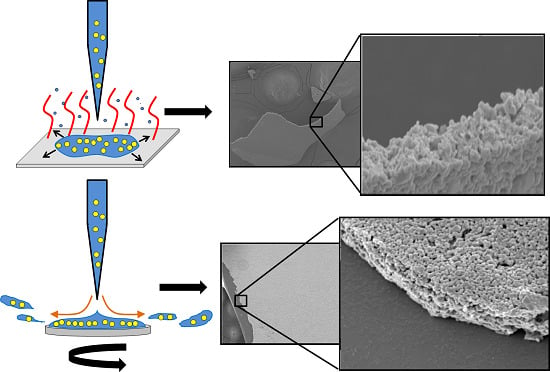
Physiochemical Characterization of Iodine (V) Oxide Part II: Morphology and Crystal Structure of Particulate Films
Abstract
:1. Introduction
2. Background
3. Results and Discussion
3.1. Suspensions of Iodine Oxides in Alcohols



3.2. Effect of Sonication on Particle Morphology
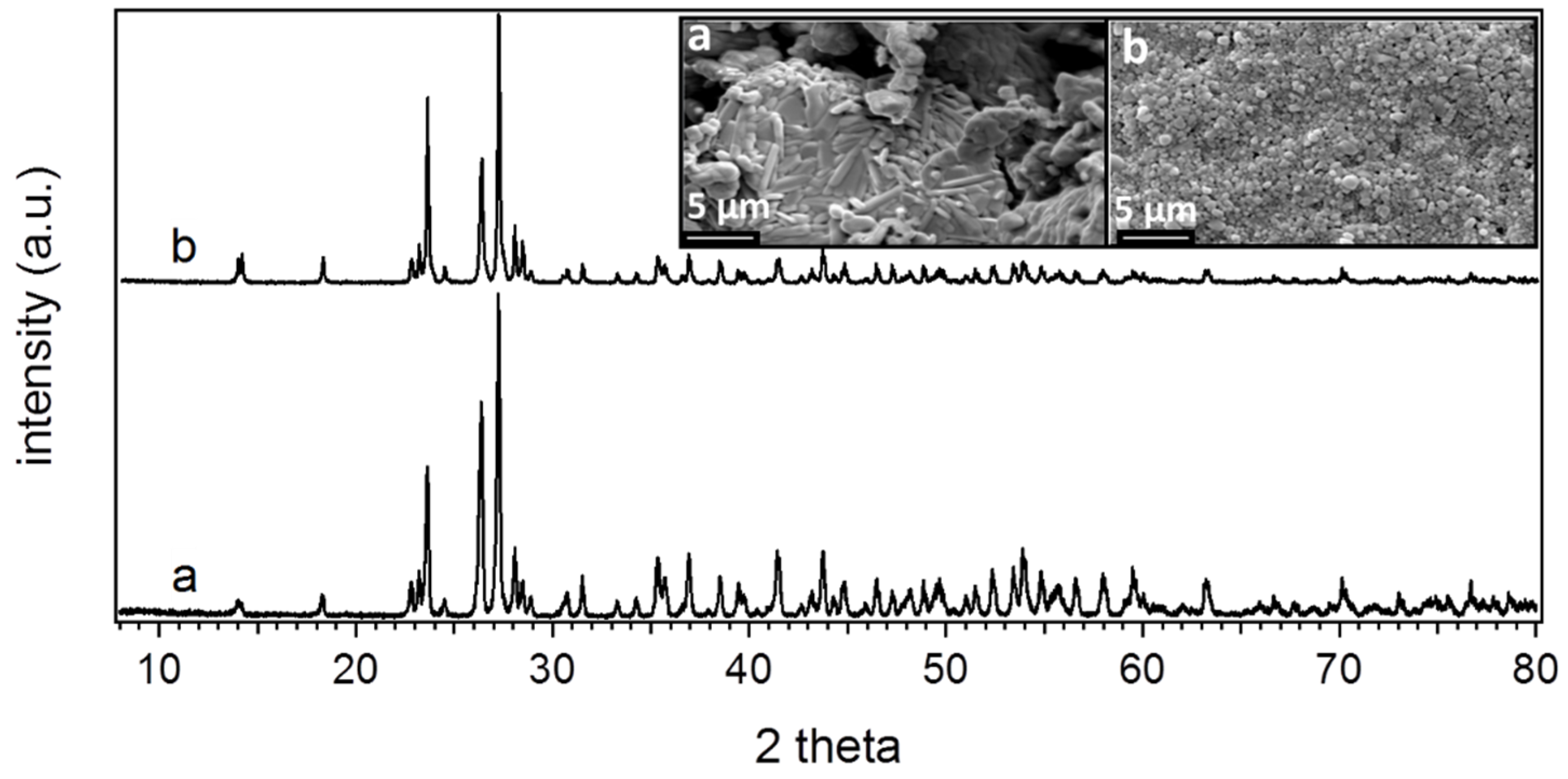
3.3. Effect of Humidity of Crystal Growth

3.4. Crystallinity, Morphology and Structure of Spin-Cast Films



| Spin Cast Rate | Film Mass | Film Thickness | Avg. Particle Diameter | %TMD | % Orientation |
|---|---|---|---|---|---|
| (b) 0 rpm * | 9.9 ± 0.1 mg | 3.4 ± 0.4 μm (1 layer) | 0.41 ± 0.12 μm | 88 ± 4.4% | 0/NA |
| (c) 500 rpm | 5.8 ± 0.1 mg | 4.1 ± 0.7 μm ( 5 layers) | 0.61 ± 0.19 μm | 43 ± 6.6% | 7/15 |
| (d) 1500 rpm | 2.8 ± 0.1 mg | 4.1 ± 0.7 μm (~1 layer) | 0.90 ± 0.33 μm | 21 ± 7.5% | 20/25 |
| (e) 2500 rpm | 2.0 ± 0.1 mg | 1.5 ± 0.4 μm (~1 layer) | 0.86 ± 0.28 μm | 40 ± 11.4% | 26/31 |

4. Experimental Section
4.1. Materials
4.2. Characterization
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Martirosyan, K.S. Nanoenergetic gas-generators: Principles and applications. J. Mater. Chem. 2011, 21, 9400–9405. [Google Scholar] [CrossRef]
- Russell, R.; Bless, S.; Pantoya, M. Impact-driven thermite reactions with iodine pentoxide and silver oxide. J. Energ. Mater. 2011, 29, 175–192. [Google Scholar] [CrossRef]
- Jenkins, C.M.; Horie, Y.; Lindsay, C.M.; Butler, G.C.; Lambert, D.; Welle, E.J. Cylindrical converging shock initiation of reactive materials. In Shock Compression of Condensed Matter–2011, Proceedings of the American Physical Society Topical Group on Shock Compression of Condensed Matter, Chicago, IL, USA, 26 June–1 July 2011; AIP Publishing: College Park, MD, USA, 2012; Volume 1426, pp. 197–200. [Google Scholar]
- Ivanov, V.G.; Ivanov, G.V.; Lapin, P.V.; Kuznetsov, V.P. Role of iodation in the combustion of metal oxides with iodine pentoxide. Combust. Explos. Shock Waves 1981, 17, 607–614. [Google Scholar] [CrossRef]
- Little, B.K.; Avjian, E.K.; Bogle, M.; Nittinger, J.C.; Emery, S.B.; Schrand, A.; Lindsay, C.M. Trade-off between sensitivity and performance in nano-aluminum/iodine (V) oxide, joint army navy NASA air force. In Proceedings of the 38th Propellant and Explosives Development and Characterization Meeting, Monterey, CA, USA, 3–6 December 2012.
- Sherwood, P.M.A.; Turner, J.J. Mass spectrum of iodine pentoxide and a novel reaction with copper. J. Chem. Soc. A Inorg. Phys. Theor. 1970, 2349–2350. [Google Scholar] [CrossRef]
- Clark, B.R.; Pantoya, M.L. The aluminum and iodine pentoxide reaction for the destruction of spore forming bacteria. Phys. Chem. Chem. Phys. 2010, 12, 12653–12657. [Google Scholar] [CrossRef] [PubMed]
- Little, B.K.; Emery, S.B.; Nittinger, J.C.; Fantasia, R.C.; Lindsay, C.M. Physiochemical characterization of Iodine (V) oxide, part 1: Hydration rates. J. Propellants Explos. Pyrotech. 2014, 35, 1–10. [Google Scholar] [CrossRef]
- Selte, K.; Kjekshus, A. Iodine oxides part II: On the system H2O-I2O5. Acta Chem. Scand. 1968, 22, 3309–3320. [Google Scholar] [CrossRef]
- Kumar, R.; Saunders, R.W.; Mahajan, A.S.; Plane, J.M.; Murray, B.J. Physical properties of iodate solutions and the deliquescence of crystalline I2O5 and HIO3. Atmos. Chem. Phys. Discuss. 2010, 10, 20823–20856. [Google Scholar] [CrossRef]
- Little, B.K. Observations made in the laboratory.
- Rossie, C.; Zhang, K.; Estève, D.; Alphonse, P.; Tailhades, P.; Vahlas, C.J. Nanoenergetic materials for MEMS: A review. J. Microelectromech. Syst. 2007, 16, 919–931. [Google Scholar] [CrossRef] [Green Version]
- Manesh, N.A.; Basu, S.; Kumar, R. Experimental flame speed in multi-layered nano-energetic materials. Combust. Flame 2010, 157, 476–480. [Google Scholar] [CrossRef]
- Ding, M.S.; Krieger, F.C.; Swank, J.A. Developing NanoFoil-Heated Thin-Film Thermal Battery. Available online: http://oai.dtic.mil/oai/oai?verb=getRecord&metadataPrefix=html&identifier=ADA586188 (accessed on 16 September 2015).
- Barron, S.C.; Knepper, R.; Walker, N.; Weihs, T.P. Characterization of self-propagating formation reactions in Ni/Zr multilayered foils using reaction heats, velocities, and temperature-time profiles. J. Appl. Phys. 2011, 109. [Google Scholar] [CrossRef]
- Trenkle, J.C.; Koerner, L.J.; Tate, M.W.; Gruner, S.M.; Weihs, T.P.; Hufnagel, T.C. Phase transformation during rapid heating of Al/Ni multilayer foils. Appl. Phys. Lett. 2008, 93. [Google Scholar] [CrossRef]
- Weihs, T.P.; Barmack, K.; Coffey, K. Fabrication and characterization of reactive multilayer films and foils. In Metallica Films for Electronic, Optical and Magnetic Applications: Structure, Processing and Properties; Woodhead Publishing Limited: Philadelphia, PA, USA, 2014; pp. 160–243. [Google Scholar]
- Dlott, D.D. Thinking Big (and Small) about Energetic Materials. Mater. Sci. Technol. 2006, 22, 463–473. [Google Scholar] [CrossRef]
- Prakash, A.; McCormick, A.V.; Zachariah, M.R. Thermo-kinetic study of core-shell Nanothermites. In Shock Compression of Condensed Matter–2005, Proceedings of the Conference of the American Physical Society Topical Group on Shock Compression of Condensed Matter, Baltimore, MD, USA, 31 July–5 August 2005; pp. 1006–1009.
- Prakash, A.; McCormick, A.V.; Zachariah, M.R. Tuning the reactivity of energetic nanoparticles by creation of a core-shell nanostructure. Nano Lett. 2005, 5, 1357–1360. [Google Scholar] [CrossRef] [PubMed]
- Jingyu, F.; Guoqiang, J.; Qing, L.; Zachariah, M.R. Passivated iodine pentoxide oxidizer for potential biocidal nanoenergetic applications. ACS Appl. Mater. Interfaces 2013, 5, 8875–8880. [Google Scholar]
- Emery, S.; Rider, K.B.; Lindsay, C.M. Stabilized Magnesium/Perfluoropolyether Nanocomposite Films by Helium Droplet Cluster Assembly. Propellants Explos. Pyrotech. 2014, 39, 161–165. [Google Scholar] [CrossRef]
- Becker, C.R.; Currano, L.J.; Churaman, W.A.; Stoldt, C.R. Thermal analysis of the exothermic reaction between galvanic porous silicon and sodium perchlorate. ACS Appl. Mater. Interfaces 2010, 2, 2998–3003. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, K.T.; Zhu, C.; Tanaka, D.J.; Kuntz, J.D.; Duoss, E.B.; Gash, A.E. Electrophoretic deposition of thermites onto micro-engineered electrodes prepared by direct-ink writing. J. Phys. Chem. B 2013, 117, 1686–1693. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Jian, G.; DeLisio, J.B.; Wang, H.; Zachariah, M.R. Electrospray deposition of energetic polymer nanocomposites with high mass particle loadings: A prelude to 3D printing of rocket motors. Adv. Eng. Mater. 2015, 17, 95–101. [Google Scholar] [CrossRef]
- Patel, V.K.; Ganguli, A.; Kant, R.; Battacharya, S. Micropatterning of nanoenergetic films of Bi2O3/Al for pyrotechnics. RSC Adv. 2015, 5, 14967–14973. [Google Scholar] [CrossRef]
- Torrey, J.D.; Kirschling, T.L.; Greenlee, L.F. Processing and characterization of nanoparticle coatings for quartz crystal microbalance measurements. J. Res. Nat. Inst. Stand. Technol. 2015, 120, 1–10. [Google Scholar] [CrossRef]
- Panigrahi, S.; Waugh, S.; Rout, S.K.; Hassan, A.K. Ray study of spin coated organic thin film under spectrophotometer. Indian J. Phys. 2004, 78, 823–826. [Google Scholar]
- Larson, R.G.; Rehg, T.J. Spin coating. In Liquid Film Coating: Scientific Principles and Their Technological Implications; Kistler, S.F., Schweizer, P.M., Eds.; Chapman & Hall: London, UK, 1997; pp. 709–734. [Google Scholar]
- Pichumani, M.; Bagheri, P.; Poduska, K.M.; González-Vinas, W.; Yethiraj, A. Dynamics, crystallization and structures in colloid spin coating. Soft Matter 2013, 9, 3220–3229. [Google Scholar] [CrossRef]
- Leventis, N.; Chandrasekaran, N.; Sadekar, A.G.; Sotiriou-Leventis, C.; Lu, H. One-Pot synthesis of interpenetrating inorganic/organic networks of CuO/resocinol-formaldehyde aerogels: Nanostructured energetic materials. J. Am. Chem. Soc. 2009, 131, 4576–4577. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.; Subramanian, S.; Bhattacharya, S.; Gao, Y.; Apperson, S.; Shende, R.; Guha, S.; Arif, M.; Bai, M.; Gangopadhyay, K.; et al. Crystallization of amorphous silicon by self-propagation of nanoengineered thermites. J. Appl. Phys. 2007, 101. [Google Scholar] [CrossRef]
- Apperson, S.; Bhattacharya, S.; Gao, Y.; Subramanian, S.; Hasan, S.; Hossain, M.; Shende, R.V.; Redner, P.; Kapoor, D.; Nicolich, S.; et al. On-Chip initiation and burn rate measurements of thermite energetic reactions. MRS Proc. 2006, 896. [Google Scholar] [CrossRef]
- Sullivan, K.T.; Kuntz, J.D.; Gash, A.E. Fine patterning of thermites for mechanistic studies and microenergetic applications. Int. J. Energ. Mater. Chem. Propuls. 2013, 12, 511–528. [Google Scholar] [CrossRef]
- Thiruvengadathan, R.; Bezmelnitsyn, A.; Apperson, S.J.; Tappmeyer, D.; Redner, P.; Balas, W.A.; Nicolich, S.; Kapoor, D.; Gangopadhyay, K.; Gangopadhyay, S. Combustion behavior of nanoenergetic material systems. In Energetic Materials: Thermophyscial Properties, Predictions, and Experimental Measurements; Boddu, V., Redner, P., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 239–262. [Google Scholar]
- Plummer, A.; Kuznetsov, V.; Joyner, T.; Shapter, J.; Voelcker, N.H. The burning rate of energetic films on nanostructured porous silicon. Small 2011, 7, 3392–3398. [Google Scholar] [CrossRef] [PubMed]
- Eapen, B.Z.; Hoffmann, V.K.; Schoenitz, M.; Dreizin, E.L. Combustion of aerosolized spherical Al powders and flakes in air. Combust. Sci. Technol. 2010, 176, 1055–1069. [Google Scholar] [CrossRef]
- Rider, K.B.; Little, B.K.; Emery, S.B.; Lindsay, C.M. Thermal analysis of magnesium/perfluoropolyether pyrolants. Propellants Explos. Pyrotech. 2013, 38, 433–440. [Google Scholar] [CrossRef]
- Shaw, W.L.; Dlott, D.D.; Williams, R.A.; Dreizin, E.L. Ignition of nanocomposite thermites by electric spark and shock wave. Propellants Explos. Pyrotech. 2014, 39, 444–453. [Google Scholar] [CrossRef]
- Hamons, J.A.; Wang, W.; Ilavsky, J.; Pantoya, M.; Weeks, B.; Vaugh, M.W. Small angle X-ray scattering analysis of the effect of cold compaction of Al/MoO3 thermite composites. Phys. Chem. Chem. Phys. 2008, 10, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Yen, N.H.; Wang, L.Y. Reactive Metals in Explosives. Propellants Explos. Pyrotech. 2012, 37, 143–155. [Google Scholar] [CrossRef]
- Luque de Castro, M.D.; Priego-Capote, F. Ultrasound-assisted Crystallization (Sonocrystallization). Ultrason. Sonochem. 2007, 14, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Ruecroft, G.; Hipkiss, D.; Ly, T.; Maxted, N.; Cains, W. Sonocrystallization: The Use of Ultrasound for Improved Industrial Crystallization. Org. Process. Res. Dev. 2005, 9, 923–932. [Google Scholar] [CrossRef]
- Suslick, K.S. Sonochemistry. Science 1990, 247, 1439–1442. [Google Scholar] [CrossRef] [PubMed]
- Hayes, W.M. CRC Handbook of Chemistry and Physics, 96th ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Lebedkin, S.F.; Klimov, A.D. Quantum Yield Wavelength Dependence of Iodine Photodissociation in Hexane and Benzene Studied by the Time-Resolved Thermal Lens Methods. Chem. Phys. Lett. 1992, 190, 313–314. [Google Scholar] [CrossRef]
- Lapworth, A. The effect of halogens on compounds, which contain the carbonyl group. J. Chem. Soc. 1904, 85, 30–42. [Google Scholar] [CrossRef]
- Morrison, R.T.; Boyd, R.N. Organic Chemistry, 5th ed.; Allyn and Bacon, Inc.: Boston, MA, USA, 1987; pp. 912–913. [Google Scholar]
- Drugfuture.com—Iodine Pentoxide. Available online: http:://www.drugfuture.com/chemdata/iodine-pentoxide.html (accessed on 8 August 2015).
- Bray, W.C.; Lamb, A.B. Process of Bearing Iodic Acid. U.S. Patent 151938, 17 July 1922. [Google Scholar]
- Fischer, A. Redetermination of HI3O8, an adduct of formula HIO3•I2O5. Acta Cryst. Sec. E 2005, 61, 278–279. [Google Scholar] [CrossRef]
- Mihi, A.; Ocana, M.; Miguez, H. Oriented collodidal-crystal thin films by spin-coating microspheres dispersed in volatile media. Adv. Mater. 2006, 18, 2244–2229. [Google Scholar] [CrossRef]
- Toolan, T.W.; Fuji, S.; Ebbens, S.J.; Nakamura, Y.; Howse, J.R. On the Mechanisms of Colloidal Self-Assembly During Spin-Coating. Soft Matter 2014, 10, 8804–8812. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Little, B.K.; Emery, S.B.; Lindsay, C.M. Physiochemical Characterization of Iodine (V) Oxide Part II: Morphology and Crystal Structure of Particulate Films. Crystals 2015, 5, 534-550. https://doi.org/10.3390/cryst5040534
Little BK, Emery SB, Lindsay CM. Physiochemical Characterization of Iodine (V) Oxide Part II: Morphology and Crystal Structure of Particulate Films. Crystals. 2015; 5(4):534-550. https://doi.org/10.3390/cryst5040534
Chicago/Turabian StyleLittle, Brian K., Samuel B. Emery, and C. Michael Lindsay. 2015. "Physiochemical Characterization of Iodine (V) Oxide Part II: Morphology and Crystal Structure of Particulate Films" Crystals 5, no. 4: 534-550. https://doi.org/10.3390/cryst5040534