Glycine Betaine Recognition through Cation−π Interactions in Crystal Structures of Glycine Betaine Complexes with C-Ethyl-pyrogallol[4]arene and C-Ethyl-resorcin[4]arene as Receptors
Abstract
:1. Introduction
2. Results and Discussion
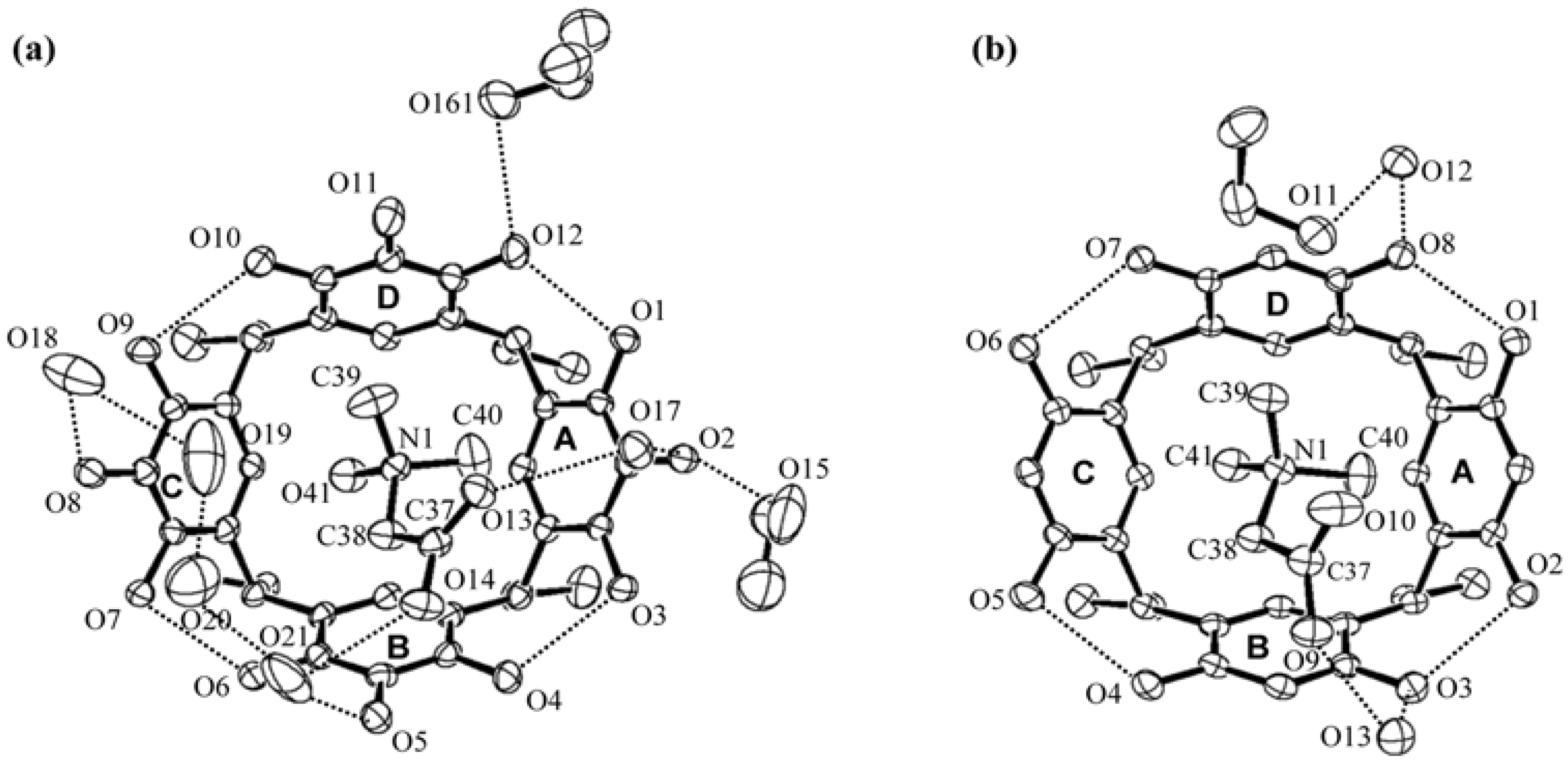
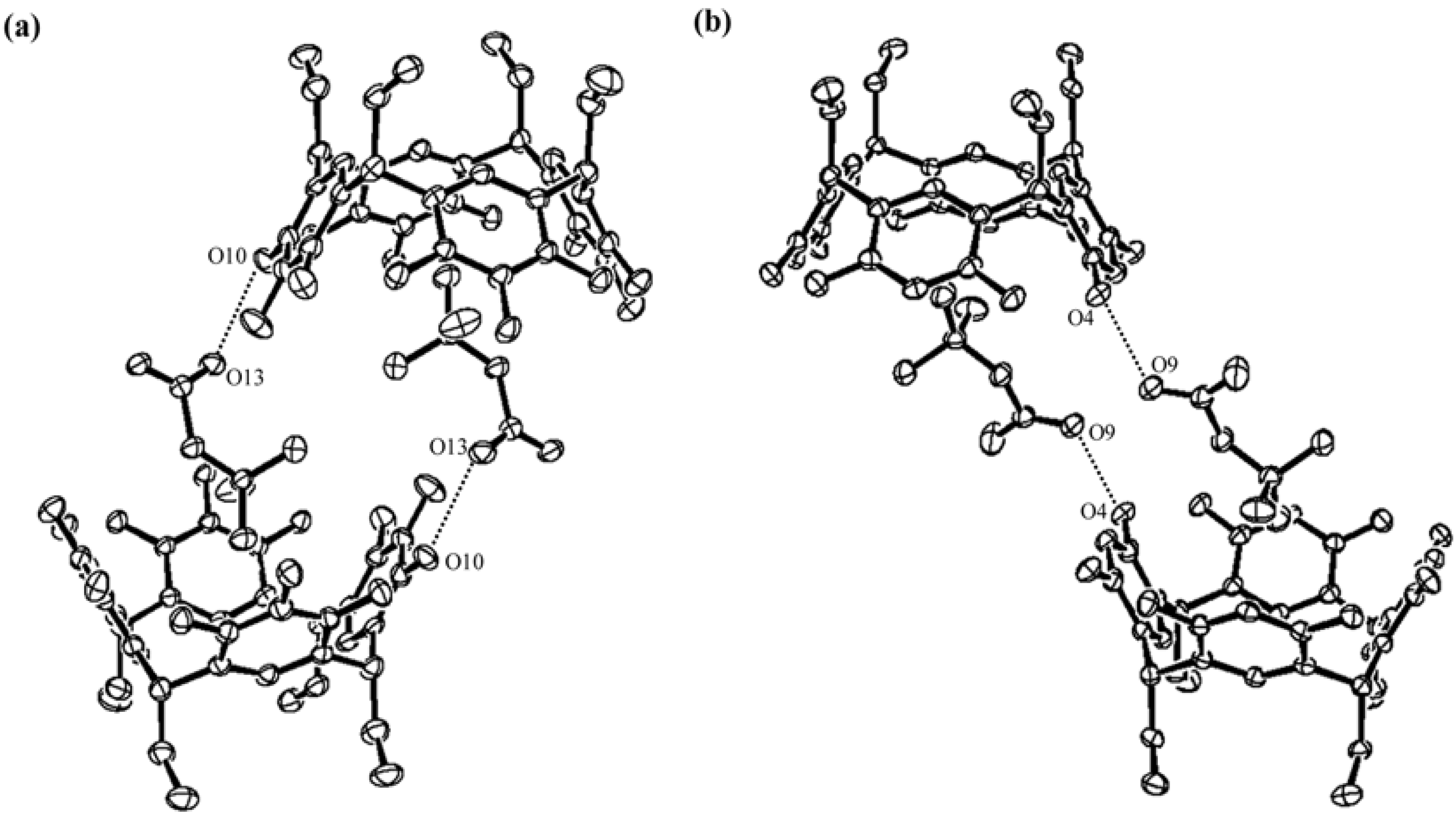
 ) and here (
) and here (  ). The almost ζ values are seen between 160° and 180°, both in CSD and PDB, while the tendency of the distribution of the η values are different. The η values observed in CSD structures are almost over 150°, especially gathered near 180°. The η values observed in PDB structures can fall into three groups; the first group is between 90° and 110°, the second between 110° and 150° and the third group over 150°. The third group has the similar tendency of distribution as the structures in CSD. The structures determined here are categorized into the third group.
). The almost ζ values are seen between 160° and 180°, both in CSD and PDB, while the tendency of the distribution of the η values are different. The η values observed in CSD structures are almost over 150°, especially gathered near 180°. The η values observed in PDB structures can fall into three groups; the first group is between 90° and 110°, the second between 110° and 150° and the third group over 150°. The third group has the similar tendency of distribution as the structures in CSD. The structures determined here are categorized into the third group.
 ) and here (
) and here (  ). The almost ζ values are seen between 160° and 180°, both in CSD and PDB, while the tendency of the distribution of the η values are different. The η values observed in CSD structures are almost over 150°, especially gathered near 180°. The η values observed in PDB structures can fall into three groups; the first group is between 90° and 110°, the second between 110° and 150° and the third group over 150°. The third group has the similar tendency of distribution as the structures in CSD. The structures determined here are categorized into the third group.
). The almost ζ values are seen between 160° and 180°, both in CSD and PDB, while the tendency of the distribution of the η values are different. The η values observed in CSD structures are almost over 150°, especially gathered near 180°. The η values observed in PDB structures can fall into three groups; the first group is between 90° and 110°, the second between 110° and 150° and the third group over 150°. The third group has the similar tendency of distribution as the structures in CSD. The structures determined here are categorized into the third group.
3. Experimental Section
4. Conclusions
Acknowledgments
Conflict of Interest
References and Notes
- Landfald, B.; Strøm, A.R. Choline-glycine betaine pathway confers a high level of osmotic tolerance in Escherichia coli. J. Bacteriol. 1986, 165, 849–855. [Google Scholar]
- Ebisuzaki, K.; Williams, J.N. Preparation and partial purification of soluble choline dehydrogenase from liver mitochondria. Biochem. J. 1955, 60, 644–646. [Google Scholar]
- Tsuge, H.; Nakano, Y.; Onishi, H.; Futamura, Y.; Ohashi, K. A novel purification and some properties of rat liver mitochondrial choline dehydrogenase. Biochim. Biophys. Acta 1980, 614, 274–284. [Google Scholar] [CrossRef]
- Finkelstein, J.D.; Martin, J.J. Methionine metabolism in mammals. J. Biol. Chem. 1984, 259, 9508–9513. [Google Scholar]
- Finkelstein, J.D.; Kyle, W.E.; Harris, B.J. Methionine metabolism in mammals. Regulation of homocysteine methyltransferases in rat tissue. Arch. Biochem. Biophys. 1971, 146, 84–92. [Google Scholar] [CrossRef]
- McKeever, M.P.; Weir, D.G.; Molloy, A.; Scott, J.M. Betaine-homocysteine methyltransferase: Organ distribution in man, pig and rat and subcellular distribution in the rat. Clin. Sci. 1991, 81, 551–556. [Google Scholar]
- DiBello, P.M.; Dayal, S.; Kaveti, S.; Zhang, D.; Kinter, M.; Lentz, S.R.; Jacobsen, D.W. The nutrigenetics of hyperhomocysteinemia. Mol. Cell. Proteomics 2010, 9, 471–485. [Google Scholar] [CrossRef]
- Schiefner, A.; Breed, J.; Bösser, L.; Kneip, S.; Gade, J.; Holtmann, G.; Diederichs, K.; Welte, W.; Bremer, E. Cation-π interactions as determinants for binding of the compatible solutes glycine betaine and proline betaine by the periplasmic ligand-binding protein ProX from Escherichia coli. J. Biol. Chem. 2004, 279, 5588–5596. [Google Scholar]
- Trikha, J.; Theil, E.C.; Allewell, N.M. High resolution crystal structures of amphibian red-cell l ferritin: Potential roles for structural plasticity and solvation in function. J. Mol. Biol. 1995, 248, 949–967. [Google Scholar] [CrossRef]
- Schiefner, A.; Holtmann, G.; Diederichs, K.; Welte, W.; Bremer, E. Structural basis for the binding of compatible solutes by ProX from the hyperthermophilic archaeon Archaeoglobus fulgidus. J. Biol. Chem. 2004, 279, 48270–48281. [Google Scholar]
- Hitomi, K.; Oyama, T.; Han, S.; Arvai, A.S.; Getzoff, E.D. Tetrameric architecture of the circadian clock protein KaiB. J. Biol. Chem. 2005, 280, 19127–19135. [Google Scholar]
- Horn, C.; Sohn-Bösser, L.; Breed, J.; Welte, W.; Schmitt, L.; Bremer, E. Molecular determinants for substrate specificity of the ligand-binding protein OpuAC from Bacillus subtilis for the compatible solutes glycine betaine and proline betaine. J. Mol. Biol. 2006, 357, 592–606. [Google Scholar] [CrossRef]
- Wolters, J.C.; Berntsson, R.P.-A.; Gul, N.; Karasawa, A.; Thunnissen, A.-M.W.H.; Slotboom, D.-J.; Poolman, B. Ligand binding and crystal structures of the substrate-binding domain of the ABC transporter OpuA. PLoS One 2010, 5. [Google Scholar] [CrossRef]
- Tschapek, B.; Pittelkow, M.; Sohn-Bösser, L.; Holtmann, G.; Smits, S.H.J.; Gohlke, H.; Bremer, E.; Schmitt, L. Arg149 is involved in switching the low affinity, open state of the binding protein AfProX into its high affinity, closed state. J. Mol. Biol. 2011, 411, 36–52. [Google Scholar] [CrossRef]
- Du, Y.; Shi, W.-W.; He, Y.-X.; Yang, Y.-H.; Zhou, C.-Z.; Chen, Y. Structures of the substrate-binding protein provide insights into the multiple compatible solute binding specificities of the Bacillus subtilis ABC transporter OpuC. Biochem. J. 2011, 436, 283–289. [Google Scholar] [CrossRef]
- Marshall, H.; Venkat, M.; Seng, N.S.; Cahn, J.; Juers, D.H. The use of trimethylamine N-oxide as a primary precipitating agent and related methylamine osmolytes as cryoprotective agents for macromolecular crystallography. Acta Cryst. 2012, D68, 69–81. [Google Scholar]
- Ressel, S.; van Scheltinga, A.C.; Vonrhein, C.; Ott, V.; Ziegler, C. Molecular basis of transport and regulation in the Na+/betaine symporter BetP. Nature 2009, 458, 47–52. [Google Scholar]
- Gardberg, A.; Fox, D.; Staker, B.; Stewart, L. Crystal Structure Of Glycine Betaine, L-Proline ABC Transporter, Glycine/Betaine/L-Proline-Binding Protein (ProX) from Borrelia Burgdorferi; PDB (Protein Data Bank): Upton, NY, USA, 2013; No. 3TMG. [Google Scholar]
- Tan, K.; Shackelford, G.; Joachimiak, A. The Crystal Structure of A Possible Acetyltransferase Clostridium Difficile 630; PDB: Upton, NY, USA, 2013; No. 3DSB. [Google Scholar]
- Ressl, S.; Scheltinga, A.C.T.V.; Vonrhein, C.; Ott, V.; Ziegler, C. Crystal Structure of the Sodium-Coupled Glycine Glutamicum with Bond Substrate; PDB: Upton, NY, USA, 2013; No. 2WIT. [Google Scholar]
- Fujisawa, I.; Takeuchi, D.; Kato, R.; Murayama, K.; Aoki, K. Crystal structures of resorcin[4]arene and tetramethylated resorcin[4]arene complexes incorporating L-carnitine. Bull. Chem. Soc. Jpn. 2011, 84, 1133–1135. [Google Scholar] [CrossRef]
- Fujisawa, I.; Takeuchi, D.; Kitamura, Y.; Okamoto, R.; Aoki, K. Crystal structure of an L-carnitine complex with pyrogallol[4]arene. J. Phys. Conf. Ser. 2012, 352. [Google Scholar] [CrossRef]
- Murayama, K.; Aoki, K. Molecular recognition involving multiple cation−π interactions: The inclusion of the acetylcholine trimethylammonium moiety in resorcin[4]arene. Chem. Commun. 1997, 1, 119–120. [Google Scholar] [CrossRef]
- Luostarinen, M.; Åhman, A.; Nissinen, M.; Rissanen, K. Ethyl pyrogall[6]arene and pyrogall[4]arene: Synthesis, structural analysis and derivatization. Supramol. Chem. 2004, 16, 505–512. [Google Scholar] [CrossRef]
- Murayama, K.; Aoki, K. Resorcin[4]arene dimer linked by eight water molecules and incorporating a tetraethylammonium ion: guest-driven capsule formation via cation-π interactions. Chem. Commun. 1998, 5, 607–608. [Google Scholar] [CrossRef]
- Mansikkamäki, H.; Nissinen, M.; Schalley, C.A.; Rissanen, K. Self-assembling resorcinarene capsules: Solid and gas phase studies on encapsulation of small alkyl ammonium cations. New J. Chem. 2003, 27, 88–97. [Google Scholar] [CrossRef]
- Mäkinen, M.; Vainiotalo, P.; Nissinen, M.; Rissanen, K. Ammonium ion mediated resorcarene capsules: ESI-FTICRMS study on gas-phase structure and ammonium ion affinity of tetraethyl resorcarene and its per-methylated derivative. J. Am. Soc. Mass Spectrom. 2003, 14, 143–151. [Google Scholar]
- Aoyama, Y.; Tanaka, Y.; Sugahara, S. Molecular recognition. 5. Molecular recognition of sugars via hydrogen-bonding interaction with a synthetic polyhydroxy macrocycle. J. Am. Chem. Soc. 1989, 111, 5397–5404. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar]
- Kabuto, C.; Akine, S.; Nemoto, T.; Kwon, E. Release of software (Yadokari-XG 2009) for crystal structure analyses. J. Cryst. Soc. Jpn. 2009, 51, 218–224. [Google Scholar] [CrossRef]
- Farrugia, L.J. ORTEP-3 for windows—A version of ORTEP-III with a graphical user interface (GUI). J. Appl. Cryst. 1997, 30, 565. [Google Scholar] [CrossRef]
- CCDC CIF Depository Request Form for data published from 1994. Available online: http://www.ccdc.cam.ac.uk/conts/retrieving.html (accessed on 7 April 2013).
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fujisawa, I.; Aoki, K. Glycine Betaine Recognition through Cation−π Interactions in Crystal Structures of Glycine Betaine Complexes with C-Ethyl-pyrogallol[4]arene and C-Ethyl-resorcin[4]arene as Receptors. Crystals 2013, 3, 306-314. https://doi.org/10.3390/cryst3020306
Fujisawa I, Aoki K. Glycine Betaine Recognition through Cation−π Interactions in Crystal Structures of Glycine Betaine Complexes with C-Ethyl-pyrogallol[4]arene and C-Ethyl-resorcin[4]arene as Receptors. Crystals. 2013; 3(2):306-314. https://doi.org/10.3390/cryst3020306
Chicago/Turabian StyleFujisawa, Ikuhide, and Katsuyuki Aoki. 2013. "Glycine Betaine Recognition through Cation−π Interactions in Crystal Structures of Glycine Betaine Complexes with C-Ethyl-pyrogallol[4]arene and C-Ethyl-resorcin[4]arene as Receptors" Crystals 3, no. 2: 306-314. https://doi.org/10.3390/cryst3020306



