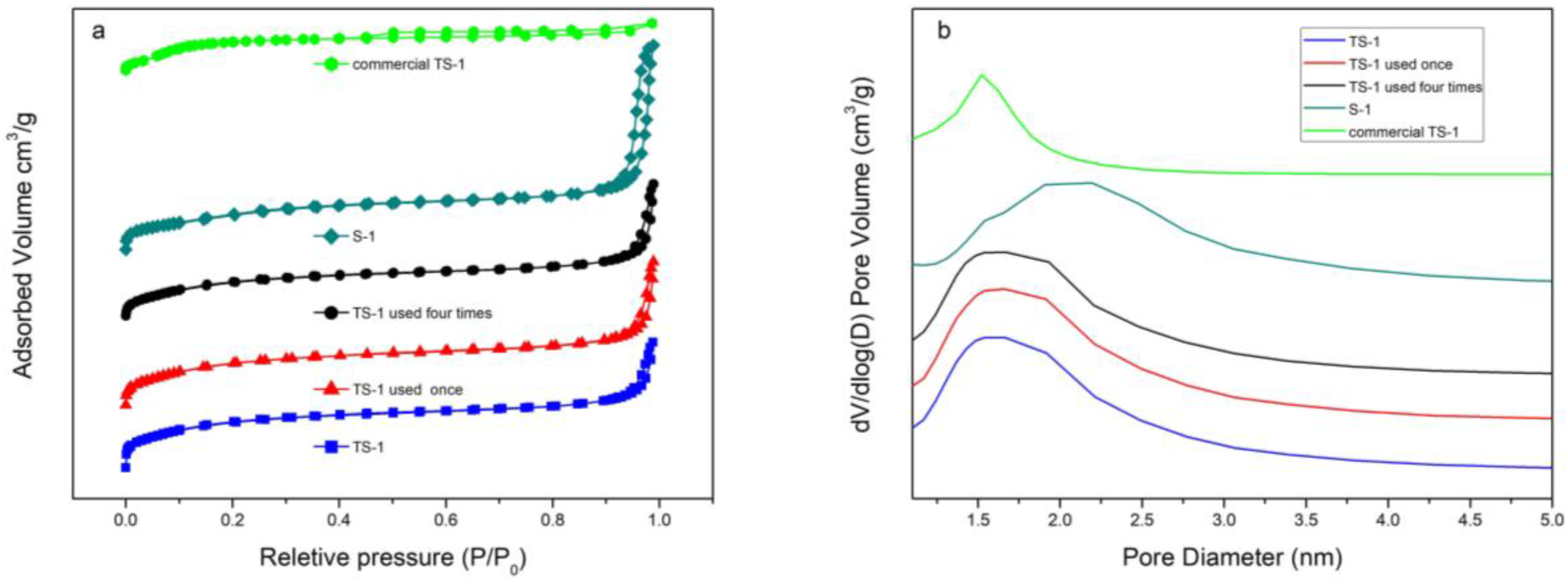
2.2.6. N2 Adsorption-Desorption Isotherms and Pore Size Distribution Curves
3. Experimental Section
3.1. Materials
3.2. Methods
3.2.1. Catalyst Preparation
3.2.2. Catalyst Characterization
3.2.3. Catalytic Activity Test
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yamanaka, H.; Hamada, R.; Nibuta, H.; Nishiyama, S.; Tsuruya, S. Gas-phase catalytic oxidation of benzene over Cu-supported ZSM-5 catalysts: An attempt of one-step production of phenol. J. Mol. Catal. A 2002, 178, 89–95. [Google Scholar] [CrossRef]
- Schmidt, R.J. Industrial catalytic processes-phenol production. Appl. Catal. A 2005, 280, 89–103. [Google Scholar] [CrossRef]
- Lemke, K.; Ehrich, H.; Lohse, U.; Berndt, H.; Jähnisch, K. Selective hydroxylation of benzene to phenol over supported vanadium oxide catalysts. Appl. Catal. A 2003, 243, 41–51. [Google Scholar] [CrossRef]
- Ehrich, H.; Berndt, H.; Pohl, M.M.; Jähnisch, K.; Baerns, M. Oxidation of benzene to phenol on supported Pt-VOx and Pd-VOx catalysts. Appl. Catal. A 2002, 230, 271–280. [Google Scholar] [CrossRef]
- Fukuzumi, S.; Ohkubo, K. One-step selective hydroxylation of benzene to phenol. Asian J. Org. Chem. 2015, 4, 836–845. [Google Scholar] [CrossRef]
- Choi, J.S.; Kim, T.H.; Saidutta, M.B.; Sung, J.S.; Kim, K.I.; Jasra, R.V.; Song, S.D.; Rhee, Y.W. Benzene hydroxylation to phenol catalyzed by transition metals supported on MCM-41 and activated carbon. J. Ind. Eng. Chem. 2004, 10, 445–453. [Google Scholar]
- Balducci, L.; Bianchi, D.; Bortolo, R.; D’Aloisio, R.; Ricci, M.; Tassinari, R.; Ungarelli, R. Direct oxidation of benzene to phenol with hydrogen peroxide over a modified titanium silicalite. Angew. Chem. 2003, 42, 4937–4940. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Shi, F.; Gu, Y.; Deng, Y. Highly selective and green aqueous–ionic liquid biphasic hydroxylation of benzene to phenol with hydrogen peroxide. Green Chem. 2003, 5, 224–226. [Google Scholar] [CrossRef]
- Niwa, S.S.; Eswaramoorthy, M.; Nair, J.; Raj, A.; Itoh, N.; Shoji, H.; Namba, T.; Mizukami, F. A one-step conversion of benzene to phenol with a palladium membrane. Science 2002, 295, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Louis, B.; Reuse, P.; Kiwi-Minsker, L.; Renken, A. Synthesis of ZSM-5 coatings on stainless steel grids and their catalytic performance for partial oxidation of benzene by N2O. Appl. Catal. A 2001, 210, 103–109. [Google Scholar] [CrossRef]
- Gu, Y.Y.; Zhao, X.H.; Zhang, G.R.; Ding, H.M.; Shan, Y.K. Selective hydroxylation of benzene using dioxygen activated by vanadium–copper oxide catalysts supported on SBA-15. Appl. Catal. A 2007, 328, 150–155. [Google Scholar] [CrossRef]
- Murata, K.; Yanyong, R.; Inaba, M. Effects of vanadium supported on ZrO2 and sulfolane on the synthesis of phenol by hydroxylation of benzene with oxygen and acetic acid on palladium catalyst. Catal. Lett. 2005, 102, 143–147. [Google Scholar] [CrossRef]
- Selli, E.; Rossetti, I.; Meloni, D.; Sini, F.; Forni, L. Effect of surface acidity on the behaviour of Fe-MFI catalysts for benzene hydroxylation to phenol. Appl. Catal. A 2004, 262, 131–136. [Google Scholar] [CrossRef]
- Taramasso, M.; Perego, G.; Notari, B. Preparation of Porous Crystalline Synthetic Material Comprised of Silicon and Titanium Oxides. U.S. Patent No 4,410,501, 18 October 1983. [Google Scholar]
- Bianchi, D.; D’Aloisio, R.; Bortolo, R.; Ricci, M. Oxidation of mono- and bicyclic aromatic compounds with hydrogen peroxide catalyzed by titanium silicalites TS-1 and TS-1b. Appl. Catal. A 2007, 327, 295–299. [Google Scholar] [CrossRef]
- Zou, H.; Sun, Q.; Fan, D.; Fu, W.; Liu, L.; Wang, R. Facile synthesis of yolk/core-shell structured TS-1@mesosilica composites for enhanced hydroxylation of phenol. Catalysts 2015, 5, 2134–2146. [Google Scholar] [CrossRef]
- Fang, Y.; Hu, H. Mesoporous TS-1: Nanocasting synthesis with CMK-3 as template and its performance in catalytic oxidation of aromatic thiophene. Catal. Commun. 2007, 8, 817–820. [Google Scholar] [CrossRef]
- Liu, T.; Wang, L.; Wan, H.; Guan, G. A magnetically recyclable TS-1 for ammoximation of cyclohexanone. Catal. Commun. 2014, 49, 20–24. [Google Scholar] [CrossRef]
- Gao, X.; An, J.; Gu, J.; Li, L.; Li, Y. A green template-assisted synthesis of hierarchical TS-1 with excellent catalytic activity and recyclability for the oxidation of 2,3,6-trimethylphenol. Microporous Mesoporous Mater. 2017, 239, 381–389. [Google Scholar] [CrossRef]
- Schmidt, I.; Krogh, A.; Wienberg, K.; Carlsson, A.; Brorson, M.; Jacobsen, C.J.H. Catalytic epoxidation of alkenes with hydrogen peroxide over first mesoporous titanium-containing zeolite. Chem. Commun. 2000, 2157–2158. [Google Scholar] [CrossRef]
- Serrano, D.P.; Sanz, R.; Pizarro, P.; Moreno, I. Tailoring the properties of hierarchical TS-1 zeolite synthesized from silanized protozeolitic units. Appl. Catal. A 2012, 435–436, 32–42. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, K.; Dong, D.; Li, D.; Hill, M.R.; Hill, A.J.; Wang, H. Synthesis of hierarchical porous zeolite nay particles with controllable particle sizes. Microporous Mesoporous Mater. 2010, 127, 167–175. [Google Scholar] [CrossRef]
- Xu, J.; Liu, H.; Yang, R.; Li, G.; Hu, C. Hydroxylation of benzene by activated carbon catalyst. Chin. J. Catal. 2012, 33, 1622–1630. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, H.; Li, G.; Hu, C. Continuous flow reactor for hydroxylation of benzene to phenol by hydrogen peroxide. Chin. J. Chem. Phys. 2012, 25, 585–591. [Google Scholar] [CrossRef]
- Wcław, A.; Nowińska, K.; Schwieger, W. Benzene to phenol oxidation over iron exchanged zeolite ZSM-5. Appl. Catal. A 2004, 270, 151–156. [Google Scholar] [CrossRef]
- Shahid, A.; Lopez-Orozco, S.; Marthala, V.R.; Hartmann, M.; Schwieger, W. Direct oxidation of benzene to phenol over hierarchical ZSM-5 zeolites prepared by sequential post synthesis modification. Microporous Mesoporous Mater. 2016, 237, 151–159. [Google Scholar] [CrossRef]
- Sato, K.; Hanaoka, T.; Niwa, S.; Stefan, C.; Namba, T.; Mizukami, F. Direct hydroxylation of aromatic compounds by a palladium membrane reactor. Catal. Today 2005, 104, 260–266. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Y.; Zhang, X.; Wang, Y.; Liu, H.; Wang, J.; Qiu, J.; Yeung, K.L. Catalytic properties of benzene hydroxylation by TS-1 film reactor and Pd–TS-1 composite membrane reactor. Catal. Today 2010, 156, 288–294. [Google Scholar] [CrossRef]
- Ye, S.; Hamakawa, S.; Tanaka, S.; Sato, K.; Esashi, M.; Mizukami, F. A one-step conversion of benzene to phenol using mems-based Pd membrane microreactors. Chem. Eng. J. 2009, 155, 829–837. [Google Scholar] [CrossRef]
- Hu, L.; Wang, C.; Ye, L.; Wu, Y.; Yue, B.; Chen, X.; He, H. Direct hydroxylation of benzene to phenol using H2O2 as an oxidant over vanadium-containing mesoporous carbon catalysts. Appl. Catal. A 2015, 504, 440–447. [Google Scholar] [CrossRef]
- Chammingkwan, P.; Hoelderich, W.F.; Mongkhonsi, T.; Kanchanawanichakul, P. Hydroxylation of benzene over TS-PQ™ catalyst. Appl. Catal. A 2009, 352, 1–9. [Google Scholar] [CrossRef]
- Ye, X.; Cui, Y.; Qiu, X.; Wang, X. Selective oxidation of benzene to phenol by Fe-CN/TS-1 catalysts under visible light irradiation. Appl. Catal. B 2014, 152–153, 383–389. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, J.; Fu, X.; Antonietti, M.; Wang, X. Fe-gC3N4-catalyzed oxidation of benzene to phenol using hydrogen peroxide and visible light. J. Am. Chem. Soc. 2009, 131, 11658–11659. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Fu, Z.; Yin, D.; Yin, D.; Liao, H. A novel micro-emulsion catalytic system for highly selective hydroxylation of benzene to phenol with hydrogen peroxide. Catal. Commun. 2005, 6, 638–643. [Google Scholar] [CrossRef]
- Feng, S.; Pei, S.; Yue, B.; Ye, L.; Qian, L.; He, H. Synthesis and characterization of V-HMS employed for catalytic hydroxylation of benzene. Catal. Lett. 2009, 131, 458–462. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, J. Direct oxidation of benzene to phenol catalyzed by vanadium substituted heteropolymolybdic acid. Transit. Met. Chem. 2006, 31, 299–305. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, T.; Li, B.; Yang, Q.; Jiang, S. Efficient hydroxylation of aromatic compounds catalyzed by an iron(II) complex with H2O2. Appl. Organomet. Chem. 2014, 28, 666–672. [Google Scholar] [CrossRef]
- Xia, S.; Yu, T.; Liu, H.; Li, G.; Hu, C. One step C–N bond formation from alkylbenzene and ammonia over Cu-modified TS-1 zeolite catalyst. Catal. Sci. Technol. 2014, 4, 3108–3119. [Google Scholar] [CrossRef]
- Yu, T.; Zhang, Q.; Xia, S.; Li, G.; Hu, C. Direct amination of benzene to aniline by reactive distillation method over copper doped hierarchical TS-1 catalyst. Catal. Sci. Technol. 2014, 4, 639–647. [Google Scholar] [CrossRef]
- Nan, M.; Luo, Y.; Li, G.; Hu, C. Improvement of the selectivity to aniline in benzene amination over Cu/TS-1 by potassium. RSC Adv. 2017, 7, 21974–21981. [Google Scholar] [CrossRef]
- Zhang, F.; Shang, H.; Jin, D.; Chen, R.; Xing, W. High efficient synthesis of methyl ethyl ketone oxime from ammoximation of methyl ethyl ketone over TS-1 in a ceramic membrane reactor. Chem. Eng. Process. 2017, 116, 1–8. [Google Scholar] [CrossRef]
- Xin, H.; Zhao, J.; Xu, S.; Li, J.; Zhang, W.; Guo, X.; Hensen, E.J.M.; Yang, Q.; Li, C. Enhanced catalytic oxidation by hierarchically structured TS-1 zeolite. J. Phys. Chem. C 2010, 114, 6553–6559. [Google Scholar] [CrossRef]
- Adedigba, A.; Sankar, G.; Catlow, C.R.A.; Du, Y.; Xi, S.; Borgna, A. On the synthesis and performance of hierarchical nanoporous TS-1 catalysts. Microporous Mesoporous Mater. 2017, 244, 83–92. [Google Scholar] [CrossRef]
- Wang, X.; Li, G.; Wang, W.; Jin, C.; Chen, Y. Synthesis, characterization and catalytic performance of hierarchical TS-1 with carbon template from sucrose carbonization. Microporous Mesoporous Mater. 2011, 142, 494–502. [Google Scholar] [CrossRef]
- Fan, W.; Duan, R.G.; Yokoi, T.; Wu, P.; Kubota, Y.; Tatsumi, T. Synthesis, crystallization mechanism, and catalytic properties of titanium-rich TS-1 free of extraframework titanium species. J. Am. Chem. Soc. 2008, 130, 10150–10164. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.; Cao, Y.; Guo, Z.; Jia, Q.; Tian, F.; Liu, L. The roles of different titanium species in TS-1 zeolite in propylene epoxidation studied by in situ UV raman spectroscopy. Phys. Chem. Chem. Phys. 2016, 18, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xiong, G.; Su, J.; Li, P.; Guo, H. In situ UV raman spectroscopic study on the reaction intermediates for propylene epoxidation on TS-1. J. Phys. Chem. C 2012, 116, 9122–9131. [Google Scholar] [CrossRef]
- Petrini, G.; Cesana, A.; Alberti, G.D.; Genoni, F.; Leofanti, G.; Padovan, M.; Paparatto, G.; Roffia, P. Deactivation phenomena on Ti-silicalite. Stud. Surf. Sci. Catal. 1991, 68, 761–766. [Google Scholar]
- Su, J.; Xiong, G.; Zhou, J.; Liu, W.; Zhou, D.; Wang, G.; Wang, X.; Guo, H. Amorphous Ti species in titanium silicalite-1: Structural features, chemical properties, and inactivation with sulfosalt. J. Catal. 2012, 288, 1–7. [Google Scholar] [CrossRef]
- Huybrechts, D.R.C.; Buskens, P.L.; Jacobs, P.A. Physicochemical and catalytic properties of titanium silicalites. J. Mol. Catal. 1992, 71, 129–147. [Google Scholar] [CrossRef]
- Wang, B.; Lin, M.; Peng, X.; Zhu, B.; Shu, X. Hierarchical TS-1 synthesized effectively by post-modification with tpaoh and ammonium hydroxide. RSC Adv. 2016, 6, 44963–44971. [Google Scholar] [CrossRef]
- Wu, G.; Xiao, J.; Zhang, L.; Wang, W.; Hong, Y.; Huang, H.; Jiang, Y.; Li, L.; Wang, C. Copper-modified TS-1 catalyzed hydroxylation of phenol with hydrogen peroxide as the oxidant. RSC Adv. 2016, 6, 101071–101078. [Google Scholar] [CrossRef]
- Song, W.; Zuo, Y.; Xiong, G.; Zhang, X.; Jin, F.; Liu, L.; Wang, X. Transformation of SiO2 in titanium silicalite-1/SiO2 extrudates during tetrapropylammonium hydroxide treatment and improvement of catalytic properties for propylene epoxidation. Chem. Eng. J. 2014, 253, 464–471. [Google Scholar] [CrossRef]
- Hu, Q.; Dou, B.; Tian, H.; Li, J.; Li, P.; Hao, Z. Mesoporous silicalite-1 nanospheres and their properties of adsorption and hydrophobicity. Microporous Mesoporous Mater. 2010, 129, 30–36. [Google Scholar] [CrossRef]
- Yu, T.; Yang, R.; Xia, S.; Li, G.; Hu, C. Direct amination of benzene to aniline with H2O2 and NH3·H2O over Cu/SiO2 catalyst. Catal. Sci. Technol. 2014, 4, 3159–3167. [Google Scholar] [CrossRef]
- Du, Q.; Guo, Y.; Duan, H.; Li, H.; Chen, Y.; Liu, H. Synthesis of hierarchical TS-1 zeolite via a novel three-step crystallization method and its excellent catalytic performance in oxidative desulfurization. Fuel 2017, 188, 232–238. [Google Scholar] [CrossRef]
- Sanz, R.; Serrano, D.P.; Pizarro, P.; Moreno, I. Hierarchical TS-1 zeolite synthesized from SiO2 TiO2 xerogels imprinted with silanized protozeolitic units. Chem. Eng. J. 2011, 171, 1428–1438. [Google Scholar] [CrossRef]
- Dai, J.; Zhong, W.; Yi, W.; Liu, M.; Mao, L.; Xu, Q.; Yin, D. Bifunctional H2WO4/TS-1 catalysts for direct conversion of cyclohexane to adipic acid: Active sites and reaction steps. Appl. Catal. B 2016, 192, 325–341. [Google Scholar] [CrossRef]
- Wang, B.; Lin, M.; Zhu, B.; Peng, X.; Xu, G.; Shu, X. The synthesis, characterization and catalytic activity of the hierarchical TS-1 with the intracrystalline voids and grooves. Catal. Commun. 2016, 75, 69–73. [Google Scholar] [CrossRef]
- Lee, W.S.; Cem Akatay, M.; Stach, E.A.; Ribeiro, F.H.; Nicholas Delgass, W. Enhanced reaction rate for gas-phase epoxidation of propylene using H2 and O2 by Cs promotion of Au/TS-1. J. Catal. 2013, 308, 98–113. [Google Scholar] [CrossRef]
- Kumar, R.; Bhaumik, A. Triphase, solvent-free catalysis over the TS-1/H2O2 system in selective oxidation reactions. Microporous Mesoporous Mater. 1998, 21, 497–504. [Google Scholar] [CrossRef]
- Bhaumik, A.; Mukherjee, P.; Kumar, R. Triphase catalysis over titanium–silicate molecular sieves under solvent-free conditions: I. Direct hydroxylation of benzene. J. Catal. 1998, 178, 101–107. [Google Scholar] [CrossRef]
- Zhong, W.; Tao, Q.; Jing, D.; Mao, L.; Xu, Q.; Zou, G.; Liu, X.; Yin, D.; Zhao, F. Visible-light-responsive sulfated vanadium-doped TS-1 with hollow structure: Enhanced photocatalytic activity in selective oxidation of cyclohexane. J. Catal. 2015, 330, 208–221. [Google Scholar] [CrossRef]
- Tekla, J.; Tarach, K.; Olejniczak, Z.; Girman, V.; Góra-Marek, K. Effective hierarchization of TS-1 and its catalytic performance in cyclohexene epoxidation. Microporous Mesoporous Mater. 2016, 233, 16–25. [Google Scholar] [CrossRef]
- Zuo, Y.; Song, W.; Dai, C.; He, Y.; Wang, M.; Wang, X.; Guo, X. Modification of small-crystal titanium silicalite-1 with organic bases: Recrystallization and catalytic properties in the hydroxylation of phenol. Appl. Catal. A 2013, 453, 272–279. [Google Scholar] [CrossRef]
- Xiao, F.; Han, Y.; Yu, Y.; Meng, X.; Yang, M.; Wu, S. Hydrothermally stable ordered mesoporous titanosilicates with highly active catalytic sites. J. Am. Chem. Soc. 2010, 33, 888–889. [Google Scholar] [CrossRef]
- Feng, X.; Sheng, N.; Liu, Y.; Chen, X.; Chen, D.; Yang, C.; Zhou, X. Simultaneously enhanced stability and selectivity for propene epoxidation with H2 and O2 on Au catalysts supported on nano-crystalline mesoporous TS-1. ACS Catal. 2017, 7, 2668–2675. [Google Scholar] [CrossRef]

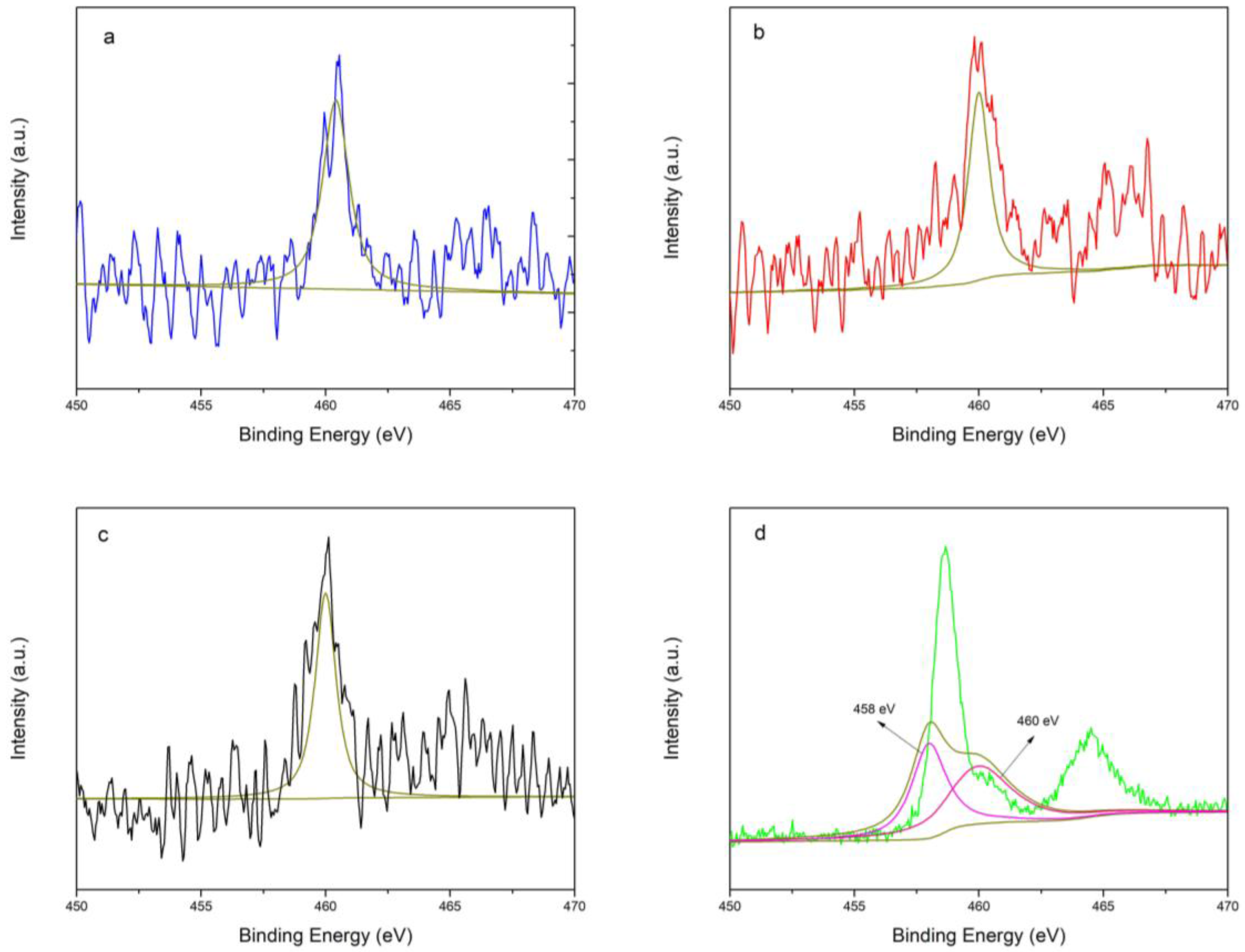
| Sample | 458 eV (wt.%) | 460 eV (wt.%) | Total (wt.%) |
|---|---|---|---|
| TS-1 | - | 0.21 | 0.21 |
| TS-1(used once) | - | 0.26 | 0.26 |
| TS-1(used four times) | - | 0.26 | 0.26 |
| commercial TS-1 | 0.92 | 0.34 | 1.26 |
| Sample | SBET (m2g−1) | Vmic (cm3g−1) | Vmeso (cm3g−1) | ΦPa (Å) |
|---|---|---|---|---|
| TS-1 | 476 | 0.17 | 0.16 | 23.0 |
| TS-1(used once) | 480 | 0.17 | 0.17 | 23.4 |
| TS-1(used four times) | 460 | 0.16 | 0.16 | 23.1 |
| S-1 | 458 | 0.16 | 0.31 | 36.8 |
| commercial TS-1 | 411 | 0.084 | 0.11 | 17.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Xiong, J.; Pang, C.; Li, G.; Hu, C. Direct Hydroxylation of Benzene to Phenol over TS-1 Catalysts. Catalysts 2018, 8, 49. https://doi.org/10.3390/catal8020049
Luo Y, Xiong J, Pang C, Li G, Hu C. Direct Hydroxylation of Benzene to Phenol over TS-1 Catalysts. Catalysts. 2018; 8(2):49. https://doi.org/10.3390/catal8020049
Chicago/Turabian StyleLuo, Yuecheng, Jiahui Xiong, Conglin Pang, Guiying Li, and Changwei Hu. 2018. "Direct Hydroxylation of Benzene to Phenol over TS-1 Catalysts" Catalysts 8, no. 2: 49. https://doi.org/10.3390/catal8020049




