1,5-Diaminopentane As A Structure-Directing Agent for Zincophosphate Networks: Zn3(PO4)2(C5H14N2)2·3H2O and C5H16N2·Zn3(PO4)2(HPO4)·H2O
Abstract
:1. Introduction
2. Results and Discussion
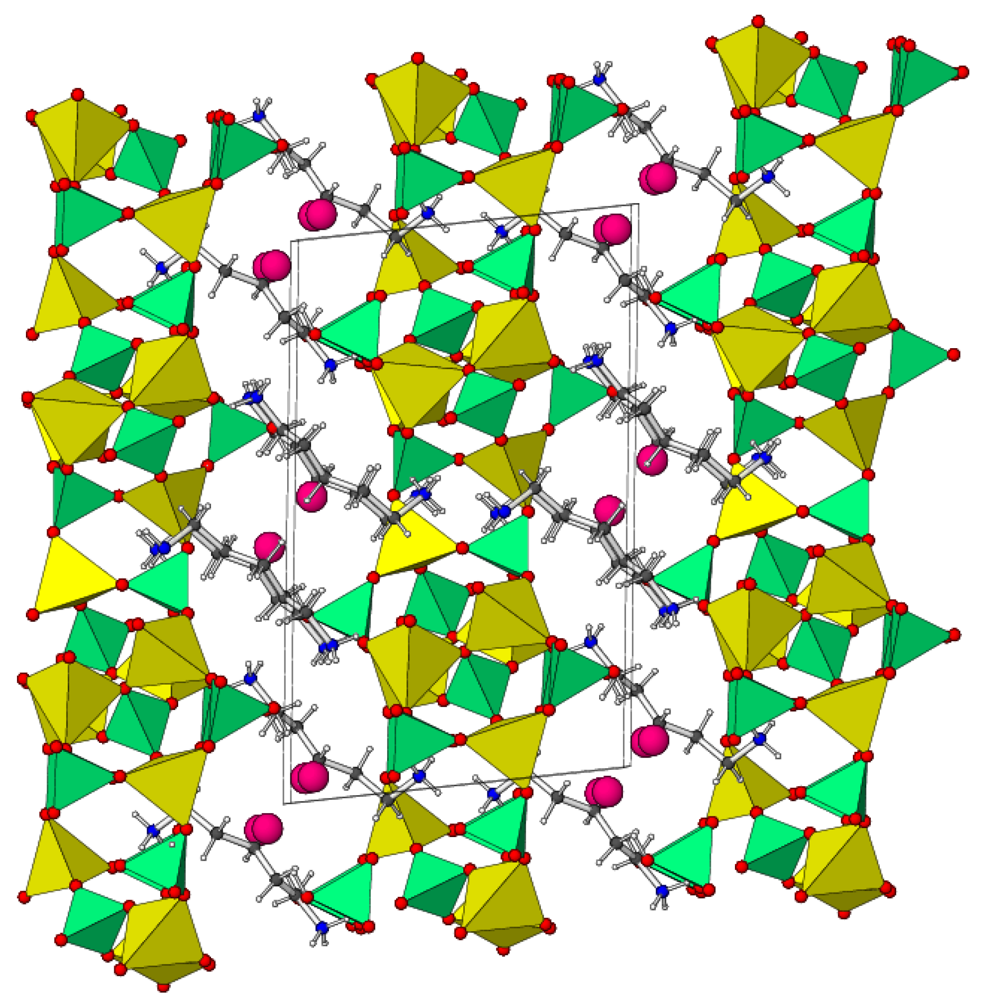
2.1. Crystal Structure of Zn3(PO4)2(C5H14N2)2·3H2O (1)
| Bond | Distance | Bond | Distance |
|---|---|---|---|
| Zn1-O5 | 1.964 (9) | Zn1-N3 | 2.037 (13) |
| Zn2-O3 | 1.914 (9) | Zn2-N2#1 | 2.027 (11) |
| Zn3-O8#2 | 1.914 (10) | Zn3-O1 | 1.933 (7) |
| Zn3-O6 | 1.943 (11) | Zn3-N1 | 2.032 (10) |
| Zn4-O2 | 1.939 (10) | Zn4-O7 | 1.942 (7) |
| Zn4-O4#3 | 1.949 (9) | Zn4-N4#4 | 2.055 (10) |
| P1-O4 | 1.516 (12) | P1-O2 | 1.542 (8) |
| P1-O3 | 1.543 (8) | P1-O1 | 1.550 (8) |
| P2-O5 | 1.526 (8) | P2-O8 | 1.537 (13) |
| P2-O7 | 1.549 (8) | P2-O6 | 1.551 (8) |
| Bond | Angle | Bond | Angle |
| P1-O1-Zn3 | 130.4 (5) | P1-O2-Zn4 | 115.3 (5) |
| P1-O3-Zn2 | 125.2 (7) | P1-O4-Zn4#2 | 128.2 (6) |
| P2-O5-Zn1 | 121.7 (7) | P2-O6-Zn3 | 114.3 (5) |
| P2-O7-Zn4 | 132.6 (6) | P2-O8-Zn3#3 | 128.5 (6) |
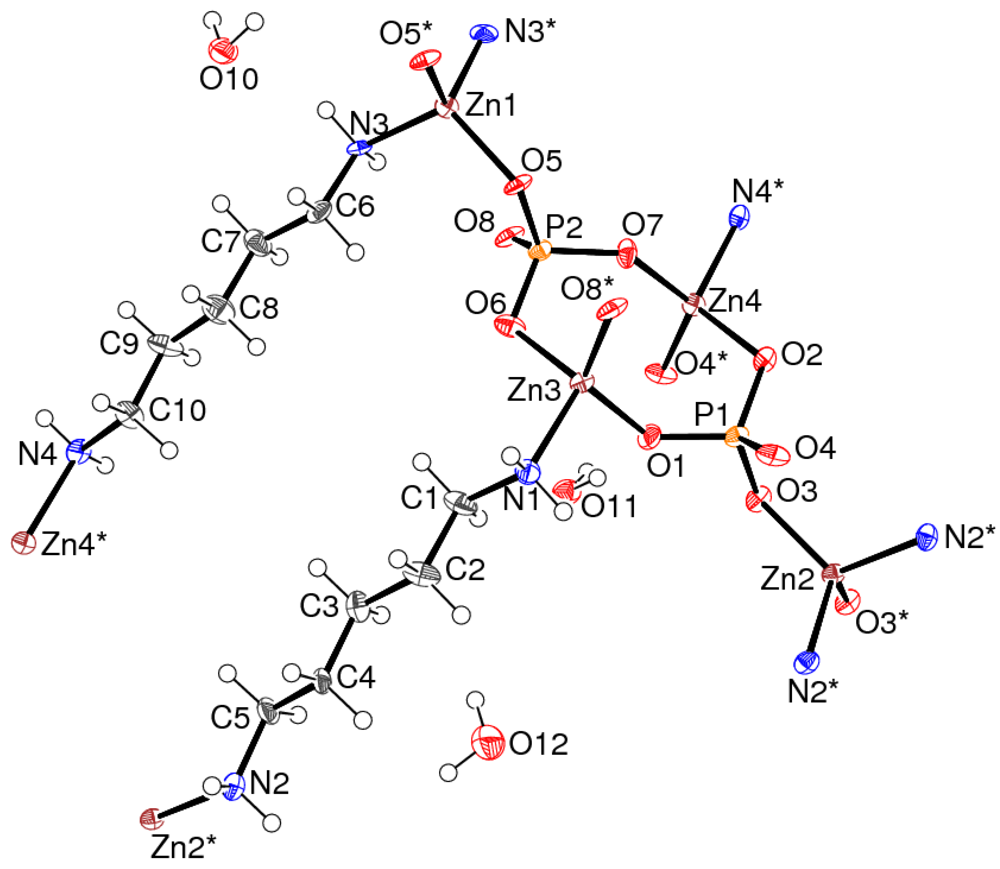
 where ϑ is the O-X-O bond angle (°) [12], indicate that all the Zn-centred tetrahedra in 1 are significantly distorted: values of 98.4°2, 79.8°2, 81.2°2 and 85.9°2 arise for Zn1, Zn2, Zn3 and Zn4, respectively. All the O atoms also link to an adjacent P atom (mean Zn-O-P = 124.5°) and the N atoms are all parts of neutral dap molecules. Both PO4 groups in 1 are close to regular tetrahedra (mean P1-O = 1.538 Å, angular variance = 2.7°2; mean P2-O = 1.541 Å, angular variance = 1.4°2) and all the O atoms link to an adjacent Zn atom, thus there are no terminal or “dangling” P=O or P-OH bonds [13] in 1. The geometrical parameters for the two unique dap molecules are unexceptional [14], and both molecules are in essentially extended conformations (mean absolute values of the N-C-C-C and C-C-C-C torsion angles = 177.6° and 174.7°, respectively).
where ϑ is the O-X-O bond angle (°) [12], indicate that all the Zn-centred tetrahedra in 1 are significantly distorted: values of 98.4°2, 79.8°2, 81.2°2 and 85.9°2 arise for Zn1, Zn2, Zn3 and Zn4, respectively. All the O atoms also link to an adjacent P atom (mean Zn-O-P = 124.5°) and the N atoms are all parts of neutral dap molecules. Both PO4 groups in 1 are close to regular tetrahedra (mean P1-O = 1.538 Å, angular variance = 2.7°2; mean P2-O = 1.541 Å, angular variance = 1.4°2) and all the O atoms link to an adjacent Zn atom, thus there are no terminal or “dangling” P=O or P-OH bonds [13] in 1. The geometrical parameters for the two unique dap molecules are unexceptional [14], and both molecules are in essentially extended conformations (mean absolute values of the N-C-C-C and C-C-C-C torsion angles = 177.6° and 174.7°, respectively).
| Bond | D-H | H∙∙∙A | D∙∙∙A | D-H∙∙∙A |
|---|---|---|---|---|
| N1-H1A∙∙∙O11#2 | 0.92 | 2.24 | 3.138 (14) | 164 |
| N1-H1B∙∙∙O6#2 | 0.92 | 2.46 | 2.981 (16) | 116 |
| N2-H2C...O3#5 | 0.92 | 2.30 | 3.131 (16) | 149 |
| N2-H2D...O11#1 | 0.92 | 2.47 | 3.245 (11) | 142 |
| N2-H2D∙∙∙O3#1 | 0.92 | 2.58 | 3.196 (16) | 125 |
| N3-H3C∙∙∙O10 | 0.92 | 2.44 | 3.158 (11) | 136 |
| N3-H3C∙∙∙O5#6 | 0.92 | 2.49 | 3.152 (16) | 129 |
| N3-H3D∙∙∙O5#3 | 0.92 | 2.28 | 3.081 (17) | 145 |
| N4-H4D∙∙∙O10#7 | 0.92 | 2.08 | 2.971 (14) | 164 |
| O10-H1W∙∙∙O7#6 | 0.90 | 1.89 | 2.723 (15) | 152 |
| O10-H2W∙∙∙O8#8 | 0.90 | 2.14 | 2.928 (11) | 145 |
| O11-H3W∙∙∙O4#3 | 0.91 | 2.30 | 2.973 (11) | 131 |
| O11-H4W∙∙∙O1 | 0.90 | 1.84 | 2.734 (15) | 171 |
| O12-H5W∙∙∙O11#1 | 0.90 | 2.07 | 2.941 (10) | 164 |
| O12-H6W∙∙∙O12#9 | 0.90 | 1.97 | 2.838 (7) | 160 |
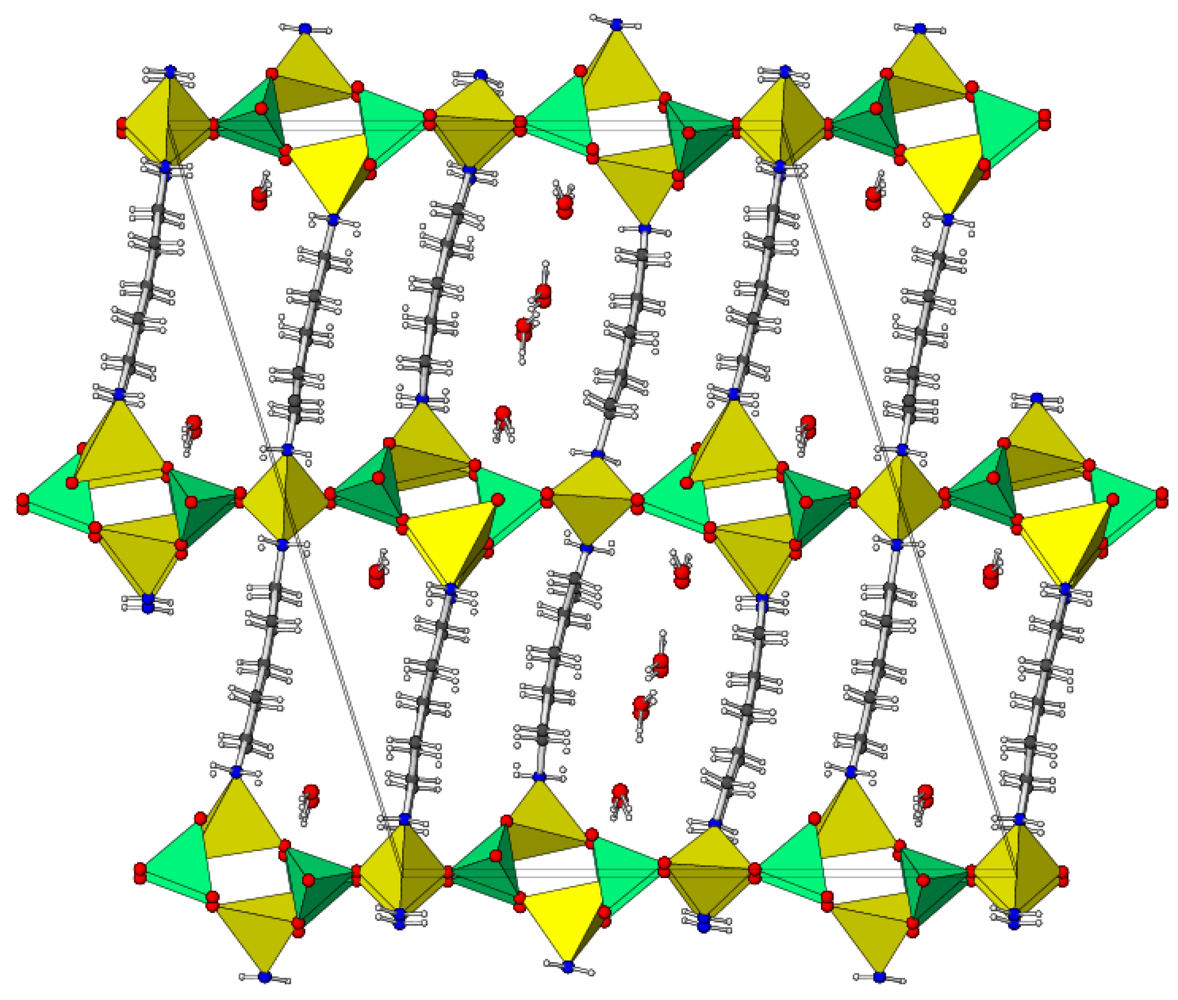
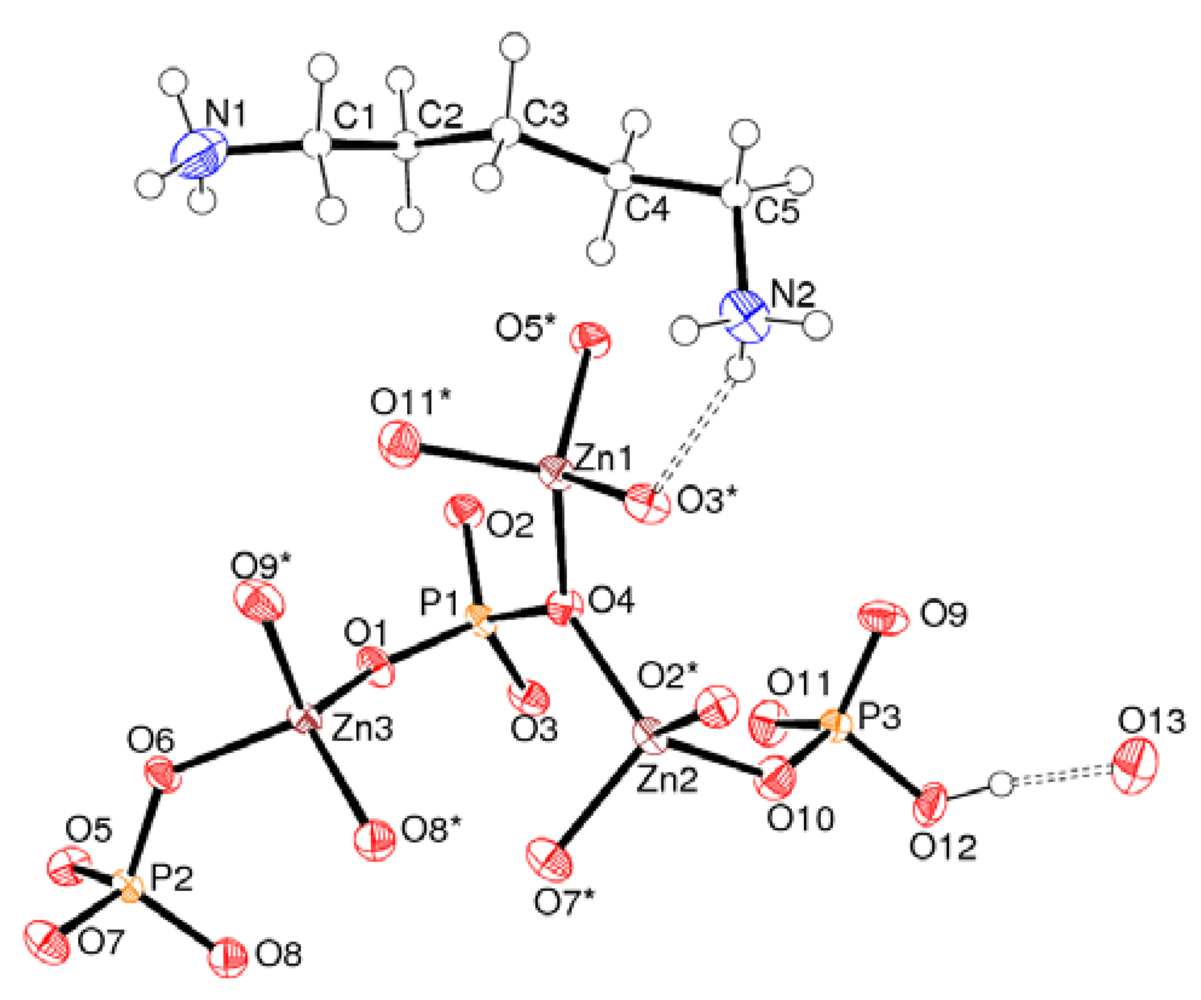
2.2. Crystal Structure of C5H16N2·Zn3(PO4)2(HPO4)·H2O (2)
| Bond | Distance | Bond | Distance |
|---|---|---|---|
| Zn1-O5#1 | 1.908 (4) | Zn1-O3#2 | 1.929 (4) |
| Zn1-O11#2 | 1.930 (4) | Zn1-O4 | 2.005 (4) |
| Zn2-O10 | 1.909 (4) | Zn2-O7#3 | 1.929 (4) |
| Zn2-O2#2 | 1.952 (4) | Zn2-O4 | 1.966 (4) |
| Zn3-O1 | 1.923 (4) | Zn3-O9#2 | 1.929 (4) |
| Zn3-O6 | 1.944 (4) | Zn3-O8#3 | 1.959 (4) |
| P1-O1 | 1.514 (4) | P1-O2 | 1.519 (4) |
| P1-O3 | 1.530 (4) | P1-O4 | 1.570 (4) |
| P2-O5 | 1.532 (4) | P2-O6 | 1.537 (4) |
| P2-O8 | 1.537 (4) | P2-O7 | 1.539 (4) |
| P3-O9 | 1.509 (4) | P3-O10 | 1.517 (4) |
| P3-O11 | 1.522 (4) | P3-O12 | 1.588 (4) |
| Bond | Angle | Bond | Angle |
| P1-O1-Zn3 | 139.8 (2) | P1-O2-Zn2#4 | 131.5 (2) |
| P1-O3-Zn1#4 | 131.2 (2) | P1-O4-Zn2 | 119.0 (2) |
| P1-O4-Zn1 | 120.1 (2) | Zn2-O4-Zn1 | 118.50 (18) |
| P2-O5-Zn1#5 | 129.7 (2) | P2-O6-Zn3 | 132.9 (2) |
| P2-O7-Zn2#3 | 130.5 (2) | P2-O8-Zn3#3 | 132.1 (2) |
| P3-O9-Zn3#4 | 136.2 (3) | P3-O10-Zn2 | 134.4 (3) |
| P3-O11-Zn1#4 | 138.6 (3) | – | – |
| Bond | D-H | H∙∙∙A | D∙∙∙A | D-H∙∙∙A |
|---|---|---|---|---|
| O12-H12∙∙∙O13 | 0.93 | 1.77 | 2.644 (7) | 155 |
| N1-H1∙∙∙O11#6 | 0.89 | 2.29 | 2.915 (9) | 127 |
| N1-H2∙∙∙O12#2 | 0.89 | 2.22 | 2.874 (9) | 130 |
| N1-H3∙∙∙O2#7 | 0.89 | 1.92 | 2.806 (8) | 174 |
| N2-H4∙∙∙O8#2 | 0.89 | 1.99 | 2.823 (6) | 155 |
| N2-H5∙∙∙O3#2 | 0.89 | 2.11 | 2.902 (6) | 148 |
| N2-H6∙∙∙O7#8 | 0.89 | 2.01 | 2.837 (7) | 154 |

3. Experimental Section
3.1. Synthesis
3.2. Single-Crystal Data Collection and Analysis
4. Conclusions
References
- Vaidhyanathan, R.; Natarajan, S. New open-framework layered tin(II) phosphates intercalated with amines. J. Mater. Chem. 1999, 9, 1807–1811. [Google Scholar] [CrossRef]
- Cowley, A.R.; Chippindale, A.M. Synthesis and characterisation of the first unidimensional cobalt hydrogen phosphate [H3N(CH2)3NH3][Co(HPO4)2]. J. Chem. Soc. Dalton Trans. 1999, 13, 2147–2149. [Google Scholar]
- Jouffret, L.; Rivenet, M.; Abraham, F. A new series of pillared uranyl-vanadates based on uranophane-type sheets in the uranium-vanadium-linear alkyl diamine systems. J. Solid State Chem. 2010, 183, 84–92. [Google Scholar] [CrossRef]
- Yang, W.; Li, J.; Xu, J.; Xong, H.; Wang, L.; Yu, J.; Xu, R. Hydrothermal synthesis of isostructural open-framework manganese and iron borophosphates: Effect of the organic templates in determining the pore shapes. Solid State Sci. 2011, 13, 757–761. [Google Scholar] [CrossRef]
- Chidambaram, D.; Neeraj, S.; Natarajan, S.; Rao, C.N.R. Open-framework zinc phosphates synthesized in the presence of structure-directing organic amines. J. Solid State Chem. 1999, 147, 154–169. [Google Scholar] [CrossRef]
- Chavez, A.V.; Nenoff, T.M.; Hannooman, L.; Harrison, W.T.A. Tetrahedral-network organo-zincophosphates: Syntheses and structures of (N2C6H14)·Zn(HPO4)2·H2O, H3N(CH2)3NH3-Zn2(HPO4)3 and (N2C6H14)· Zn3(HPO4)4. J. Solid State Chem. 1999, 147, 584–591. [Google Scholar] [CrossRef]
- Fu, R.; Hu, S.; Wu, X. Mix-solvothermal syntheses, characterization, and properties of new zinc diphosphonates with Zn-O-P chains, layers, and 3D frameworks. Cryst. Growth Des. 2007, 7, 1134–1144. [Google Scholar] [CrossRef]
- Rodgers, J.A.; Harrison, W.T.A. H3N(CH2)6NH3·Zn4(PO4)2(HPO4)2·3H2O: A novel three-dimensional zinc phosphate framework containing 5- and 20-rings. J. Mater. Chem. 2000, 10, 2853–2856. [Google Scholar] [CrossRef]
- Holtby, A.S.; Harrison, W.T.A.; Yilmaz, V.T.; Büyükgüngör, O. Büyükgüngör, O. New three-dimensional (3,4)-net zincophosphites templated by linear diammonium cations: H3N(CH2)5NH3·Zn3(HPO3)4 and β-H3N(CH2)6NH3·Zn3(HPO3)4. Solid State Sci. 2007, 9, 149–154. [Google Scholar] [CrossRef]
- Song, H.H.; Zheng, L.M.; Wang, Z.; Yan, C.H.; Xin, X.Q. Zinc diphosphonates templated by organic amines: Syntheses and characterizations of [NH3(CH2)2NH3]Zn(hedpH2)2·2H2O and [NH3(CH2)nNH3]Zn2(hedpH)2·2H2O (n = 4, 5, 6) (hedp = 1-hydroxyethylidenediphosphonate). Inorg. Chem. 2001, 40, 5024–5029. [Google Scholar] [CrossRef]
- Fan, J.; Hanson, B.E. Novel zinc phosphate topologies defined by organic ligands. Inorg. Chem. 2005, 44, 6998–7008. [Google Scholar] [CrossRef]
- Robinson, K.; Gibbs, G.V.; Ribbe, P.H. Quadratic elongation-quantitative measure of distortion in coordination polyhedra. Science 1971, 172, 567–570. [Google Scholar]
- Harrison, W.T.A.; Nenoff, T.M.; Eddy, M.M.; Martin, T.E.; Stucky, G.D. Organic templates in zincophosphate synthesis-Zn5(PO4)2(HPO4)4·H2O·2H2N2C6H12 containing two distinct 8-ring channels and Zn3(PO4)(HPO4)2·HN2C6H12 containing tetrahedral 3-rings and Zn-N bonds. J. Mater. Chem. 1992, 2, 1127–1134. [Google Scholar] [CrossRef]
- Belhouchet, M.; Savariault, J.M.; Mhiri, T. Crystal structure and thermal behavior of two new organic monosulfates NH3(CH2)5NH3SO4·1.5H2O and NH3(CH2)9NH3SO4·H2O. J. Phys. Chem. Solids 2005, 66, 1294–1301. [Google Scholar] [CrossRef]
- Mandal, S.; Natarajan, S. Solvothermal synthesis and structures of one- and two-dimensional zinc phosphates. Inorg. Chim. Acta 2004, 357, 1437–1443. [Google Scholar] [CrossRef]
- Harrison, W.T.A.; Rodgers, J.A.; Phillips, M.L.F.; Nenoff, T.E. In situ template generation for zincophosphate synthesis leading to guanylurea zinc phosphate, C2H7N4O·ZnPO4, containing template-to-template N–H·O hydrogen bonds. Solid State Sci. 2002, 4, 969–972. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar]
- Spek, A.L. Structure validation in chemical crystallography. Acta Cryst. 2009, D65, 148–155. [Google Scholar]
- Nenoff, T.M.; Harrison, W.T.A.; Gier, T.E.; Calabrese, J.C.; Stucky, G.D. The low-temperature synthesis and characterization of two layered materials containing 3-ring groupings: NaH(ZnPO4)2 and CsH(ZnPO4)2. J. Solid State Chem. 1993, 107, 285–295. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Harrison, W.T.A.; Currie, W.R. 1,5-Diaminopentane As A Structure-Directing Agent for Zincophosphate Networks: Zn3(PO4)2(C5H14N2)2·3H2O and C5H16N2·Zn3(PO4)2(HPO4)·H2O. Crystals 2012, 2, 974-983. https://doi.org/10.3390/cryst2030974
Harrison WTA, Currie WR. 1,5-Diaminopentane As A Structure-Directing Agent for Zincophosphate Networks: Zn3(PO4)2(C5H14N2)2·3H2O and C5H16N2·Zn3(PO4)2(HPO4)·H2O. Crystals. 2012; 2(3):974-983. https://doi.org/10.3390/cryst2030974
Chicago/Turabian StyleHarrison, William T.A., and William R. Currie. 2012. "1,5-Diaminopentane As A Structure-Directing Agent for Zincophosphate Networks: Zn3(PO4)2(C5H14N2)2·3H2O and C5H16N2·Zn3(PO4)2(HPO4)·H2O" Crystals 2, no. 3: 974-983. https://doi.org/10.3390/cryst2030974