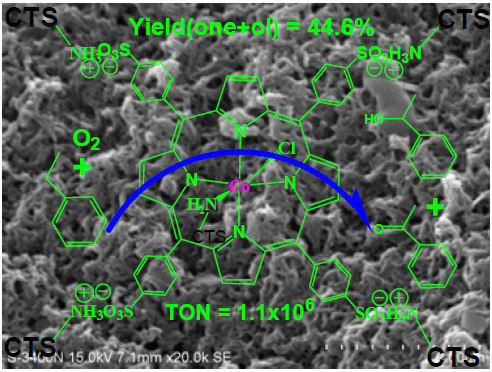
Effect of Mesoporous Chitosan Action and Coordination on the Catalytic Activity of Mesoporous Chitosan-Grafted Cobalt Tetrakis(p-Sulfophenyl)Porphyrin for Ethylbenzene Oxidation
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. H2 TPPS and Co TPPS Synthesis
2.3. Preparation of Co TPPS/mesp-CTS Catalyst Material
2.4. Characterization Equipment
2.5. Measurement of Catalytic Performance forCo TPPS/Mesp-CTS
3. Results and Discussion
3.1. Characterization for the Co TPPS/mesp-CTS Material
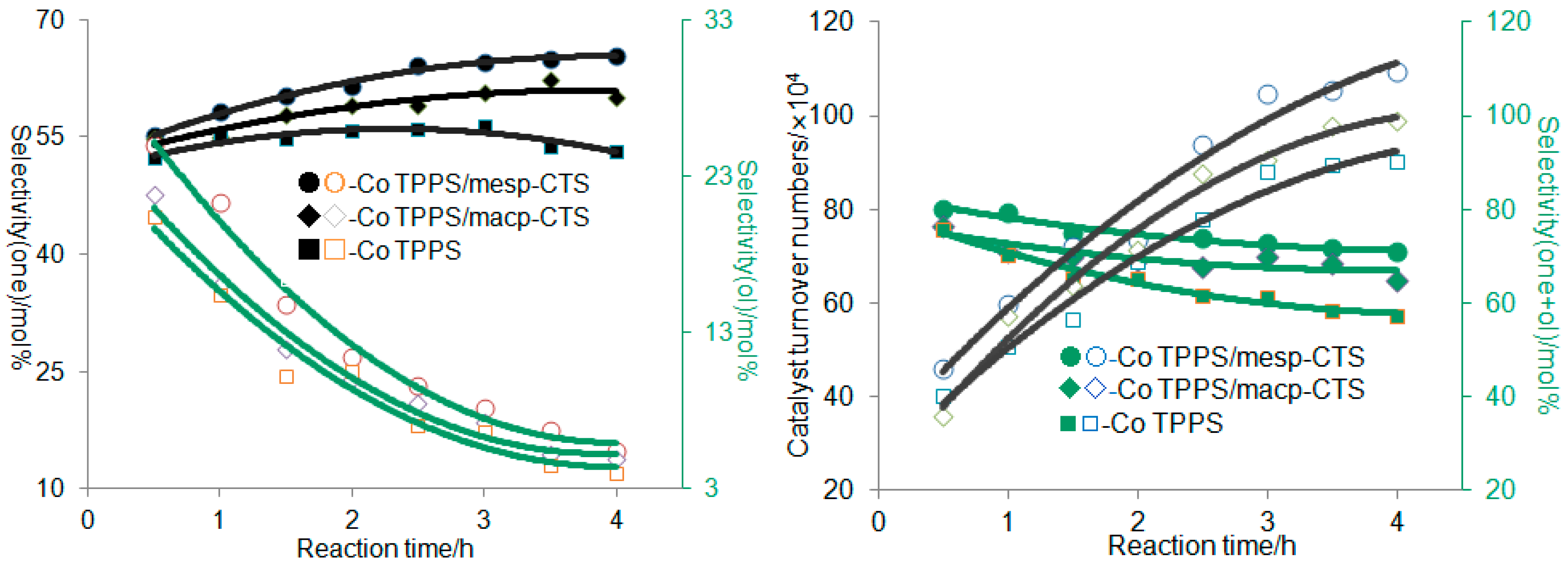
3.2. Oxidation of Ethylbenzene over Co TPPS/mesp-CTS
 mesp-CTS/TPPS CoOH], which forms the hydroxyl radical,
mesp-CTS/TPPS CoOH], which forms the hydroxyl radical,  , as well as [mesp-CTS/TPPS Co], which proceeds into the next recycle. The major reaction products, 1-phenylethanol and acetophenone, are generated in radical chain transfer processes; 1-phenylethanol is generated first, followed by acetophenone, as shown in Scheme 1 and Figure 7 (left).
, as well as [mesp-CTS/TPPS Co], which proceeds into the next recycle. The major reaction products, 1-phenylethanol and acetophenone, are generated in radical chain transfer processes; 1-phenylethanol is generated first, followed by acetophenone, as shown in Scheme 1 and Figure 7 (left).4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Yang, S.L.; Peng, L.; Huang, P.P.; Wang, X.S.; Sun, Y.B.; Cao, C.Y.; Song, W.G. Nitrogen, phosphorus, and sulfur co-doped hollow carbon shell as superior metal-free catalyst for selective oxidation of aromatic alkanes. Angew. Chem. Int. Ed. 2016, 55, 4016–4020. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Yang, J.; Liu, Y.Y.; Ma, J.F. Porphyrin-based mixed-valent Ag(I)/Ag(II) and Cu(I)/Cu(II) networks as efficient heterogeneous catalysts for the azide–alkyne “click” reaction and promising oxidation of ethylbenzene. Chem. Commun. 2016, 52, 1373–1376. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Cheng, H.Y.; Sun, L.S.; Liang, F.; Zhang, C.; Ying, Z.; Lin, W.W.; Zhao, F.Y. Synthesis of acetophenone from aerobic catalytic oxidation of ethylbenzene over Ti–Zr–Co alloy catalyst: Influence of annealing conditions. Appl. Catal. A Gen. 2016, 512, 9–14. [Google Scholar] [CrossRef]
- Xie, R.F.; Fan, G.L.; Yang, L.; Li, F. Hierarchical flower-like Co–Cu mixed metal oxide microspheres as highly efficient catalysts for selective oxidation of ethylbenzene. Chem. Eng. J. 2016, 288, 169–178. [Google Scholar] [CrossRef]
- Selvamani, A.; Selvaraj, M.; Krishnan, P.S.; Gurulakshmi, M.; Shanthi, K. Low temperature vapor phase selective oxidation of ethylbenzene over Ce1−xMnxO2 nanotubes. Appl. Catal. A Gen. 2015, 495, 92–103. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Duan, Y.; Zhang, L.; Yu, M.M.; Li, J. Synthesis, crystal structure of two new Zn(II), Cu(II) porphyrins and their catalytic activities to ethylbenzene oxidation. Inorg. Chem. Commun. 2015, 58, 53–56. [Google Scholar] [CrossRef]
- Chen, A.B.; Yu, Y.F.; Wang, R.J.; Yu, Y.H.; Zang, W.W.; Tang, P.; Ma, D. Nitrogen-doped dual mesoporous carbon for the selective oxidation of ethylbenzene. Nanoscale 2015, 7, 14684–14690. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.M.; Chen, K.X.; Zhang, W.S.; Yao, J.; Li, H.R. Efficient metal-free oxidation of ethylbenzene with molecular oxygen utilizing the synergistic combination of NHPI analogues. J. Mol. Catal. A Chem. 2015, 402, 79–82. [Google Scholar] [CrossRef]
- Li, H.Y.; Ma, H.; Wang, X.H.; Gao, J.; Chen, C.; Shi, S.; Qu, M.J.; Feng, N.; Xu, J. Efficient oxidation of ethylbenzene catalyzed by cobalt zeolitic imidazolate framework ZIF-67 and NHPI. J. Energy Chem. 2014, 23, 742–746. [Google Scholar] [CrossRef]
- Wang, J.; Liu, H.Y.; Gu, X.M.; Wang, H.H.; Su, D.S. Synthesis of nitrogen-containing ordered mesoporous carbon as a metal-free catalyst for selective oxidation of ethylbenzene. Chem. Commun. 2014, 50, 9182–9184. [Google Scholar] [CrossRef] [PubMed]
- Bay, S.; Baumeister, T.; Hashmi, A.S.K.; Röder, T. Safe and fast flow synthesis of functionalized oxazoles with molecular oxygen in a microstructured reactor. Org. Process Res. Dev. 2016, 20, 1297–1304. [Google Scholar] [CrossRef]
- Hashmi, A.S.; Blanco Jaimes, M.C.; Schuster, A.M.; Rominger, F. From propargylic amides to functionalized oxazoles: Domino gold catalysis/oxidation by dioxygen. J. Org. Chem. 2012, 77, 6394–6408. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.P.D.; Martins, L.M.D.R.S.; Carabineiro, S.A.C.; Lauterbach, T.; Rominger, F.; Hashmi, A.S.K.; Pombeiro, A.J.L.; Figueiredo, J.L. Homogeneous and heterogenised new gold C-scorpionate complexes as catalysts for cyclohexane oxidation. Catal. Sci. Technol. 2013, 3, 3056–3069. [Google Scholar] [CrossRef]
- Yang, L.; Fan, B.B.; Cui, X.Y.; Shi, X.F.; Li, R.F. Solvent-free aerobic oxidation of ethylbenzene over Mn-containing silylated MgAl layered double hydroxides. J. Ind. Eng. Chem. 2015, 21, 689–695. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, S.F.; Liu, Z.G. Influence of the synergistic effect between Co–N–C and ceria on the catalytic performance for selective oxidation of ethylbenzene. Phys. Chem. Chem. Phys. 2015, 17, 14012–14020. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.L.; Chen, Y.; Liu, Z.G. Cobalt catalysts embedded in N-doped carbon derived from cobalt porphyrin via a one-pot method for ethylbenzene oxidation. J. Mol. Catal. A Chem. 2015, 408, 91–97. [Google Scholar] [CrossRef]
- Lv, W.M.; Yang, L.; Fan, B.B.; Zhao, Y.; Chen, Y.F.; Lu, N.Y.; Li, R.F. Silylated MgAl LDHs intercalated with MnO2 nanowires: Highly efficient catalysts for the solvent-free aerobic oxidation of ethylbenzene. Chem. Eng. J. 2015, 263, 309–316. [Google Scholar] [CrossRef]
- Wang, R.X.; Gao, B.J.; Jiao, W.Z. A novel method for immobilization of Co tetraphenylporphyrins on P(4VP-co-St)/SiO2: Efficient catalysts for aerobic oxidation of ethylbenzenes. Appl. Surf. Sci. 2009, 255, 4109–4113. [Google Scholar] [CrossRef]
- Shen, D.H.; Ji, L.T.; Liu, Z.G.; Sheng, W.B.; Guo, C.C. Ethylbenzene oxidation over hybrid metalloporphyrin@silica nanocomposite microspheres. J. Mol. Catal. A Chem. 2013, 379, 15–20. [Google Scholar]
- Zhao, M.; Ou, S.; Wu, C.D. Porous metal-organic frameworks for heterogeneous biomimetic catalysis. Acc. Chem. Res. 2014, 47, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.Y.; Zhang, Y.; Feng, J.; Li, W.Y.; Yu, W.W. Facile preparation and high performance of magnetically separable metalloporphyrin. Chem. Eng. J. 2015, 263, 385–391. [Google Scholar] [CrossRef]
- Brule, E.; Miguel, Y.R.; Hiic, K.K. Chemoselective epoxidation of dienes using polymer-supported manganese porphyrin catalysts. Tetrahedron 2004, 60, 5913–5918. [Google Scholar] [CrossRef]
- Mukherjee, M.; Ray, A.R. Biomimetic oxidation of l-arginine with hydrogen peroxide catalyzed by the resin-supported iron (III) porphyrin. J. Mol. Catal. A Chem. 2007, 266, 207–214. [Google Scholar] [CrossRef]
- Zhang, W.J.; Jiang, P.P.; Wang, Y.; Zhang, J.; Zheng, J.W.; Zhang, P.B. Selective oxidation over a metalloporphyrinic metal–organic framework catalyst and insights into the mechanism of bicarbonate ion as co-catalyst. Chem. Eng. J. 2014, 257, 28–35. [Google Scholar] [CrossRef]
- Kumar, D.; Latifi, R.; Kumar, S.; Rybak-Akimova, E.V.; Sainna, M.A.; de Visser, S.P. Rationalization of the barrier height for p-Z-styrene epoxidation by iron(IV)-oxo porphyrin cation radicals with variable axial ligands. Inorg. Chem. 2013, 52, 7968–7979. [Google Scholar] [CrossRef] [PubMed]
- Song, W.J.; Ryu, Y.O.; Song, R.; Nam, W. Oxoiron(IV) porphyrin p-cation radical complexes with a chameleon behavior in cytochrome P450 model reactions. J. Biol. Inorg. Chem. 2005, 10, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Yuan, R.X.; Peng, Y.; Chen, X.F.; Zhao, S.K.; Wei, S.J.; Guo, W.X.; Chen, X. Oxygen oxidation of ethylbenzene over manganese porphyrin is promoted by the axial nitrogen coordination in powdered chitosan. RSC Adv. 2016, 6, 48571–48579. [Google Scholar] [CrossRef]
- Zeng, K.; Huang, G.; Yuan, R.X.; Wang, W.L.; Guo, Y.A.; Zhao, S.K. Catalysis of nano-porous chitosan grafted manganese tetra(p-carboxylphenyl)porphyrin for oxidation of ethylbenzene. J. Guangxi Univ. (Nat. Sci. Ed.) 2015, 40, 505–513. [Google Scholar]
- Cai, J.L.; Huang, G.; Mo, L.Q.; Wei, Y.X.; Guo, Y.A. Catalysis of chitosan-supported cobalt tetrakis(p-sulphophenyl)porphyrin for oxidation of ethylbenzene. J. Guangxi Univ. (Nat. Sci. Ed.) 2014, 39, 1385–1392. [Google Scholar]
- Adler, A.D.; Longo, F.R.; Finarelli, J.D.; Goldmacher, J.; Assour, J.; Korssakow, L. A simplified synthesis for meso-tetraphenylporphin. J. Org. Chem. 1967, 32, 476. [Google Scholar] [CrossRef]
- Haber, J.; Kłosowski, M.; Połtowicz, J. Co-oxidation of styrene and iso-butyraldehyde in the presence of polyaniline-supported metalloporphyrins. J. Mol. Catal. A Chem. 2003, 201, 167–178. [Google Scholar] [CrossRef]
- Rorrer, G.L.; Hsien, T.Y.; Way, J.D. Synthesis of porous-magnetic chitosan beads for removal of cadmium ions from waste water. Ind. Eng. Chem. Res. 1993, 32, 2170–2178. [Google Scholar] [CrossRef]
- Huang, G.; Xiang, F.; Li, T.M.; Jiang, Y.X.; Guo, Y.A. Selective oxidation of toluene over the new catalyst cobalt tetra (4-hydroxyl) phenylporphyrin supported on zinc oxide. Catal. Commun. 2011, 12, 886–889. [Google Scholar] [CrossRef]
- Huang, G.; Mo, L.Q.; Cai, J.L.; Cao, X.; Peng, Y.; Guo, Y.A.; Wei, S.J. Environmentally friendly and efficient catalysis of cyclohexane oxidation by iron meso-tetrakis(pentafluorophenyl)porphyrinimmobilized on zinc oxide. Appl. Catal. B Environ. 2015, 162, 364–371. [Google Scholar] [CrossRef]
- Claure, M.T.; Chai, S.H.; Dai, S.; Unocic, K.A.; Alamgir, F.M.; Agrawal, P.K.; Jones, C.W. Tuning of higher alcohol selectivity and productivity in CO hydrogenation reactions over K/MoS2 domains supported on mesoporous activated carbon and mixed MgAl oxide. J. Catal. 2015, 324, 88–97. [Google Scholar] [CrossRef]
- Singh, V.; Tiwari, A.; Tripath, D.N.; Sanghi, R. Microwave enhanced synthesis of chitosan-graft –polyacrylamide. Polymer 2006, 47, 254–260. [Google Scholar] [CrossRef]
- Lim, S.H.; Hudson, S.M. Synthesis and antimicrobial activity of a water-soluble chitosan derivative with a fiber-reactive group. Carbohydr. Res. 2004, 339, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Chen, D.M.; He, T.J.; Liu, F.C. Raman and infrared spectral study of meso-sulfonatophenyl substituted porphyrins (TPPSn, n=1, 2A, 2O, 3, 4). Spectrochim. Acta Part A 2003, 59, 87–101. [Google Scholar] [CrossRef]
- Rong, Y.; Chen, P.; Liu, M. Self-assembly of water-soluble TPPS in organic solvents: From nanofibers to mirror imaged chiral nanorods. Chem. Commun. 2013, 49, 10498–10500. [Google Scholar] [CrossRef] [PubMed]
- Orihara, Y.; Uo, M.; Inoue, H.; Makishima, A.; Tani, T. Preparation and spectroscopy of lead-tin fluorophosphate glass doped with TPPS and TPPS-Sn. Phys.Chem. 1996, 100, 1582–1587. [Google Scholar] [CrossRef]
- Shen, L.L.; Qu, R.; Shi, H.J.; Huang, F.; An, Y.L.; Shi, L.Q. A biocompatible cobaltporphyrin-based complex micelle constructed via supramolecular assembly for oxygen transfer. Biomater. Sci. 2016, 4, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Annoni, E.; Pizzotti, M.; Ugo, R.; Quici, S.; Morotti, T.; Casati, N.; Macchi, P. The effect on E-stilbazoles second order NLO response by axial interaction with M.(II),10,15,20-tetraphenyl porphyrinates (M = Zn, Ru, Os); a new crystalline packing with very large holes. Inorg. Chim. Acta 2006, 359, 3029–3041. [Google Scholar] [CrossRef]
- Yan, Y.; Yao, P.P.; Mu, Q.; Wang, L.; Mu, J.; Li, X.Q.; Kang, S.Z. Electrochemical behavior of amino-modified multi-walled carbon nanotubes coordinated with cobalt porphyrin for the oxidation of nitric oxide. Appl. Surf. Sci. 2011, 258, 58–63. [Google Scholar] [CrossRef]
- Marco, J.F.; Gancedo, J.R.; Gracia, M.; Gautier, J.L.; Rios, E.I.; Palmer, R.; Greaves, C.; Berry, F.J. Cation distribution and magnetic structure of the ferrimagnetic spinel NiCo2O4. J. Mater. Chem. 2001, 11, 3087–3093. [Google Scholar] [CrossRef]
- Liang, H.W.; Wei, W.; Wu, Z.S.; Feng, X.L.; Mullen, K. Mesoporous metal-nitrogen-doped carbon electrocatalysts for highly efficient oxygen reduction reaction. J. Am. Chem. Soc. 2013, 135, 16002–16005. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zheng, L.C.; Li, Y.W.; Free, M.; Yang, M.Z. Adsorptive recovery of palladium(II) from aqueous solution onto cross-linked chitosan/montmorillonite membrane. RSC Adv. 2016, 6, 51757–51767. [Google Scholar] [CrossRef]
- Roy, P.S.; Samanta, A.; Mukherjee, M.; Roy, B.; Mukherjee, A. Designing novel pH-induced chitosan–gum odina complex coacervates for colon targeting. Ind. Eng. Chem. Res. 2013, 52, 15728–15745. [Google Scholar] [CrossRef]
- Maachou, H.; Genet, M.J.; Aliouche, D.; Dupont-Gillainb, C.C.; Rouxhet, P.G. XPS analysis of chitosan–hydroxyapatite biomaterials: From elements to compounds. Surf. Interface Anal. 2013, 45, 1088–1097. [Google Scholar] [CrossRef]
- Berner, S.; Lidbaum, H.; Ledung, G.; Ahlund, J.; Nilson, K.; Schiessling, J.; Gelius, U.; Backvall, J.E.; Puglia, C.; Oscarsson, S. Electronic and structural studies of immobilized thiol-derivatized cobalt porphyrins on gold surfaces. Appl. Surf. Sci. 2007, 253, 7540–7548. [Google Scholar] [CrossRef]
- Xin, L.; Yang, F.; Rasouli, S.; Qiu, Y.; Li, Z.F.; Uzunoglu, A.; Sun, C.J.; Liu, Y.Z.; Ferreira, P.; Li, W.Z.; et al. Understanding Pt nanoparticle anchoring on graphene supports through surface functionalization. ACS Catal. 2016, 6, 2642–2653. [Google Scholar] [CrossRef]
- Varadwaj, G.B.B.; Rana, S.; Parida, K.; Nayakc, B.B. A multi-functionalized montmorillonite for co-operative catalysis in one-pot Henry reaction and water pollution remediation. J. Mater. Chem. A 2014, 2, 7526–7534. [Google Scholar] [CrossRef]
- Han, M.G.; Cho, S.K.; Oh, S.G.; Im, S.S. Preparation and characterization of polyaniline nanoparticles synthesized from DBSA micellar solution. Synth. Met. 2002, 126, 53–60. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Xu, C.; Zhou, Y.F. Crosslinked chitosan nanofiber mats fabricated by one-step electrospinning and ion-imprinting methods for metal ions adsorption. Sci. China Chem. 2015, 59, 95–105. [Google Scholar] [CrossRef]
- Fidalgo-Marijuan, A.; Barandika, G.; Bazan, B.; Urtiaga, M.K.; Arriortua, M.I. Thermal stability and crystallochemical analysis for CoII-based coordination polymers with TPP and TPPS porphyrins. CrystEngComm 2013, 15, 4181–4188. [Google Scholar] [CrossRef]
- Huang, G.; Luo, J.; Deng, C.C.; Guo, Y.A.; Zhao, S.K.; Zhou, H.; Wei, S. Catalytic oxidation of toluene with molecular oxygen over manganese tetraphenylporphyrin supported on chitosan. Appl. Catal. A Gen. 2008, 338, 83–86. [Google Scholar] [CrossRef]
- Black, J.F. Metal-catalyzed autoxidation. The unrecognized consequences of metal-hydoperoxide complex formation. J. Am. Chem. Soc. 1978, 100, 527–535. [Google Scholar] [CrossRef]
- Guo, C.C.; Liu, Q.; Wang, X.T.; Hu, H.Y. Selective liquid phase oxidation of toluene with air. Appl. Catal. A Gen. 2005, 282, 55–59. [Google Scholar] [CrossRef]
- Guo, C.C.; Liu, X.Q.; Liu, Y.; Liu, Q.; Chu, M.F.; Zhang, X.B. Studies of simple μ-oxo-bisiron(III)porphyrin as catalyst of cyclohexane oxidation with air in absence of cocatalysts or coreductants. J. Mol. Catal. A Chem. 2003, 192, 289–294. [Google Scholar] [CrossRef]
- Kasaikina, O.T.; Kortenska, V.D.; Kartasheva, Z.S.; Kuznetsova, G.M.; Maximova, T.V.; Sirota, T.V.; Yanishlieva, N.V. Hydrocarbon and lipid oxidation in micro heterogeneous systems formed by surfactants or nanodispersed Al2O3, SiO2 and TiO2. Colloids Surf. A Physicochem. Eng. Aspects 1999, 149, 29–38. [Google Scholar] [CrossRef]
- Evans, S.; Smith, J.R.L. The oxidation of ethylbenzene and other alkylaromatics by dioxygen catalysed by iron(III) tetrakis(pentafluorophenyl)porphyrin and related iron porphyrins. J. Chem. Soc. Perkin Trans. 2000, 2, 1541–1552. [Google Scholar] [CrossRef]
- Evans, S.; Smith, J.R.L. The oxidation of ethylbenzene by dioxygen catalysed by supported iron porphyrins derived from iron(III) tetrakis(pentafluorophenyl)porphyrin. J. Chem. Soc. Perkin Trans 2001, 2, 174–180. [Google Scholar] [CrossRef]
- Lyons, J.E.; Ellis, P.E.; Myers, H.K. Halogenated metalloporphyrin complexes as catalysts for selective reactions of acyclic alkanes with molecular oxygen. J. Catal. 1995, 155, 59–73. [Google Scholar] [CrossRef]
- Zhou, X.T.; Ji, H.B. Highly efficient oxidative cleavage of carbon-carbon double bond over meso-tetraphenyl cobalt porphyrin catalyst in the presence of molecular oxygen. Chin. J. Chem. 2012, 30, 2103–2108. [Google Scholar] [CrossRef]
- Scherson, D.A.; Gupta, S.L.; Fierro, C.; Yeager, E.B.; Kordesch, M.E.; Eldridge, J.; Hoffman, R.W.; Blue, J. Cobalt tetramethoxyphenyl porphyrin-emission mossbauer spectroscopy and O2, reduction electrochemical studies. Electrochim. Acta. 1983, 28, 1205–1209. [Google Scholar] [CrossRef]
- Walker, F.A. ESR studies of Co(II) tetraphenylporphyrins and their oxygen adducts: Complex formation with aromatic molecules and sterically hindered lewis base. J. Magn. Reson. 1974, 15, 201–218. [Google Scholar] [CrossRef]
- Weselucha-Birczynska, A.; Nakamotob, K.; Proniewicz, L.M. Simultaneous observation of ν(O–O), ν(Co–O2) and δ(CoOO) in resonance Raman spectra of five- and six-coordinate dioxygen adducts of Co(TPP-d8). J. Mol. Struct. 1992, 275, 95–103. [Google Scholar] [CrossRef]
- Lee, C.H.; Lin, H.C.; Cheng, S.H.; Lin, T.S.; Mou, C.Y. Hydroxo-bridged dinuclear cupric complexes encapsulated in various mesoporous silicas to mimic the catalytic activity of catechol oxidases: Reactivity and selectivity study. J. Phys. Chem. C 2009, 113, 16058–16069. [Google Scholar] [CrossRef]
- Feng, X.; Sheng, N.; Liu, Y.B.; Che, X.B.; Chen, D.; Yang, C.H.; Zhou, X.G. Simultaneously enhanced stability and selectivity for propene epoxidation with H2 and O2 on Au catalysts supported on nano-crystalline mesoporous TS-1. ACS Catal. 2017, 7, 2668–2675. [Google Scholar] [CrossRef]







| Material | BET Surface Area (m/g) | BJH Total Pore Volume (cm/g) | BJH Adsorption Average Pore Diameter (nm) |
|---|---|---|---|
| mesp-CTS | 104.2 | 0.64 | 23.1 |
| Co TPPS/mesp-CTS | 102.2 | 0.64 | 23.0 |
| XPS Spectra | Existential form of the Key Element | Binding Energy/eV | ||
|---|---|---|---|---|
| Co TPPS/mesp-CTS | Co TPPS | mesp-CTS | ||
| Co 2p | Co–N | 798.0 | 797.6 | - |
| 786.6 | 782.1 | - | ||
| Cl 2p | Cl–Co | 196.4 | 199.6 | - |
| N 1s | N–C= (N=C) | 399.8 | 399.9 | - |
| N–Co | 398.0 | 398.6 | - | |
| NH2–C | 398.1 | - | 397.7 | |
| N=C | 400.5 | - | 400.6 | |
| NH–C=O | 400.9 | - | 400.9 | |
| NH3–C | 401.5 | - | - | |
| C 1s | C=O, O–C–O | 287.8 | - | 288.0 |
| C–N, C=N, | ||||
| C–O, C–O–C | 285.0 | 284.8 | 285.0 | |
| C–C, C=C | 283.8 | 283.2 | 283.6 | |
| O 1s | O–S | 531.3 | 532.2 | - |
| O=S | – | 531.8 | - | |
 | |||||||
| Catalyst | Run | TON (×106) | Yield (%) | Selectivity (%) | |||
| -on | -ol | -al | (-ac) + (-es) | ||||
| Co TPPS/mesp-CTS | 1 | 1.10 | 42.8 | 65.4 | 5.4 | 3.4 | 25.8 |
| 2 | 1.65 | 50.0 | 65.4 | 6.0 | 3.6 | 25.0 | |
| 3 | 2.04 | 43.7 | 62.4 | 4.5 | 3.1 | 30.0 | |
| 4 | 5.05 | 42.0 | 62.5 | 3.5 | 3.2 | 30.8 | |
| Average | 2.46 | 44.6 | 63.9 | 4.9 | 3.3 | 27.9 | |
| Co TPPS b | 1 | 0.90 | 34.8 | 53.3 | 4.0 | 3.1 | 39.6 |
| Co TPPS/macp-CTS b | 1 | 0.99 | 36.8 | 60.1 | 4.9 | 4.0 | 31.0 |
| No catalyst | 4.7 | 43.3 | 13.3 | 8.8 | 34.6 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, G.; Mo, L.Q.; Wei, Y.X.; Zhou, H.; Guo, Y.A.; Wei, S.J. Effect of Mesoporous Chitosan Action and Coordination on the Catalytic Activity of Mesoporous Chitosan-Grafted Cobalt Tetrakis(p-Sulfophenyl)Porphyrin for Ethylbenzene Oxidation. Catalysts 2018, 8, 199. https://doi.org/10.3390/catal8050199
Huang G, Mo LQ, Wei YX, Zhou H, Guo YA, Wei SJ. Effect of Mesoporous Chitosan Action and Coordination on the Catalytic Activity of Mesoporous Chitosan-Grafted Cobalt Tetrakis(p-Sulfophenyl)Porphyrin for Ethylbenzene Oxidation. Catalysts. 2018; 8(5):199. https://doi.org/10.3390/catal8050199
Chicago/Turabian StyleHuang, Guan, Lin Qiang Mo, Yan Xun Wei, Hong Zhou, Yong An Guo, and Su Juan Wei. 2018. "Effect of Mesoporous Chitosan Action and Coordination on the Catalytic Activity of Mesoporous Chitosan-Grafted Cobalt Tetrakis(p-Sulfophenyl)Porphyrin for Ethylbenzene Oxidation" Catalysts 8, no. 5: 199. https://doi.org/10.3390/catal8050199