Nanostructured Oxides Synthesised via scCO2-Assisted Sol-Gel Methods and Their Application in Catalysis
Abstract
:1. Introduction
1.1. Nanostructured Oxides and Their Synthesis
1.2. Sol-Gel Method
1.3. Supercritical CO2 and its Properties as Reaction Medium and Drying Agent
2. Nanostructured Oxides Synthesised via scCO2-Assisted Sol-Gel Methods
2.1. ScCO2 as Solvent
2.2. ScCO2 as Anti-Solvent
2.3. Combination of Sol-Gel Methods and scCO2 Drying to Produce Aerogels
3. Catalytic Applications of Nanostructured Oxides Prepared by scCO2-Assisted Methods
3.1. Photocatalysis
3.2. Chemocatalytic Oxidation of Organic Compounds
3.3. Chemocatalytic Oxidation and Reduction of Inorganic Compounds
3.4. Nanostructured Oxides as Catalyst Supports
3.4.1. Supported Ni-Based Catalysts
Reforming Reactions
Other Catalytic Reactions
3.4.2. Supported Noble-Metal Nanoparticles Catalysts
4. Conclusions and Perspectives
Acknowledgments
Conflicts of Interest
References
- Patzke, G.R.; Zhou, Y.; Kontic, R.; Conrad, F. Oxide nanomaterials: Synthetic developments, mechanistic studies, and technological innovations. Angew. Chem. Int. Ed. 2011, 50, 826–859. [Google Scholar] [CrossRef] [PubMed]
- Alonso, E.; Montequi, I.; Cocero, M. Effect of synthesis conditions on photocatalytic activity of TiO2 powders synthesized in supercritical CO2. J. Supercrit. Fluids 2009, 49, 233–238. [Google Scholar] [CrossRef]
- Seo, J.G.; Youn, M.H.; Bang, Y.; Song, I.K. Effect of Ni/Al atomic ratio of mesoporous Ni-Al2O3 aerogel catalysts on their catalytic activity for hydrogen production by steam reforming of liquefied natural gas (LNG). Int. J. Hydrogen Energy 2010, 35, 12174–12181. [Google Scholar] [CrossRef]
- Lan, Y.; Li, X.; Li, G.; Luo, Y. Sol-gel method to prepare graphene/Fe2O3 aerogel and its catalytic application for the thermal decomposition of ammonium perchlorate. J. Nanopart. Res. 2015, 17, 395. [Google Scholar] [CrossRef]
- Krumm, M.; Pueyo, C.L.; Polarz, S. Monolithic zinc oxide aerogels from organometallic sol-gel precursors. Chem. Mater. 2010, 22, 5129–5136. [Google Scholar] [CrossRef]
- Gash, A.E.; Tillotson, T.M.; Satcher, J.H., Jr.; Hrubesh, L.W.; Simpson, R.L. New sol-gel synthetic route to transition and main-group metal oxide aerogels using inorganic salt precursors. J. Non-Cryst. Solids 2001, 285, 22–28. [Google Scholar] [CrossRef]
- Zeng, T.; Zhang, X.; Wang, S.; Niu, H.; Cai, Y. Spatial confinement of a Co3O4 catalyst in hollow metal-organic frameworks as a nanoreactor for improved degradation of organic pollutants. Environ. Sci. Technol. 2015, 49, 2350–2357. [Google Scholar] [CrossRef] [PubMed]
- Novak, Z.; Kotnik, P.; Knez, Ž. Preparation of WO3 aerogel catalysts using supercritical CO2 drying. J. Non-Cryst. Solids 2004, 350, 308–313. [Google Scholar] [CrossRef]
- Cabañas, A.; Enciso, E.; Carbajo, M.C.; Torralvo, M.J.; Pando, C.; Renuncio, J.A.R. Synthesis of SiO2-aerogel inverse opals in supercritical carbon dioxide. Chem. Mater. 2005, 17, 6137–6145. [Google Scholar] [CrossRef]
- Sui, R.; Rizkalla, A.; Charpentier, P.A. Experimental study on the morphology and porosity of TiO2 aerogels synthesized in supercritical carbon dioxide. Microporous Mesoporous Mater. 2011, 142, 688–695. [Google Scholar] [CrossRef]
- Chowdhury, M.B.; Sui, R.; Lucky, R.A.; Charpentier, P.A. One-pot procedure to synthesize high surface area alumina nanofibers using supercritical carbon dioxide. Langmuir 2009, 26, 2707–2713. [Google Scholar] [CrossRef] [PubMed]
- Gleiter, H. Nanostructured materials: Basic concepts and microstructure. Acta Mater. 2000, 48, 1–29. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Lelong, G.; Saboungi, M.-L. Recent progress in the synthesis and selected applications of MCM-41: A short review. J. Exp. Nanosci. 2006, 1, 375–395. [Google Scholar] [CrossRef]
- Sui, R.; Charpentier, P. Synthesis of metal oxide nanostructures by direct sol-gel chemistry in supercritical fluids. Chem. Rev. 2012, 112, 3057–3082. [Google Scholar] [CrossRef] [PubMed]
- Frenzer, G.; Maier, W.F. Amorphous porous mixed oxides: Sol-gel ways to a highly versatile class of materials and catalysts. Annu. Rev. Mater. Res. 2006, 36, 281–331. [Google Scholar] [CrossRef]
- Tseng, T.K.; Lin, Y.S.; Chen, Y.J.; Chu, H. A review of photocatalysts prepared by sol-gel method for VOCs removal. Int. J. Mol. Sci. 2010, 11, 2336–2361. [Google Scholar] [CrossRef] [PubMed]
- Debecker, D.P.; Mutin, P.H. Non-hydrolytic sol-gel routes to heterogeneous catalysts. Chem. Soc. Rev. 2012, 41, 3624–3650. [Google Scholar] [CrossRef] [PubMed]
- Debecker, D.P.; Hulea, V.; Mutin, P.H. Mesoporous mixed oxide catalysts via non-hydrolytic sol-gel: A review. Appl. Catal. A 2013, 451, 192–206. [Google Scholar] [CrossRef]
- Seisenbaeva, G.A.; Kessler, V.G. Precursor directed synthesis-“molecular” mechanisms in the Soft Chemistry approaches and their use for template-free synthesis of metal, metal oxide and metal chalcogenide nanoparticles and nanostructures. Nanoscale 2014, 6, 6229–6244. [Google Scholar] [CrossRef] [PubMed]
- Kessler, V.G.; Spijksma, G.I.; Seisenbaeva, G.A.; Håkansson, S.; Blank, D.H.; Bouwmeester, H.J. New insight in the role of modifying ligands in the sol-gel processing of metal alkoxide precursors: A possibility to approach new classes of materials. J. Sol-Gel Sci. Technol. 2006, 40, 163–179. [Google Scholar] [CrossRef]
- Sanli, D.; Bozbag, S.; Erkey, C. Synthesis of nanostructured materials using supercritical CO2: Part I. Physical transformations. J. Mater. Sci. 2012, 47, 2995–3025. [Google Scholar] [CrossRef]
- Bozbag, S.; Sanli, D.; Erkey, C. Synthesis of nanostructured materials using supercritical CO2: Part II. Chemical transformations. J. Mater. Sci. 2012, 47, 3469–3492. [Google Scholar] [CrossRef]
- Zhang, X.; Heinonen, S.; Levänen, E. Applications of supercritical carbon dioxide in materials processing and synthesis. RSC Adv. 2014, 4, 61137–61152. [Google Scholar] [CrossRef]
- Duan, H.; Wang, D.; Li, Y. Green chemistry for nanoparticle synthesis. Chem. Soc. Rev. 2015, 44, 5778–5792. [Google Scholar] [CrossRef] [PubMed]
- Cansell, F.; Aymonier, C. Design of functional nanostructured materials using supercritical fluids. J. Supercrit. Fluids 2009, 47, 508–516. [Google Scholar] [CrossRef]
- Marre, S.; Jensen, K.F. Synthesis of micro and nanostructures in microfluidic systems. Chem. Soc. Rev. 2010, 39, 1183–1202. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, E.; Qiubai, S.; Zhang, Z.; Zhang, C.; Wei, G. Mini-Review: Green sustainable processes using supercritical fluid carbon dioxide. J. Environ. Sci. 2009, 21, 720–726. [Google Scholar] [CrossRef]
- Loy, D.A.; Russick, E.M.; Yamanaka, S.A.; Baugher, B.M.; Shea, K.J. Direct formation of aerogels by sol-gel polymerizations of alkoxysilanes in supercritical carbon dioxide. Chem. Mater. 1997, 9, 2264–2268. [Google Scholar] [CrossRef]
- Sui, R.; Rizkalla, A.S.; Charpentier, P.A. Kinetics study on the sol-gel reactions in supercritical CO2 by using in situ ATR-FTIR spectrometry. Cryst. Growth Des. 2008, 8, 3024–3031. [Google Scholar] [CrossRef]
- Sui, R.; Rizkalla, A.S.; Charpentier, P.A. Synthesis and formation of silica aerogel particles by a novel sol-gel route in supercritical carbon dioxide. J. Phys. Chem. B 2004, 108, 11886–11892. [Google Scholar] [CrossRef]
- Farhangi, N.; Chowdhury, R.R.; Medina-Gonzalez, Y.; Ray, M.B.; Charpentier, P.A. Visible light active Fe doped TiO2 nanowires grown on graphene using supercritical CO2. Appl. Catal. B 2011, 110, 25–32. [Google Scholar] [CrossRef]
- Lucky, R.A.; Charpentier, P.A. N-doped ZrO2/TiO2 bimetallic materials synthesized in supercritical CO2: Morphology and photocatalytic activity. Appl. Catal. B 2010, 96, 516–523. [Google Scholar] [CrossRef]
- Lucky, R.A.; Medina-Gonzalez, Y.; Charpentier, P.A. Zr doping on one-dimensional titania nanomaterials synthesized in supercritical carbon dioxide. Langmuir 2010, 26, 19014–19021. [Google Scholar] [CrossRef] [PubMed]
- Lucky, R.A.; Charpentier, P.A. A One-step approach to the synthesis of ZrO2-modified TiO2 nanotubes in supercritical carbon dioxide. Adv. Mater. 2008, 20, 1755–1759. [Google Scholar] [CrossRef]
- Lucky, R.; Charpentier, P. A thermal study on the structural changes of bimetallic ZrO2-modified TiO2 nanotubes synthesized using supercritical CO2. Nanotechnology 2009, 20, 195601. [Google Scholar] [CrossRef] [PubMed]
- Charpentier, P.A.; Li, X.; Sui, R. Study of the sol-gel reaction mechanism in supercritical CO2 for the formation of SiO2 nanocomposites. Langmuir 2009, 25, 3748–3754. [Google Scholar] [CrossRef] [PubMed]
- Salarian, M.; Xu, W.Z.; Wang, Z.; Sham, T.-K.; Charpentier, P.A. Hydroxyapatite-TiO2-based nanocomposites synthesized in supercritical CO2 for bone tissue engineering: Physical and mechanical properties. ACS Appl. Mater. Interfaces 2014, 6, 16918–16931. [Google Scholar] [CrossRef] [PubMed]
- Jensen, H.; Bremholm, M.; Nielsen, R.P.; Joensen, K.D.; Pedersen, J.S.; Birkedal, H.; Chen, Y.S.; Almer, J.; Søgaard, E.G.; Iversen, S.B. In situ high-energy synchrotron radiation study of sol-gel nanoparticle formation in supercritical fluids. Angew. Chem. Int. Ed. 2007, 46, 1113–1116. [Google Scholar] [CrossRef] [PubMed]
- Durand, V.; Drobek, M.; Duchateau, M.; Hertz, A.; Ruiz, J.-C.; Sarrade, S.; Guizard, C.; Julbe, A. Potential of sub-and supercritical CO2 reaction media for sol-gel deposition of silica-based molecular sieve membranes. Sep. Purif. Technol. 2014, 121, 30–37. [Google Scholar] [CrossRef]
- Jespersen, H.T.; Štandeker, S.; Novak, Z.; Schaumburg, K.; Madsen, J.; Knez, Ž. Supercritical fluids applied to the sol-gel process for preparation of AEROMOSILS/palladium particle nanocomposite catalyst. J. Supercrit. Fluids 2008, 46, 178–184. [Google Scholar] [CrossRef]
- Hertz, A.; Corre, Y.-M.; Sarrade, S.; Guizard, C.; Julbe, A.; Ruiz, J.-C.; Fournel, B. Yttria stabilized zirconia synthesis in supercritical CO2: Understanding of particle formation mechanisms in CO2/co-solvent systems. J. Eur. Ceram. Soc. 2010, 30, 1691–1698. [Google Scholar] [CrossRef]
- Jammaer, J.; Aprile, C.; Verbruggen, S.W.; Lenaerts, S.; Pescarmona, P.P.; Martens, J.A. A non-aqueous synthesis of TiO2/SiO2 composites in supercritical CO2 for the photodegradation of pollutants. ChemSusChem 2011, 4, 1457–1463. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Xu, Q.; Li, L. Porous TiO2/SiO2 composite prepared using PEG as template direction reagent with assistance of supercritical CO2. J. Colloid Interface Sci. 2007, 316, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, A.; Huang, Y.; Xu, Q.; Yin, J.; Zhang, T. Nanocasting synthesis of mesostructured Co3O4 via a supercritical CO2 deposition method and the catalytic performance for CO oxidation. Catal. Lett. 2012, 142, 275–281. [Google Scholar] [CrossRef]
- Jiang, H.; Huang, P.; Liu, L.; Zhang, M. Controllable synthesis of Ce1−xZrxO2 hollow nanospheres via supercritical anti-solvent precipitation. Mater. Charact. 2012, 63, 98–104. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, H.; Kuang, L.; Zhang, M. Synthesis of highly dispersed MnOx-CeO2 nanospheres by surfactant-assisted supercritical anti-solvent (SAS) technique: The important role of the surfactant. J. Supercrit. Fluids 2014, 92, 84–92. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, H.; Kuang, L.; Li, G.; Zhang, M. Synthesis of MnOx-CeO2·NOx catalysts by polyvinylpyrrolidone-assisted supercritical antisolvent precipitation. J. Mater. Res. 2014, 29, 2188–2197. [Google Scholar] [CrossRef]
- Nesterov, N.; Paharukova, V.; Yakovlev, V.; Martyanov, O. The facile synthesis of Ni-Cu catalysts stabilized in SiO2 framework via a supercritical antisolvent approach. J. Supercrit. Fluids 2016, 112, 119–127. [Google Scholar] [CrossRef]
- Tang, Z.-R.; Edwards, J.K.; Bartley, J.K.; Taylor, S.H.; Carley, A.F.; Herzing, A.A.; Kiely, C.J.; Hutchings, G.J. Nanocrystalline cerium oxide produced by supercritical antisolvent precipitation as a support for high-activity gold catalysts. J. Catal. 2007, 249, 208–219. [Google Scholar] [CrossRef]
- Hutchings, G.J. Catalyst synthesis using supercritical carbon dioxide: A green route to high activity materials. Top. Catal. 2009, 52, 982–987. [Google Scholar] [CrossRef]
- Miedziak, P.J.; Tang, Z.; Davies, T.E.; Enache, D.I.; Bartley, J.K.; Carley, A.F.; Herzing, A.A.; Kiely, C.J.; Taylor, S.H.; Hutchings, G.J. Ceria prepared using supercritical antisolvent precipitation: A green support for gold-palladium nanoparticles for the selective catalytic oxidation of alcohols. J. Mater. Chem. 2009, 19, 8619–8627. [Google Scholar] [CrossRef]
- Marin, R.P.; Kondrat, S.A.; Gallagher, J.R.; Enache, D.I.; Smith, P.; Boldrin, P.; Davies, T.E.; Bartley, J.K.; Combes, G.B.; Williams, P.B. Preparation of Fischer-Tropsch supported cobalt catalysts using a new gas anti-solvent process. ACS Catal. 2013, 3, 764–772. [Google Scholar] [CrossRef]
- Marin, R.P.; Kondrat, S.A.; Pinnell, R.K.; Davies, T.E.; Golunski, S.; Bartley, J.K.; Hutchings, G.J.; Taylor, S.H. Green preparation of transition metal oxide catalysts using supercritical CO2 anti-solvent precipitation for the total oxidation of propane. Appl. Catal. B 2013, 140, 671–679. [Google Scholar] [CrossRef]
- Tang, Z.R.; Jones, C.D.; Aldridge, J.K.; Davies, T.E.; Bartley, J.K.; Carley, A.F.; Taylor, S.H.; Allix, M.; Dickinson, C.; Rosseinsky, M.J. New nanocrystalline Cu/MnOx catalysts prepared from supercritical antisolvent precipitation. ChemCatChem 2009, 1, 247–251. [Google Scholar] [CrossRef]
- Tang, Z.-R.; Kondrat, S.A.; Dickinson, C.; Bartley, J.K.; Carley, A.F.; Taylor, S.H.; Davies, T.E.; Allix, M.; Rosseinsky, M.; Claridge, J. Synthesis of high surface area CuMn2O4 by supercritical anti-solvent precipitation for the oxidation of CO at ambient temperature. Catal. Sci. Technol. 2011, 1, 740–746. [Google Scholar] [CrossRef]
- Choi, J.; Suh, D.J. Catalytic applications of aerogels. Catal. Surv. Asia 2007, 11, 123–133. [Google Scholar] [CrossRef]
- Pierre, A.C.; Pajonk, G.M. Chemistry of aerogels and their applications. Chem. Rev. 2002, 102, 4243–4266. [Google Scholar] [CrossRef] [PubMed]
- Pajonk, G. Aerogel catalysts. Appl. Catal. 1991, 72, 217–266. [Google Scholar] [CrossRef]
- Pajonk, G. Catalytic aerogels. Catal. Today 1997, 35, 319–337. [Google Scholar] [CrossRef]
- Dorcheh, A.S.; Abbasi, M. Silica aerogel; synthesis, properties and characterization. J. Mater. Process. Technol. 2008, 199, 10–26. [Google Scholar] [CrossRef]
- Poco, J.; Satcher, J.; Hrubesh, L. Synthesis of high porosity, monolithic alumina aerogels. J. Non-Cryst. Solids 2001, 285, 57–63. [Google Scholar] [CrossRef]
- Sui, R.; Rizkalla, A.S.; Charpentier, P.A. Direct synthesis of zirconia aerogel nanoarchitecture in supercritical CO2. Langmuir 2006, 22, 4390–4396. [Google Scholar] [CrossRef] [PubMed]
- Gash, A.E.; Tillotson, T.M.; Satcher, J.H., Jr.; Poco, J.F.; Hrubesh, L.W.; Simpson, R.L. Use of epoxides in the sol-gel synthesis of porous iron (III) oxide monoliths from Fe (III) salts. Chem. Mater. 2001, 13, 999–1007. [Google Scholar] [CrossRef]
- Dong, W.; Mansour, A.; Dunn, B. Structural and electrochemical properties of amorphous and crystalline molybdenum oxide aerogels. Solid State Ionics 2001, 144, 31–40. [Google Scholar] [CrossRef]
- Popa, M.; Macovei, D.; Indrea, E.; Mercioniu, I.; Popescu, I.; Danciu, V. Synthesis and structural characteristics of nitrogen doped TiO2 aerogels. Microporous Mesoporous Mater. 2010, 132, 80–86. [Google Scholar] [CrossRef]
- Beauger, C.; Testut, L.; Berthon-Fabry, S.; Georgi, F.; Guétaz, L. Doped TiO2 aerogels as alternative catalyst supports for proton exchange membrane fuel cells: A comparative study of Nb, V and Ta dopants. Microporous Mesoporous Mater. 2016, 232, 109–118. [Google Scholar] [CrossRef] [Green Version]
- Suh, D.J.; Park, T.-J.; Lee, S.-H.; Kim, K.-L. Nickel-alumina composite aerogels as liquid-phase hydrogenation catalysts. J. Non-Cryst. Solids 2001, 285, 309–316. [Google Scholar] [CrossRef]
- Seo, J.G.; Youn, M.H.; Jung, J.C.; Song, I.K. Hydrogen production by steam reforming of liquefied natural gas (LNG) over mesoporous nickel-alumina aerogel catalyst. Int. J. Hydrogen Energy 2010, 35, 6738–6746. [Google Scholar] [CrossRef]
- Yao, N.; Cao, S.; Yeong, K.L. Mesoporous TiO2-SiO2 aerogel with hierarchal pore structures. Microporous Mesoporous Mater. 2009, 117, 570–579. [Google Scholar] [CrossRef]
- Choi, J.; Shin, C.B.; Park, T.-J.; Suh, D.J. Characteristics of vanadia-titania aerogel catalysts for oxidative destruction of 1, 2-dichlorobenzene. Appl. Catal. A 2006, 311, 105–111. [Google Scholar] [CrossRef]
- Davis, M.; Hikal, W.M.; Gümeci, C.; Hope-Weeks, L.J. Aerogel nanocomposites of ZnO-SnO2 as efficient photocatalysts for the degradation of Rhodamine B. Catal. Sci. Technol. 2012, 2, 922–924. [Google Scholar] [CrossRef]
- Neumann, B.; Elkins, T.W.; Gash, A.E.; Hagelin-Weaver, H.; Bäumer, M. Sol-gel preparation of samaria catalysts for the oxidative coupling of methane. Catal. Lett. 2015, 145, 1251–1261. [Google Scholar] [CrossRef]
- Köppel, R.A.; Stöcker, C.; Baiker, A. Copper-and silver-zirconia aerogels: Preparation, structural properties and catalytic behavior in methanol synthesis from carbon dioxide. J. Catal. 1998, 179, 515–527. [Google Scholar] [CrossRef]
- Kwak, C.; Park, T.-J.; Suh, D.J. Preferential oxidation of carbon monoxide in hydrogen-rich gas over platinum-cobalt-alumina aerogel catalysts. Chem. Eng. Sci. 2005, 60, 1211–1217. [Google Scholar] [CrossRef]
- Wagh, P.B.; Begag, R.; Pajonk, G.M.; Venkateswara Rao, A.; Haranath, D. Comparison of some physical properties of silica aerogel monoliths synthesized by different precursors. Mater. Chem. Phys. 1999, 57, 214–218. [Google Scholar]
- Guo, G.; Whitesell, J.K.; Fox, M.A. Synthesis of TiO2 photocatalysts in supercritical CO2 via a non-hydrolytic route. J. Phys. Chem. B 2005, 109, 18781–18785. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhu, J.; Liu, Y.-M.; Cao, Y.; Li, H.-X.; He, H.-Y.; Dai, W.-L.; Fan, K.-N. Photocatalytic activity of epoxide sol-gel derived titania transformed into nanocrystalline aerogel powders by supercritical drying. J. Mol. Catal. A Chem. 2006, 255, 260–268. [Google Scholar] [CrossRef]
- Li, H.; Sunol, S.; Sunol, A. Development of titanium-dioxide-based aerogel catalyst with tunable nanoporosity and photocatalytic activity. Nanotechnology 2012, 23, 294012. [Google Scholar] [CrossRef] [PubMed]
- Mumin, M.A.; Moula, G.; Charpentier, P.A. Supercritical CO2 synthesized TiO2 nanowires covalently linked with core-shell CdS-ZnS quantum dots: Enhanced photocatalysis and stability. RSC Adv. 2015, 5, 67767–67779. [Google Scholar] [CrossRef]
- Tillotson, T.; Sunderland, W.; Thomas, I.; Hrubesh, L. Synthesis of lanthanide and lanthanide-silicate aerogels. J. Sol-Gel Sci. Technol. 1994, 1, 241–249. [Google Scholar] [CrossRef]
- Livraghi, S.; Paganini, M.C.; Giamello, E.; Selloni, A.; Di Valentin, C.; Pacchioni, G. Origin of photoactivity of nitrogen-doped titanium dioxide under visible light. JACS 2006, 128, 15666–15671. [Google Scholar] [CrossRef] [PubMed]
- Serpone, N. Is the band gap of pristine TiO2 narrowed by anion-and cation-doping of titanium dioxide in second-generation photocatalysts? J. Phys. Chem. B 2006, 110, 24287–24293. [Google Scholar] [CrossRef] [PubMed]
- Bahruji, H.; Bowker, M.; Davies, P.R.; Pedrono, F. New insights into the mechanism of photocatalytic reforming on Pd/TiO2. Appl. Catal. B 2011, 107, 205–209. [Google Scholar] [CrossRef]
- Marin, R.P.; Ishikawa, S.; Bahruji, H.; Shaw, G.; Kondrat, S.A.; Miedziak, P.J.; Morgan, D.J.; Taylor, S.H.; Bartley, J.K.; Edwards, J.K. Supercritical antisolvent precipitation of TiO2 with tailored anatase/rutile composition for applications in redox catalysis and photocatalysis. Appl. Catal. A 2015, 504, 62–73. [Google Scholar] [CrossRef]
- Fort, C.; Pap, Z.; Indrea, E.; Baia, L.; Danciu, V.; Popa, M. Pt/N-TiO2 Aerogel composites used for hydrogen production via photocatalysis process. Catal. Lett. 2014, 144, 1955–1961. [Google Scholar] [CrossRef]
- Holmen, A. Direct conversion of methane to fuels and chemicals. Catal. Today 2009, 142, 2–8. [Google Scholar] [CrossRef]
- Müller, C.; Maciejewski, M.; Mallat, T.; Baiker, A. Organically modified titania–silica aerogels for the epoxidation of olefins and allylic alcohols. J. Catal. 1999, 184, 280–293. [Google Scholar] [CrossRef]
- Müller, C.; Deck, R.; Mallat, T.; Baiker, A. Hydrophobic titania-silica aerogels: Epoxidation of cyclic compounds. Top. Catal. 2000, 11, 369–378. [Google Scholar] [CrossRef]
- Coles, M.P.; Lugmair, C.G.; Terry, K.W.; Tilley, T.D. Titania-silica materials from the molecular precursor Ti[OSi(OtBu)3]4: Selective epoxidation catalysts. Chem. Mater. 2000, 12, 122–131. [Google Scholar] [CrossRef]
- Choi, J.; Suh, D.J. Complete oxidation of 1, 2-dichlorobenzene over V2O5-TiO2 and MnOx-TiO2 aerogels. Korean J. Chem. Eng. 2014, 31, 1773–1779. [Google Scholar] [CrossRef]
- Kang, M.; Choi, J.; Kim, Y.T.; Park, E.D.; Shin, C.B.; Suh, D.J.; Yie, J.E. Effects of preparation methods for V2O5-TiO2 aerogel catalysts on the selective catalytic reduction of NO with NH3. Korean J. Chem. Eng. 2009, 26, 884–889. [Google Scholar] [CrossRef]
- Kim, M.; Park, D.; Park, S.; Yang, X.; Choi, J.; Suh, D. Selective oxidation of hydrogen sulfide containing excess water and ammonia over vanadia-titania aerogel catalysts. Catal. Today 2006, 111, 212–216. [Google Scholar] [CrossRef]
- Trueba, M.; Trasatti, S.P. γ-Alumina as a support for catalysts: A review of fundamental aspects. Eur. J. Inorg. Chem. 2005, 2005, 3393–3403. [Google Scholar] [CrossRef]
- Matsuda, S.; Kato, A. Titanium oxide based catalysts-a review. Appl. Catal. 1983, 8, 149–165. [Google Scholar] [CrossRef]
- Huirache-Acuña, R.; Nava, R.; Peza-Ledesma, C.L.; Lara-Romero, J.; Alonso-Núez, G.; Pawelec, B.; Rivera-Muñoz, E.M. SBA-15 mesoporous silica as catalytic support for hydrodesulfurization catalysts-review. Materials 2013, 6, 4139–4167. [Google Scholar] [CrossRef] [PubMed]
- Davis, K. Material review: Alumina (Al2O3). Sch. Dr. Stud. Eur. Union J. 2010, 2, 109–114. [Google Scholar]
- Sui, R.; Rizkalla, A.S.; Charpentier, P.A. Formation of titania nanofibers: A direct sol-gel route in supercritical CO2. Langmuir 2005, 21, 6150–6153. [Google Scholar] [CrossRef] [PubMed]
- Pakhare, D.; Spivey, J. A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 2014, 43, 7813–7837. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Suh, D.J.; Park, T.-J.; Kim, K.-L. Effect of metal particle size on coking during CO2 reforming of CH4 over Ni-alumina aerogel catalysts. Appl. Catal. A 2000, 197, 191–200. [Google Scholar] [CrossRef]
- Bang, Y.; Seo, J.G.; Youn, M.H.; Song, I.K. Hydrogen production by steam reforming of liquefied natural gas (LNG) over mesoporous Ni-Al2O3 aerogel catalyst prepared by a single-step epoxide-driven sol-gel method. Int. J. Hydrogen Energy 2012, 37, 1436–1443. [Google Scholar] [CrossRef]
- Bang, Y.; Seo, J.G.; Song, I.K. Hydrogen production by steam reforming of liquefied natural gas (LNG) over mesoporous Ni-La-Al2O3 aerogel catalysts: Effect of La content. Int. J. Hydrogen Energy 2011, 36, 8307–8315. [Google Scholar] [CrossRef]
- Seo, J.G.; Youn, M.H.; Bang, Y.; Song, I.K. Hydrogen production by steam reforming of simulated liquefied natural gas (LNG) over mesoporous nickel-M-alumina (M = Ni, Ce, La, Y, Cs, Fe, Co, and Mg) aerogel catalysts. Int. J. Hydrogen Energy 2011, 36, 3505–3514. [Google Scholar] [CrossRef]
- Yoo, J.; Bang, Y.; Han, S.J.; Park, S.; Song, J.H.; Song, I.K. Hydrogen production by tri-reforming of methan over nickel-alumina aerogel catalyst. J. Mol. Catal. A: Chem. 2015, 410, 74–80. [Google Scholar] [CrossRef]
- Han, S.J.; Bang, Y.; Yoo, J.; Seo, J.G.; Song, I.K. Hydrogen production by steam reforming of ethanol over mesoporous Ni-Al2O3-ZrO2 xerogel catalysts: Effect of nickel content. Int. J. Hydrogen Energy 2013, 38, 8285–8292. [Google Scholar] [CrossRef]
- Song, J.H.; Han, S.J.; Yoo, J.; Park, S.; Kim, D.H.; Song, I.K. Hydrogen production by steam reforming of ethanol over Ni-Sr-Al2O3-ZrO2 aerogel catalyst. J. Mol. Catal. A Chem. 2016, 424, 342–350. [Google Scholar] [CrossRef]
- Lee, S.-H.; Suh, D.J.; Park, T.-J.; Kim, K.-L. The effect of heat treatment conditions on the textural and catalytic properties of nickel-titania composite aerogel catalysts. Catal. Commun. 2002, 3, 441–447. [Google Scholar] [CrossRef]
- Krompiec, S.; Mrowiec-Białoń, J.; Skutil, K.; Dukowicz, A.; Pająk, L.; Jarzębski, A. Nickel-alumina composite aerogel catalysts with a high nickel load: A novel fast sol-gel synthesis procedure and screening of catalytic properties. J. Non-Cryst. Solids 2003, 315, 297–303. [Google Scholar] [CrossRef]


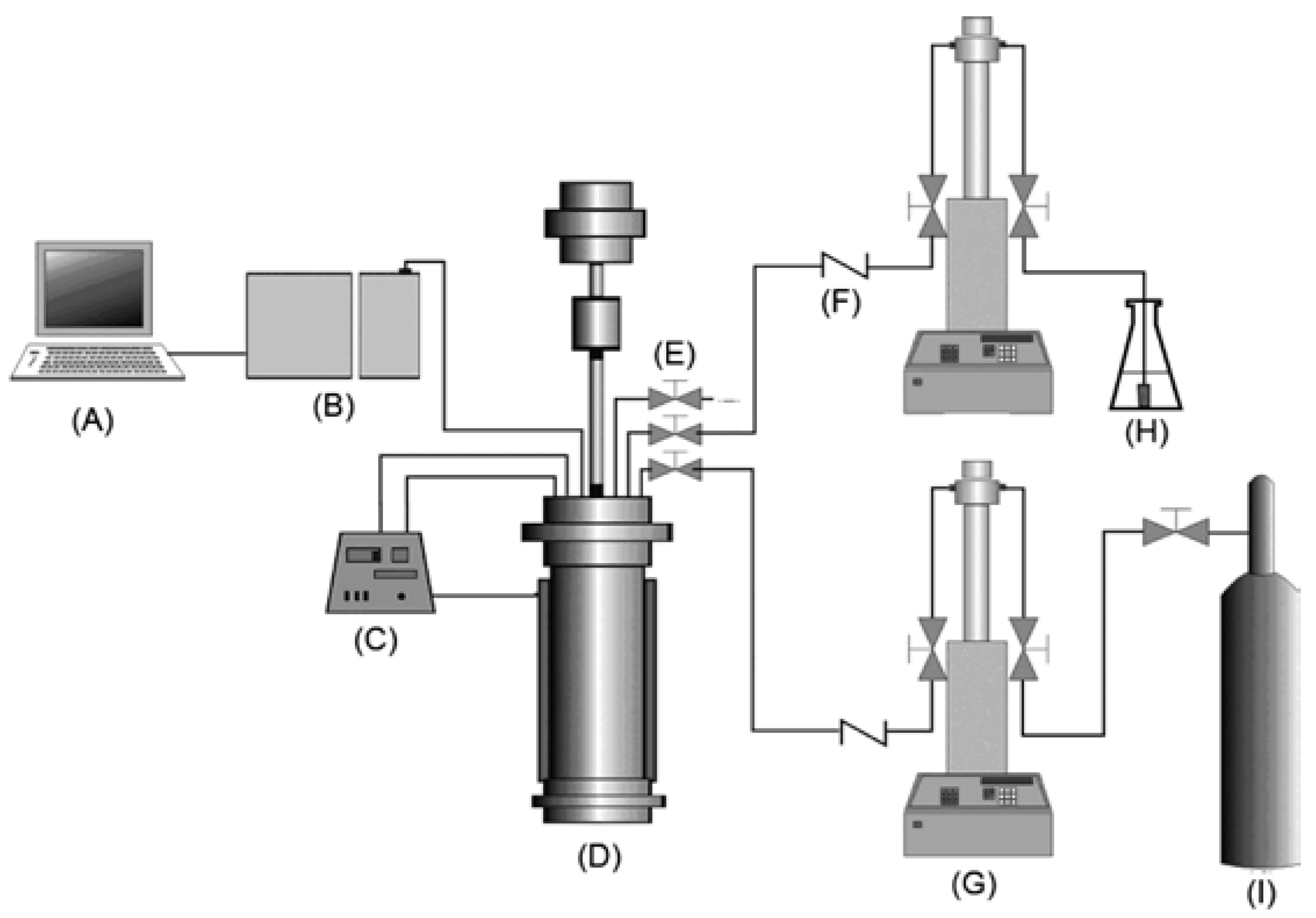
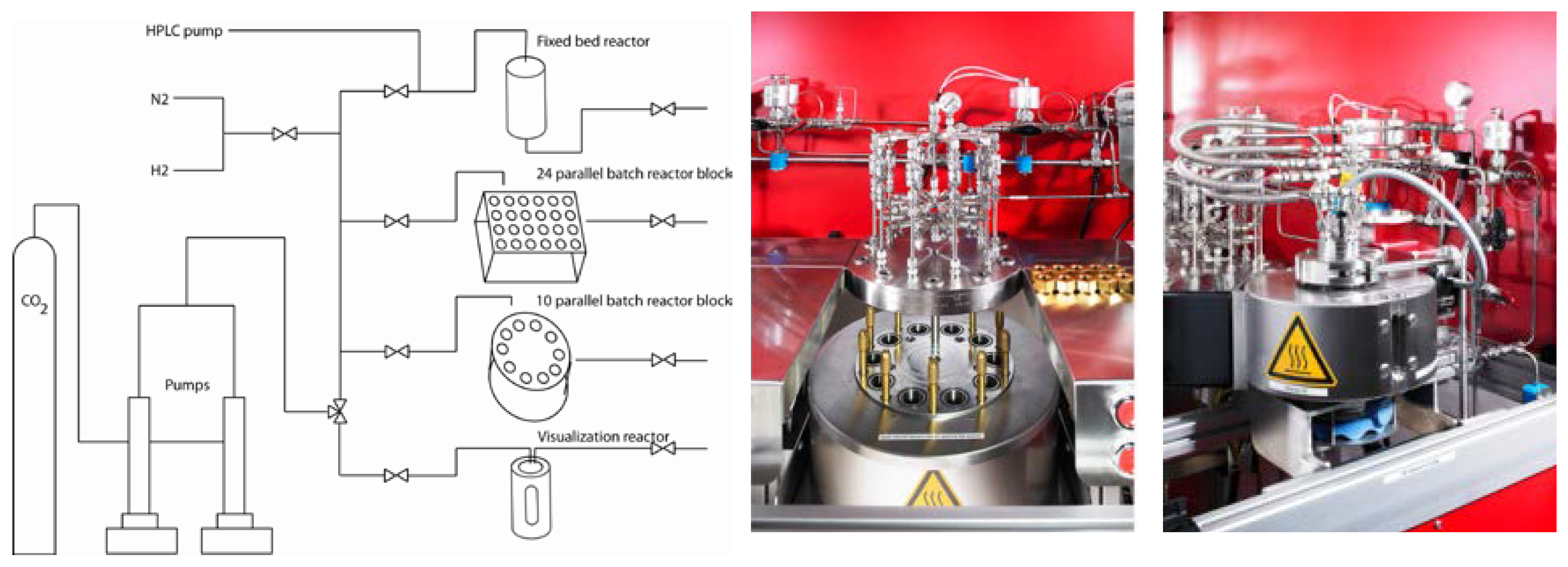




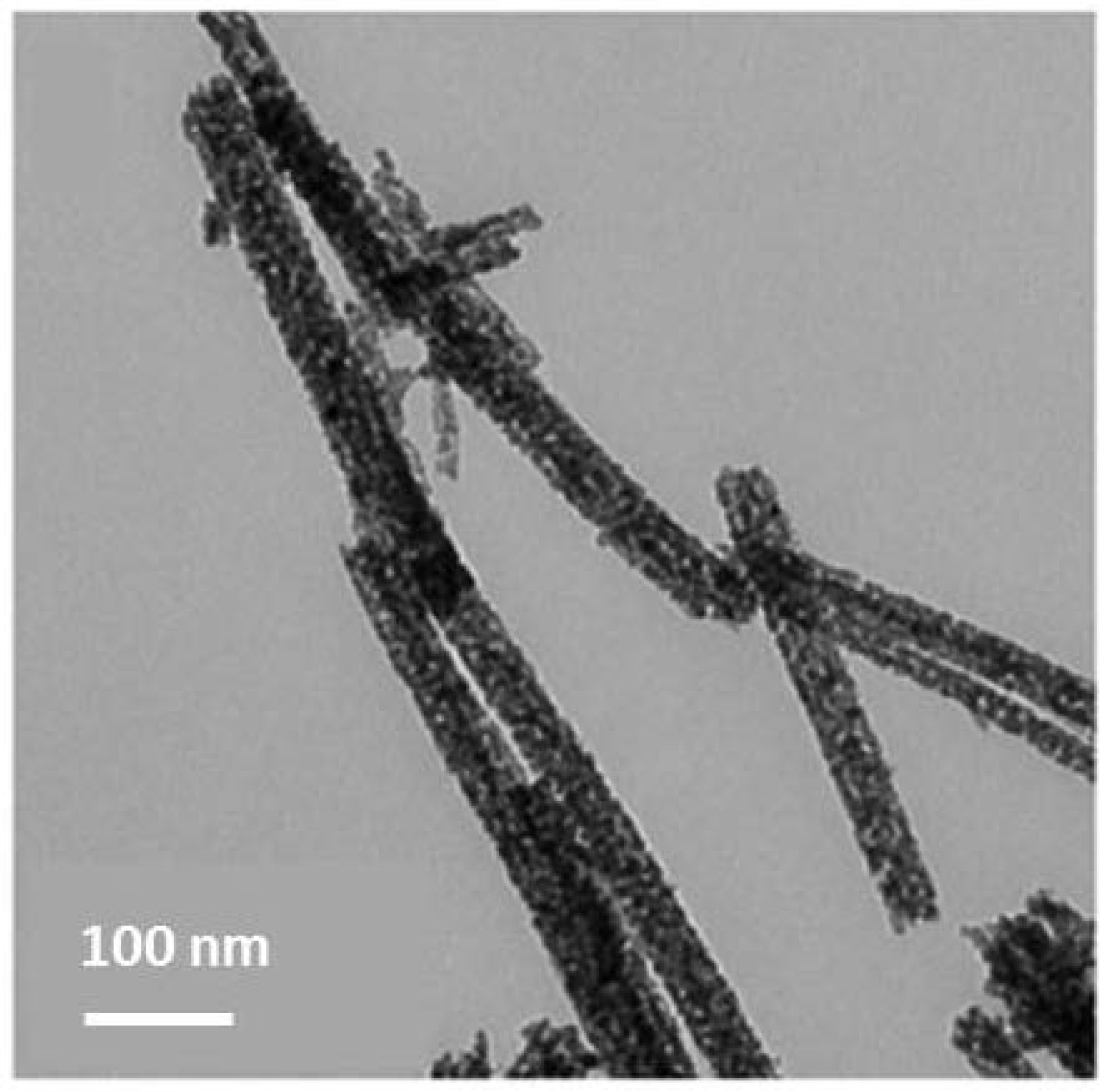






| Product | Morphology | Precursors, Reactants, Co-Solvents | Ref. |
|---|---|---|---|
| TiO2 | Aerogel | Titanium (IV) isopropoxide (TIP), acetic acid | [29] |
| Nanoparticles | TIP, deionised water, polypropylene fibre | [38] | |
| Spherical particles, micron-size rods, nanofibres | TIP, Titanium butoxide (TBO), acetic acid | [10] | |
| Spherical particles | Diisopropoxititanium bis(acetylacetonate) (DIPBAT), absolute ethanol, isopropyl alcohol | [2] | |
| SiO2 | Molecular sieve membranes | Tetraethyl orthosilicate (TEOS), ultrapure water, 2-propanol, nitric acid, yttrium (III) acetate hydrate. | [39] |
| Aerogel | TEOS, acetic acid | [29] | |
| Nanoparticles | Tetramethyl orthosilicate (TMOS), TEOS, acetone, benzoic acid, acetic acid, formic acid, water | [30] | |
| Ordered porous structure | TEOS, water, polystyrene latex | [9] | |
| ZrO2 | Aerogel | Zirconium butoxide (ZBO), acetic acid | [29] |
| Al2O3 | Nanofibres | Aluminium (III) isopropoxide (AIP), acetic acid | [11] |
| Pd-SiO2 | Nanoparticles | TMOS, formic acid, polydimethylsiloxane (PDMS), palladium(N,N′-bis(1,1,1,3,5,5,5)heptafluoro-2,4-pentanediiminate (Pd(II)HFPDI) | [40] |
| N- and N/Zr-doped TiO2 | Nanofibres and flake-like structures | TIP, zirconium (IV) propoxide (ZPO), acetic acid, isopropanol, triethylamine | [32] |
| Fe-doped TiO2/rGO | Nanowires | TIP, iron chloride, acetic acid, reduced graphite oxide (rGO) | [31] |
| ZrO2-TiO2 | Nanotubular structures | TIP, ZPO, acetic acid | [33] |
| Nanotubes | TIP, ZPO, acetic acid | [34] | |
| Nanotubes | TIP, ZPO, acetic acid | [35] | |
| Y-ZrO2 | Spherical nanoparticles | Zirconium hydroxyacetate, yttrium acetate, pentane or 2-propanol, nitric acid | [41] |
| TiO2-SiO2 | Nanostructured composites | TMOS, TIP, formic acid, Pluronic 17R4 surfactant | [42] |
| Spherical or cubic nanoparticles | Tetrabutyl titanate, TEOS, polyethylene glycol (PEG) 20000, aqueous ammonia, ethanol | [43] | |
| SiO2/polyethylene | Polymer/SiO2 nanocomposites | TMOS, TEOS, acetic acid, polyethylene | [36] |
| Hydroxyapatite-TiO2 | Nanocomposites | Calcium nitrate tetrahydrate, diammonium hydrogen phosphate, CTAB, PEG 400, TIP, glacial acetic acid, ammonium hydroxide, dichloromethane, ethanol, polycaprolactone | [37] |
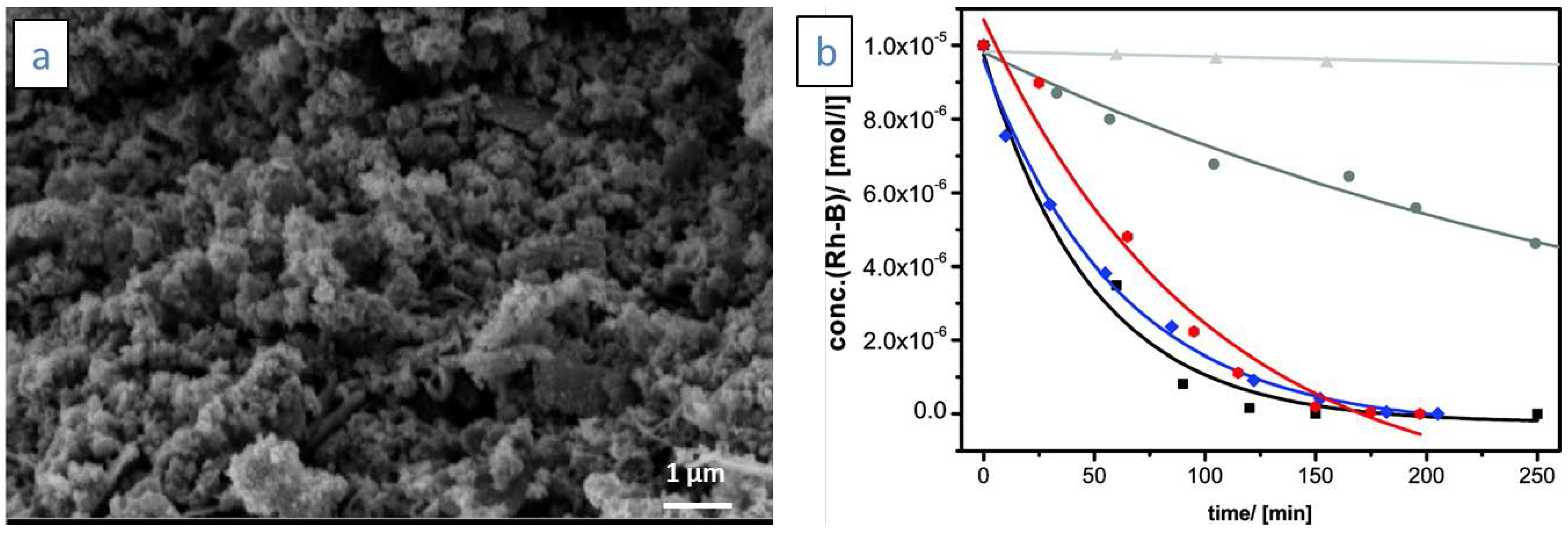
| Photocatalyst | Pollutant | Initial Concentration | Radiation Type | T/°C | t/min | Pollutant Removal/Aerogel | Pollutant Removal/Xerogel | Pollutant Removal/P25 | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| TiO2 | Phenol | 0.6 mmol/L | UV | 25 | 120 | 92 | 4.5 | 82 | [77] |
| Fe-doped TiO2/WO3 | Methylene blue | 10 ppm | UV | RT a | 360 | 77 | - | 72 | [78] |
| Visible | RT a | 720 | ~67 | - | ~16 | ||||
| CdS-ZnS-MPA-TiO2 | Methylene blue | 0.0312 mmol/L | UV | RT a | 40 | 88 | - | - | [79] |
| Visible | RT a | 240 | 85 | - | - | ||||
| 40 TiO2-SiO2 b | Phenol | 100 ppm | UV | 30 | 180 | 51 c | - | 53 d | [42] |
| Acetaldehyde | 100 ppm | UV | 40 | 40 | 49 c | - | 97 | ||
| ZnO | Rhodamine B | 0.02 mmol/L | UV-Vis | RT a | 150 | 100 | - | - | [5] |
| ZnO-SnO2 | Rhodamine B | 0.012 mmol/L | UV | RT a | 30 | 100 | - | - | [71] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, Y.; Pescarmona, P.P. Nanostructured Oxides Synthesised via scCO2-Assisted Sol-Gel Methods and Their Application in Catalysis. Catalysts 2018, 8, 212. https://doi.org/10.3390/catal8050212
Tao Y, Pescarmona PP. Nanostructured Oxides Synthesised via scCO2-Assisted Sol-Gel Methods and Their Application in Catalysis. Catalysts. 2018; 8(5):212. https://doi.org/10.3390/catal8050212
Chicago/Turabian StyleTao, Yehan, and Paolo P. Pescarmona. 2018. "Nanostructured Oxides Synthesised via scCO2-Assisted Sol-Gel Methods and Their Application in Catalysis" Catalysts 8, no. 5: 212. https://doi.org/10.3390/catal8050212





