[Pr2(pdc)3(Hpdc)(H2O)4]n·n(H3hp)·8n(H2O), a One-Dimensional Coordination Polymer Containing PrO6N3 Tri-Capped Trigonal Prisms and PrO8N Mono-Capped Square Anti-Prisms (H2pdc = Pyridine 2,6-Dicarboxylic Acid, C7H5NO4; 3hp = 3-Hydroxy Pyridine, C5H5NO)
1. Introduction
2. Results and Discussion
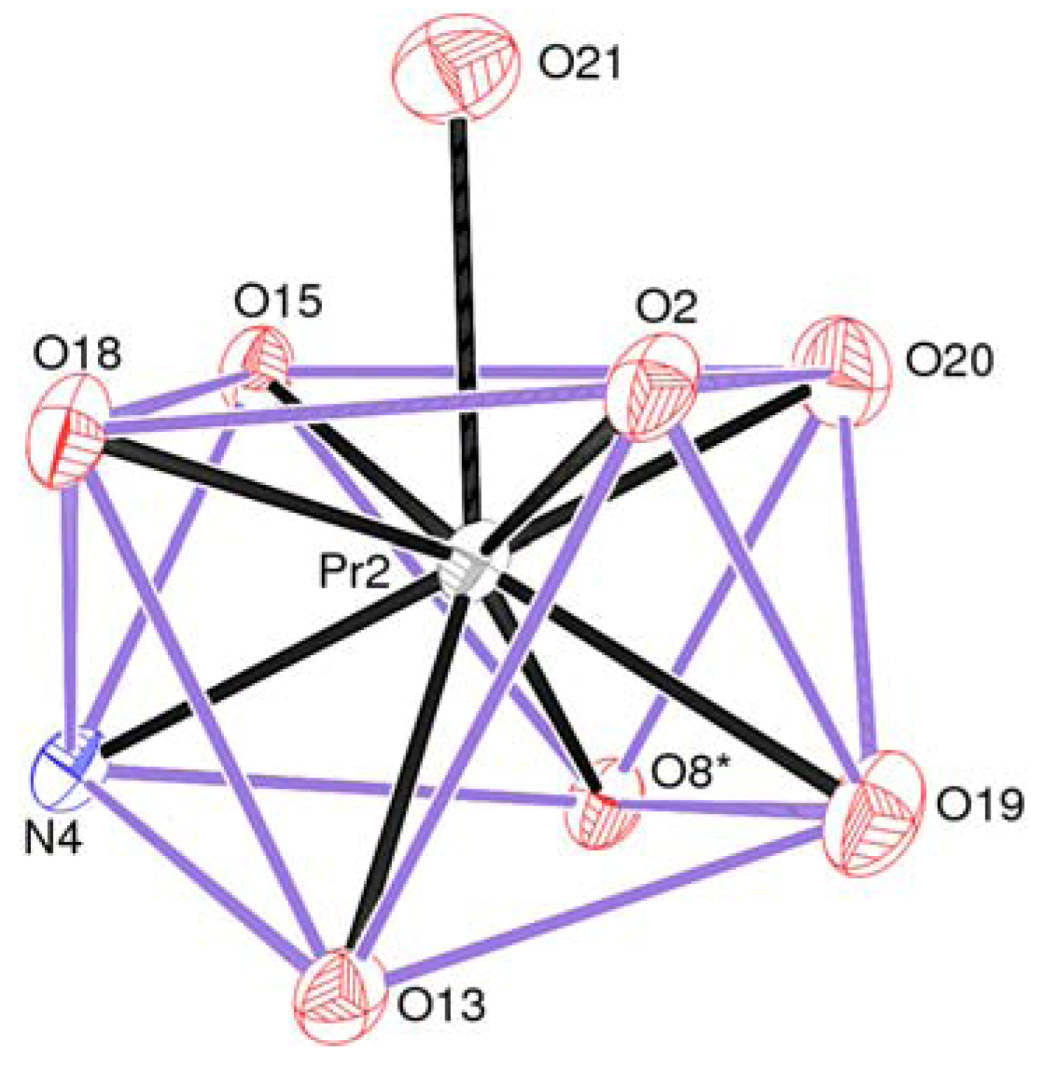
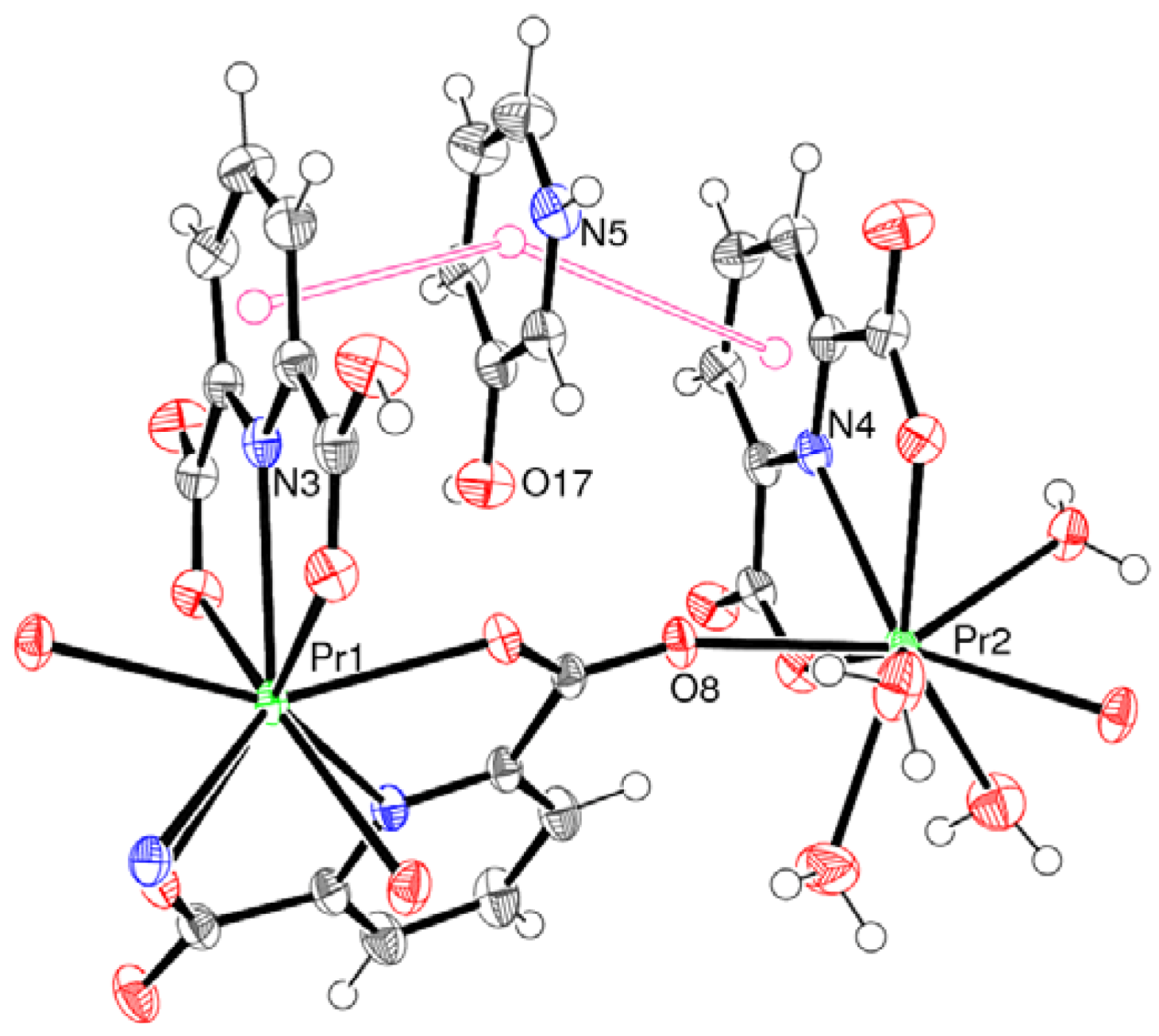
2.1. Crystal Structure of 1


| Pr1–O7 | 2.469(2) | Pr1–O11 | 2.472(2) |
| Pr1–O5 | 2.499(2) | Pr1–O3 | 2.501(2) |
| Pr1–O1 | 2.518(2) | Pr1–O9 | 2.601(2) |
| Pr1–N2 | 2.594(3) | Pr1–N1 | 2.604(3) |
| Pr1–N3 | 2.620(3) | ||
| Pr2–O8 #1 | 2.448(2) | Pr2–O2 | 2.469(2) |
| Pr2–O15 | 2.477(2) | Pr2–O13 | 2.489(2) |
| Pr2–O19 | 2.490(3) | Pr2–O20 | 2.501(3) |
| Pr2–O18 | 2.525(2) | Pr2–O21 | 2.582(3) |
| Pr2–N4 | 2.604(3) |

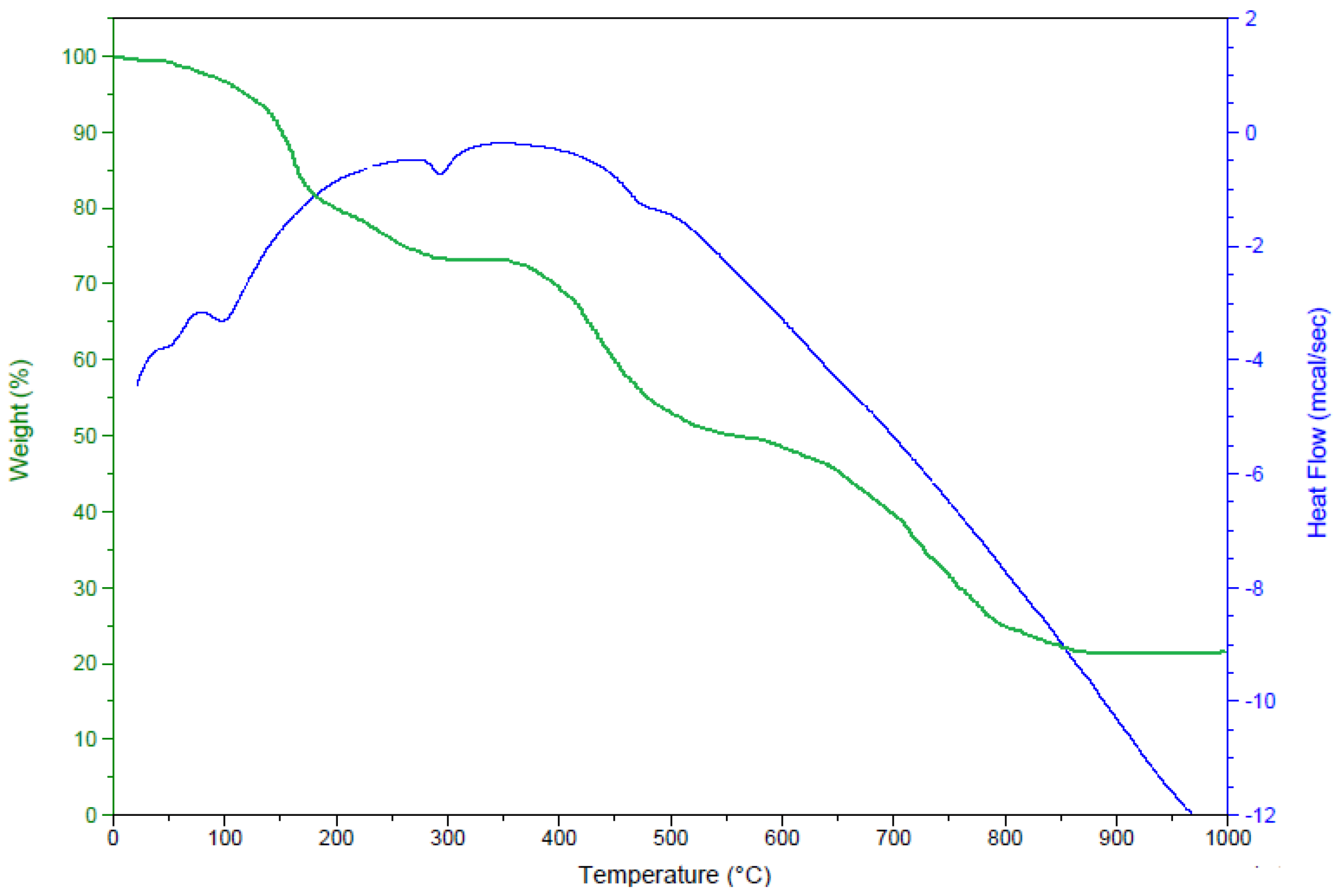
2.2. Thermal Analysis
3. Experimental Section
3.1. Instrumental
3.2. Synthesis
3.3. Single-Crystal Data Collection and Analysis
4. Conclusions
References
- Dong, Y.B.; Xu, H.X.; Ma, J.P.; Huang, R.Q. Silver(I) coordination polymers based on a nano-sized bent bis(3-acetylenylphenyl-(4-cyanophenyl))oxadiazole ligand: The role of ligand isomerism and the templating effect of polyatomic anions and solvent intermediates. Inorg. Chem. 2006, 45, 3325–3343. [Google Scholar] [CrossRef]
- Shimpi, M.R.; SeethaLekshmi, N.; Pedireddi, V.R. Supramolecular architecture in some 4-halophenylboronic acids. Cryst. Growth Des. 2007, 7, 1958–1963. [Google Scholar] [CrossRef]
- Fan, L.; Wang, E.; Li, Y.; An, H.; Xiao, D.; Wang, X. Wells-Dawson anion, a useful building block to construct one-dimensional chain as a chelate ligand coordinating with transition metal cations. J. Mol. Struct. 2007, 841, 28–33. [Google Scholar] [CrossRef]
- Albada, G.A.V.; Gorter, S.; Reedijk, J. Synthesis, spectral characterization and X-ray structure of aquasodium triaquabis(pyridine-2,6-dicarboxyl-diato)samariate trihydrate, an unique bis-pdc compound, with a sheet-type structure stabilised by sodium ions in each direction. Polyhedron 1999, 18, 1821–1824. [Google Scholar] [CrossRef]
- Prasad, T.K.; Rajasekharan, M.V. A novel water octamer in Ce(dipic)2(H2O)3·4H2O: Crystallographic, thermal, and theoretical studies. Cryst. Growth Des. 2006, 6, 488–491. [Google Scholar]
- Hamacek, J.; Zebret, S.; Bernardinelli, G. Supramolecular structure of the polymeric Eu(III) complex with pyridine-2,6-dicarboxylic acid. Polyhedron 2009, 28, 2179–2182. [Google Scholar] [CrossRef]
- Park, K.K.; Kwon, T.R.; Park, Y.J.; Jung, E.C.; Kim, W.H. Ternary complex formation of Eu(III) and Am(III) with pyridine-2,6-dicarboxylate in aqueous solutions. J. Alloy Compd. 2007, 444, 677–682. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Bharadwaj, P.K. Coexistence of water dimer and hexamer clusters in 3D metal-organic framework structures of Ce(III) and Pr(III) with pyridine-2,6-dicarboxylic acid. Inorg. Chem. 2003, 42, 8250–8254. [Google Scholar] [CrossRef]
- Zhao, B.; Yi, L.; Dai, Y.; Chen, X.-Y.; Cheng, P.; Liao, D.-Z.; Yan, S.-P.; Jiang, Z.-H. Systematic investigation of the hydrothermal syntheses of Pr(III)-PDA (PDA = pyridine-2,6-dicarboxylate anion) metal-organic frameworks. Inorg. Chem. 2005, 44, 911–920. [Google Scholar]
- APEX-II and SAINT, Bruker AXS Inc.: Madison, WI, USA.
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar]
- Spek, A.L. Structure validation in chemical crystallography. Acta Cryst. 2009, D65, 148–155. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sharif, S.; Sahin, O.; Khan, I.U.; Büyükgüngör, O.; Harrison, W.T.A. [Pr2(pdc)3(Hpdc)(H2O)4]n·n(H3hp)·8n(H2O), a One-Dimensional Coordination Polymer Containing PrO6N3 Tri-Capped Trigonal Prisms and PrO8N Mono-Capped Square Anti-Prisms (H2pdc = Pyridine 2,6-Dicarboxylic Acid, C7H5NO4; 3hp = 3-Hydroxy Pyridine, C5H5NO). Crystals 2012, 2, 1253-1260. https://doi.org/10.3390/cryst2031253
Sharif S, Sahin O, Khan IU, Büyükgüngör O, Harrison WTA. [Pr2(pdc)3(Hpdc)(H2O)4]n·n(H3hp)·8n(H2O), a One-Dimensional Coordination Polymer Containing PrO6N3 Tri-Capped Trigonal Prisms and PrO8N Mono-Capped Square Anti-Prisms (H2pdc = Pyridine 2,6-Dicarboxylic Acid, C7H5NO4; 3hp = 3-Hydroxy Pyridine, C5H5NO). Crystals. 2012; 2(3):1253-1260. https://doi.org/10.3390/cryst2031253
Chicago/Turabian StyleSharif, Shahzad, Onur Sahin, Islam Ullah Khan, Orhan Büyükgüngör, and William T. A. Harrison. 2012. "[Pr2(pdc)3(Hpdc)(H2O)4]n·n(H3hp)·8n(H2O), a One-Dimensional Coordination Polymer Containing PrO6N3 Tri-Capped Trigonal Prisms and PrO8N Mono-Capped Square Anti-Prisms (H2pdc = Pyridine 2,6-Dicarboxylic Acid, C7H5NO4; 3hp = 3-Hydroxy Pyridine, C5H5NO)" Crystals 2, no. 3: 1253-1260. https://doi.org/10.3390/cryst2031253




