1. Introduction
Calcium carbonate is largely found in natural creatures, especially marine organisms, such as echinoderms, abalone, and sea molluscs [
1,
2]. These parts of inorganic-organic composites often function as body supports, e.g., exoskeletons, to protect the creatures from injury, or form shelters to offer themselves a safe living environment. Unlike relatively regular artificial crystals, CaCO
3 crystals in biological systems present very complicated morphologies, e.g., the aragonite nanoplates in nacre [
3], the complex structure of coccolithophores [
4], calcite spines of sea urchins [
5], etc., which seem to be perfectly pre-designed and are rarely reproduced by using any advanced biomimetic methods in the laboratory. It is, therefore, of great interest to reveal detailed processes of crystal growth and to understand crucial factors which control the crystal growth in natural creatures.
Crystalline CaCO
3 exists as one of three principal anhydrous polymorphs, i.e., calcite (rhombohedral), aragonite (orthorhombic), and vaterite (hexagonal). According to the phase diagram of pure calcium carbonate, calcite has been proved to be the most thermodynamically stable phase, and vaterite, the least [
6]. Vaterite would transform into a more stable polymorph upon contact with water [
7], e.g., converting to calcite at low temperature and to aragonite at high temperature. However, in some special conditions, the stabilities of these three polymorphs can alternate. For example, Mg substitution in calcite may greatly reduce its stability, leading to a transformation to aragonite [
8].
In nature creatures, CaCO
3 microcrystals are commonly embedded in a biological matrix and both aragonite and vaterite can be the stable phases. For example, aragonite is included in many marine biominerals, such as nacreous tablets [
9,
10,
11,
12], urchin spines [
5,
13], and crossed lamellae in seashells [
14]. Metastable vaterite can also exist in the natural productions and biomineralization. According to the research of Soldati et al. [
15] and Wehrmeister et al. [
16], both vaterite and aragonite were detected in freshwater cultured pearls, and vaterite was observed to exist in an aragonite environment. In Ascidiacea, known as sea squirts, vaterite also exists in form of spicules [
17].
In addition to these crystalline phases, in many biomineralization processes, amorphous calcium carbonate (ACC), as a precursor of crystalline CaCO
3, plays an important role in the formation of crystalline phases and crystal morphologies. In a simple environment, ACC can transform to aragonite at high temperature (over 40 °C) and to calcite at low temperature [
18,
19].
Spherulite is probably one of the most interesting morphologies found in natural creatures or biominerals, because their nature of the inorganic and biological composite and their radial crystal orientations. Spherulites were found in a wide range of materials, in addition to the common carbonates, for instance, polymers [
20], metals [
21], and organic molecules [
22]. Spherulites have also attracted increasing attention from geologists [
23,
24,
25], because carbonate spherulites have high porosity and permeability and are excellent reservoirs in pre-salt fields. In the last decades, several key factors of forming spherulites have been proposed, including Brownian motion, phase field effect, and dipole field interaction.
Spherulites can form via aggregation of tiny nanocrystallites (<10 nm) in Brownian motion [
26]. However, the detailed microstructures and complicated morphologies of spherulites cannot be explained simply using Brownain motion. The phase field effect was believed to be a more convincing explanation of the spherulitic growth [
27]. According to the previous research, the spherulites were divided into two categories: (1) spherulites grow in a radial manner from a nucleus and more branches intermittently filled the space during the growth; and (2) threadlike particles grow first and new grains formed at the growth front. By splaying out the branching on both ends, a spherical morphology was achieved. In this process, two characteristic “eyes” were developed on each side of the core region [
27]. The phase field, as is claimed to be the driving force of the spherulite formation, was based on the “diffusional instabilities”. The model indicated a competition between the ordering effect of discrete local crystallographic symmetries and the randomization of the local crystallographic orientation with growth front nucleation. In 2000, Banfield et al. [
28] revealed a three-dimensional rotation of nanoparticles in their HRTEM study of natural iron oxyhydroxide biomineralization products. Neighbouring nanoparticles could aggregate and rotate to adopt parallel orientations.
According to many published papers, some elements, such as magnesium, can not only affect the stability of the crystalline CaCO
3, but also play important roles in the formation of spherulitic morphology. Tracy et al. reported that co-existence of Mg
2+ and SO
42− ions allowed a formation of spherulites of CaCO
3 [
29,
30]. ACC was also found to be important to the formation of novel morphologies, in addition to working as a transient precursor for crystalline phases of CaCO
3 [
31]. It is quite interesting to see that, in biological systems, some organisms can produce unusually stable hydrated ACC, as reported by Addadi et al. [
32]. The hydration of ACC would inevitably affected by pH in the solution [
33]. The crystallization process of ACC in biomineralization was also investigated by using an in situ small- and wide-angle X-ray scattering (SAXS/WAXS) method, and a multi-step evolution was discovered, i.e., formation in order of hydrated and disordered ACC, more ordered and dehydrated ACC, ACC dissolution and spherulitic growth of vaterite, and Ostwald ripening of the vaterite [
34].
The addition of organic compounds in a synthetic system can enhance the formation of spherulitic morphology of CaCO
3. For example, hollow spherulites of calcite crystals would precipitate in the presence of alginates [
35]. Biological matter is even better. Rodriguez-Navarro et al. believed that bacterical production of CO
2 and NH
3, and the transformation of the latter to OH−, led to the growth of vaterite crystallites on bacterial cells, or the so-called extracellular polymeric substance (EPS), to form spherulites [
36]. Greer et al. [
37] also observed such a phenomenon in a synthesis of biomimetic vateritic hexagonal prisms with gelatine, and further found that the electrical dipole field interaction between the crystallites was the driving force for aggregation and self-orientation. In other words, the nanocrystallites were regarded as dipoles and, because of the interaction with the central dipole field, the nanocrystallites embedded in a soft matter matrix could rotate and self-arrange in an unusual way.
There is another common argument of whether the spherulites of CaCO
3 grow directly from solution by central multidirectional growth or by aggregation of nano-sized precursor crystals. Andreassen and co-workers made a significant effort and found that the former is more likely the true mechanism [
38,
39]. This conclusion is probably correct for some spherulites, such as the particles constructed by a single layer of radiating nanorods [
40]. However, it may face a challenge in explaining the formation of spherulites with multiple layers of short nanorods. Further evidence is needed from HRTEM images of the specimens at intermediate stages of growth.
In the present work, spherulites found in limpet shells are investigated chiefly by using electron microscopy. Some interesting microstructures have been observed, e.g., a core rich in biological matter, multilayer short nanorods, possible evolution of the microstructures, etc. A dipole field-driven formation mechanism of the hierarchical structure of these calcium carbonate mesospherulites is proposed. Spherulites formed in a natural environment and synthesized in the laboratory, as presented in a previously-published report [
37], are compared in order to shed light to the future biomimetic synthesis of crystals with various morphologies.
2. Results
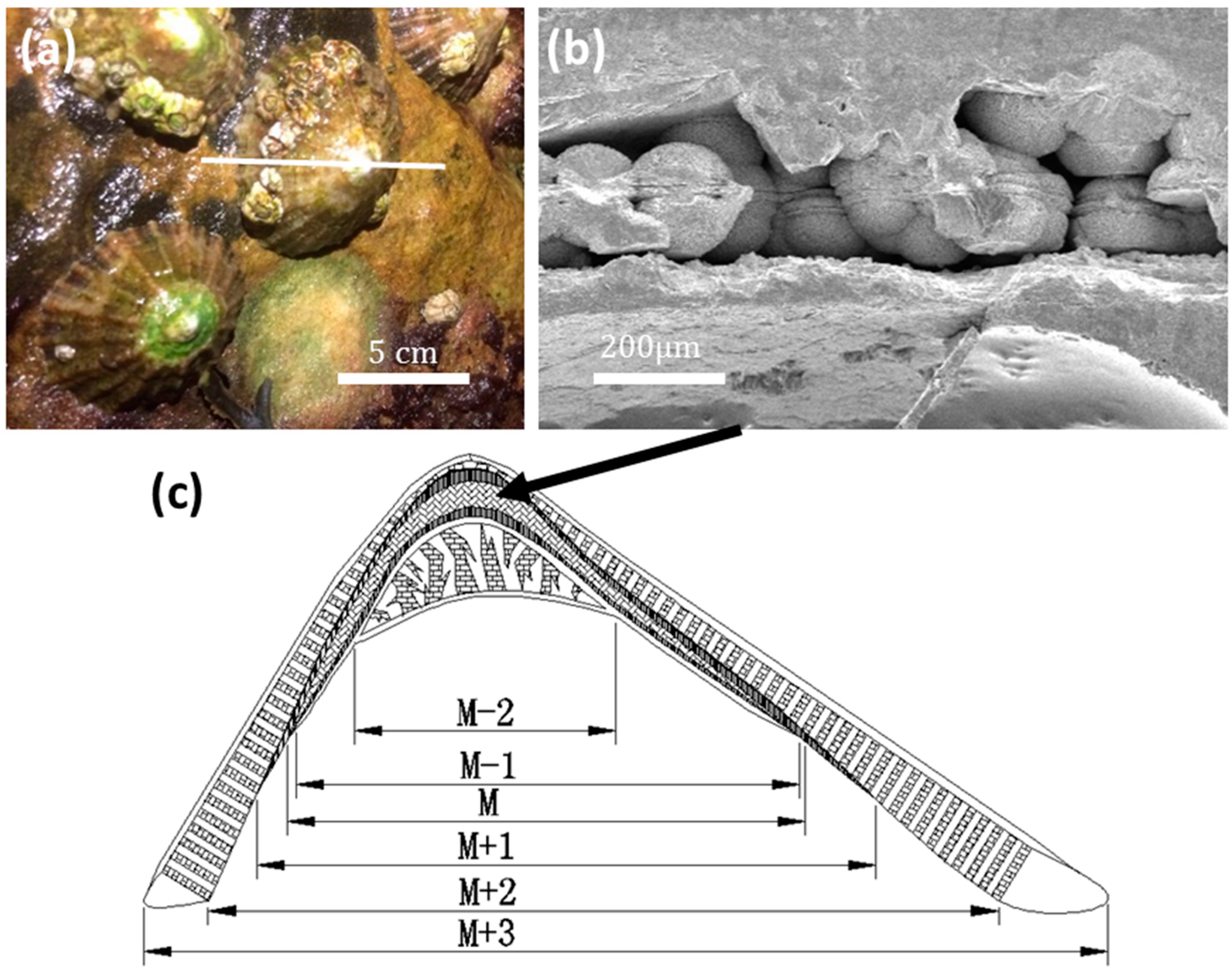
Live limpet samples were collected at East Sands, St Andrews, Scotland (
Figure 1a). According to SEM observations, a limpet shell consists of six layers (M − 2, M − 1, M, M + 1, M + 2, M + 3, from inner to outer sides), as illustrated in
Figure 1c. Spherulites about 100 μm in diameter were only found in the M layer (abbreviation of myostracum) (
Figure 1b). There is an equatorial notch at the centre of each spherulite, dividing the whole particle into two hemispheres, which was the first sign that aroused our curiosity.
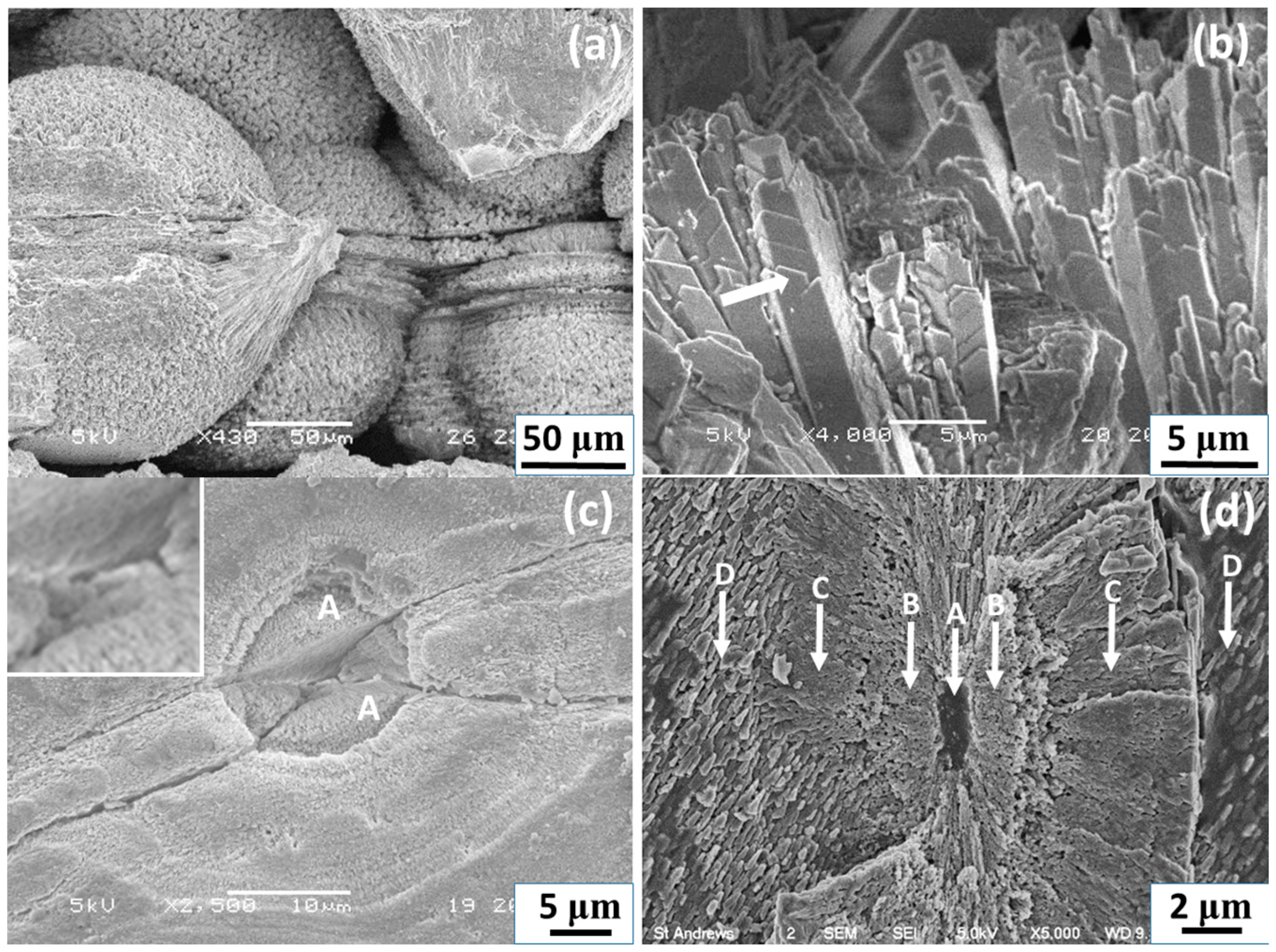
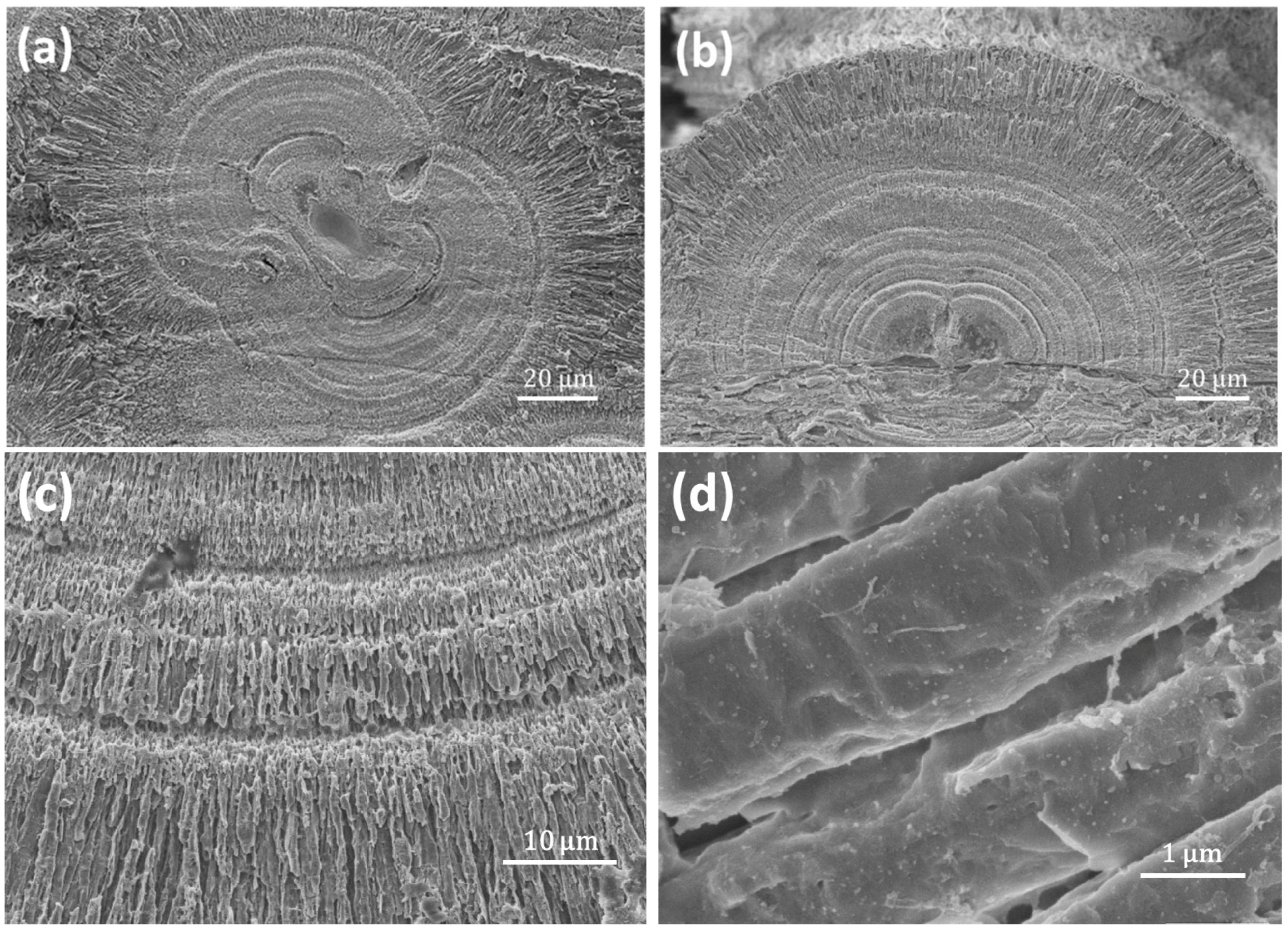
SEM images of whole spherulites show a rough surface (
Figure 2a). Examinations of the exterior and near surface area of the spherulites reveal that the outer layer of the particles is formed by radially-located mesorods, which have a shape of a hexagonal prism with about 1.5–5 μm in width and 20 μm in length, sticking out from the centre of the particles (
Figure 2b). On the side surface of the hexagonal prisms, multiple nanosheets grow from the bottom as coating skins, indicating that the starting point of the growth is at the bottom ends of the mesorods and the mesorods have a high crystallinity. Similarly, aragonite crystal mesorods were synthesized by Zhou et al. and Nan et al. [
41,
42].
The equatorial notches on the spherulites are not only a surface structural feature, but evidence of cracks cross to the particle cores.
Figure 2c shows a SEM image of a cross-section of spherulite, revealing the microstructure in the central area of the particle. It is noted that the crack starts from a small disk (~4 μm in diameter) at the centre of a core, which is about 15 μm in diameter. The spherical core is separated into two hemispheres by the smaller disk, which seems to have a sandwich shape (see inset of
Figure 2c).
Figure 2d shows a SEM cross-section image of another spherulite, which is also supposed to be at an early stage of growth because of the small size and the relatively poor ordering of the nanoparticles. In particular, radiating mesorods have not formed in the surrounding areas, marked D. It can be seen that the profile view of the central disk shows a multi-layer structure, i.e., a plate sandwiched by two layers (B). The central plate (elliptical shape in a profile view marked by A), about 1.1 μm in thickness and 4 μm in diameter, likely contains only biological substances, rather than Ca-containing inorganic molecules, as judged from the dark image contrast. A similar area was examined by using EDX to be Ca poor and carbon rich (see Figure 6 below). The two adjacent layers marked by B, 2.5 μm in thickness and 15 μm in diameter, show possible CaCO
3 crystals. Outside of layer B formed another layer marked by C, in which radially-ordered traces can be seen, implying that nanocrystallites in this layer have self-orientated, probably guided by the central disk. Outside of layer C, in the regions marked by D, there are many parallel, short, needle-like particles lying along a direction which seems to have no special relation with the mesorods in the central area. This area seems to be less affected by the central disk.
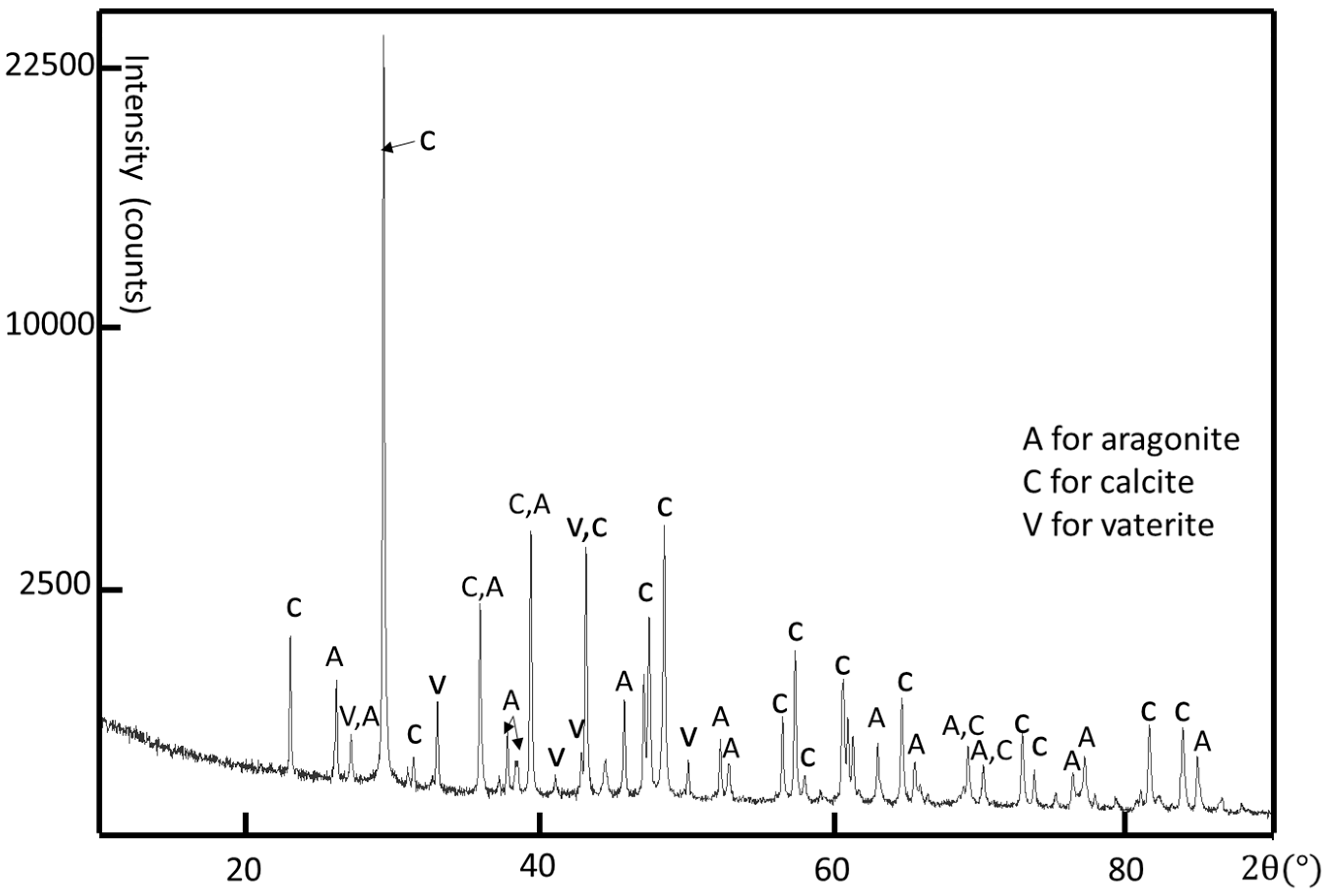
Although the crystal structure of individual crystallites can be determined by using selected area electron diffraction (SAED) patterns and high-resolution transmission electron microscopy (HRTEM) images, powder X-ray diffraction (XRD) is used to determine all possible crystalline phases in the whole shell. A limpet shell was ground into powder for XRD analysis. According to the XRD result shown in
Figure 3, all three common phases of calcium carbonate, i.e., calcite, aragonite, and vaterite, are detected. The major crystalline phases are calcite and aragonite. The calcite phase has a rhombohedral structure with
a = 4.98 Å,
c = 17.07 Å, space group R-3c (JCPDS card no. 01-080-9776) and the aragonite phase is orthorhombic with
a = 4.97 Å,
b = 7.96 Å,
c = 5.75 Å, space group Pmcn (JCPDS card no. 01-075-9984). There are some small peaks confirming the existence of hexagonal vaterite as a minor phase in the limpet shell with the unit cell parameters
a = 4.13 Å,
c = 8.49 Å, space group P63/mmc (JCPDS card no. 24-0030 [
43]).
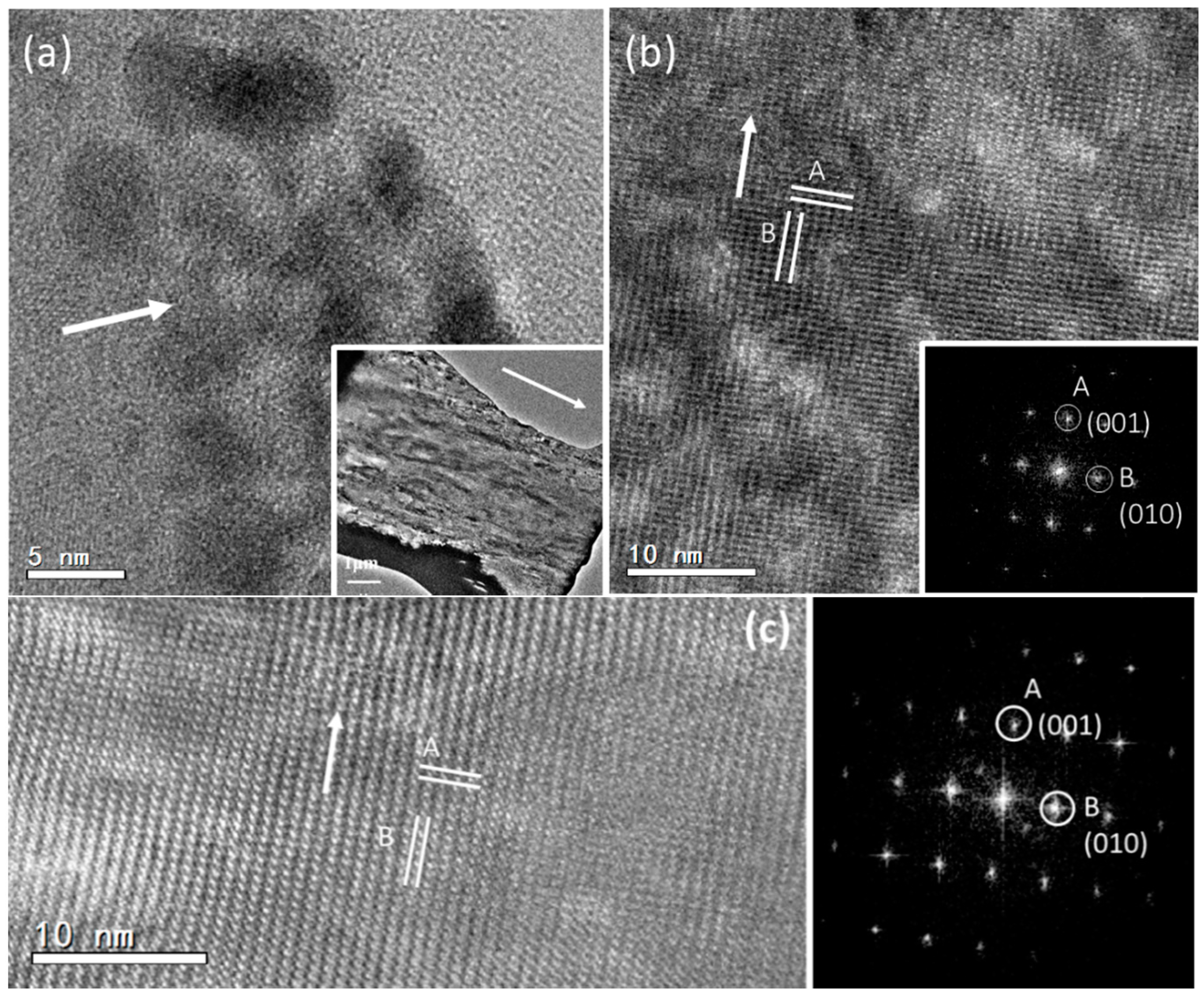
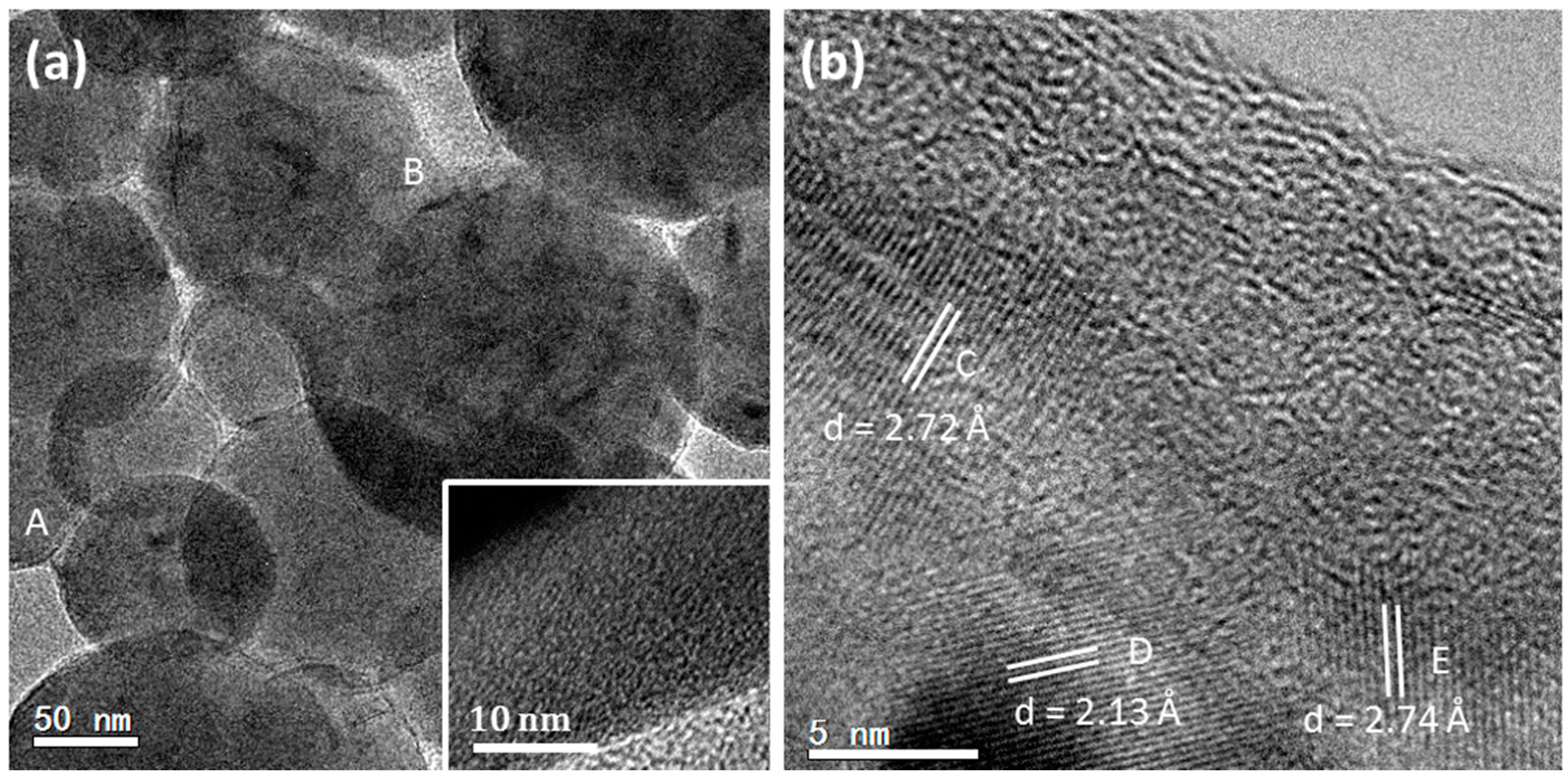
To explore the structure of the mesorods in the spherulites, a focused ion beam (FIB) technique was used to prepare samples of mesorods for the HRTEM study. A slice of a mesorod 200 nm in thickness and 15 μm in length along the long axis was cut out. Differing from the expected high crystallinity and uniform thickness, low-magnification transmission electron microscopy (TEM) images of the mesorod show a flocculent image contrast pattern, implying a large number of defects (Inset of
Figure 4a). HRTEM images reveal that the surface area of the mesorod has a poor crystallinity, containing many nanocrystallites embedded in an amorphous matrix (
Figure 4a). These nanocrystallites are randomly orientated.
In an inner area shown in
Figure 4b, nanocrystallites are also recognized. However, they are all self-orientated and connected. The corresponding FFT pattern looks like a type of single crystal. Moving towards the centre of the mesorod, high crystallinity regions can be found, as shown in
Figure 4c. The crystal orientations of the regions in
Figure 4b,c are the same. A SAED pattern, recorded from a large area of this sample covering both polycrystalline region and high crystallinity region, is similar to the FFT patterns (
Figure S1 in Supplementary Information). The two principal
d-spacings are measured from the HRTEM images in
Figure 4b,c,
dA = 5.73 Å and
dB = 7.95 Å, with an interplane angle of 90°, which can be indexed to the (001) and (010) planes of the aragonite structure. It is also confirmed that the longitudinal axis of the mesorod is parallel to the [001] zone axis of aragonite. According to the space group Pmcn of aragonite, the (001) and (010) diffraction spots should be systematically absent. Their appearance can be attributed to multiple scattering. However, lattice distortion lowering the symmetry of the aragonite structure cannot be ruled out.
Some spherulite particles (as shown in
Figure 5) are elliptical with a relatively smooth surface (denser), but without a visible notch. To gain more information of their inner structures, it is essential to have an insight of the cross-section. The particles were cut into halves and the newly-exposed surfaces were polished. A cross-section SEM image of a typical spherulite is shown in
Figure 5a. The spherulite is not a perfect spherical particle. The notch in the middle can still be recognized, although it is difficult to see from outside. The notch extends through the spherulite and separates the spherulite into symmetrical hemispheres. Along the notch, the particle becomes “thin” with a waist and looks like a short dumbbell. On the other hand, the spherulite shown in
Figure 5b has no obvious notch at all, although a similar dumbbell-like core is maintained.
The hierarchical structure of the spherulites can be described as multi-layer spherical particles and over 10 distinguished layers can be detected in each spherulite (
Figure 5a,b). One micrometre-wide low-density gaps are presented in between the layers (
Figure 5c). The layer thickness gets smaller when the layer is closer to the core.
These layers are formed with mesorods, which are all in a radial arrangement. The diameter of the mesorods also decreases when they are closer to the core (
Figure 5c). The enlarged SEM image of individual mesorods show that the mesorods in inner layers do not have a regular hexagonal morphology, as we observed from the mesorods in an outer layer (see
Figure 2b). The relatively thin mesorods seem to have a polymer-like coating layer, referring to the morphology, which likely consists of biological substances. There is also a gap between the mesorods, making the layer highly porous.
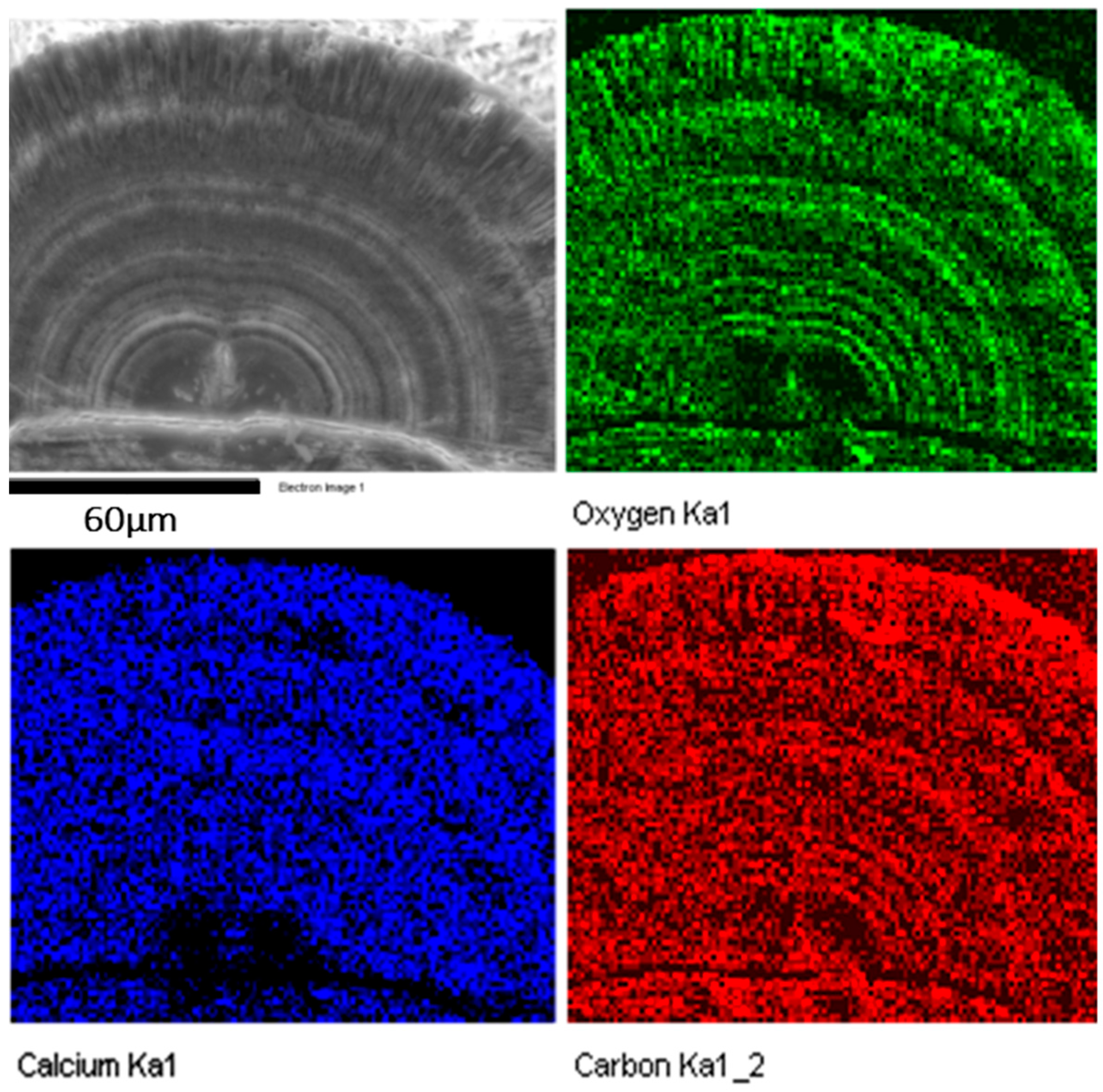
The core structure must be crucial in constructing the microstructures of spherulites. Most particles have a double-hemisphere core. The first image in
Figure 6 is a SEM image of a half spherulite. The image contrast in the two hemispheres separated by a double-layer disk particle is significantly darker than other parts, implying that these areas contain light elements or have a low density. Elemental mapping shows that the carbon map is brighter than calcium and oxygen, and the Ca and O concentrations are very low in these double-hemispherical areas in the core, further indicating that these areas contain less CaCO
3, but rich in organic materials (
Figure 6). This structure is quite different from that in
Figure 2d, where only one biomass rich area is present, i.e., the central plate inside the double-layer disk.
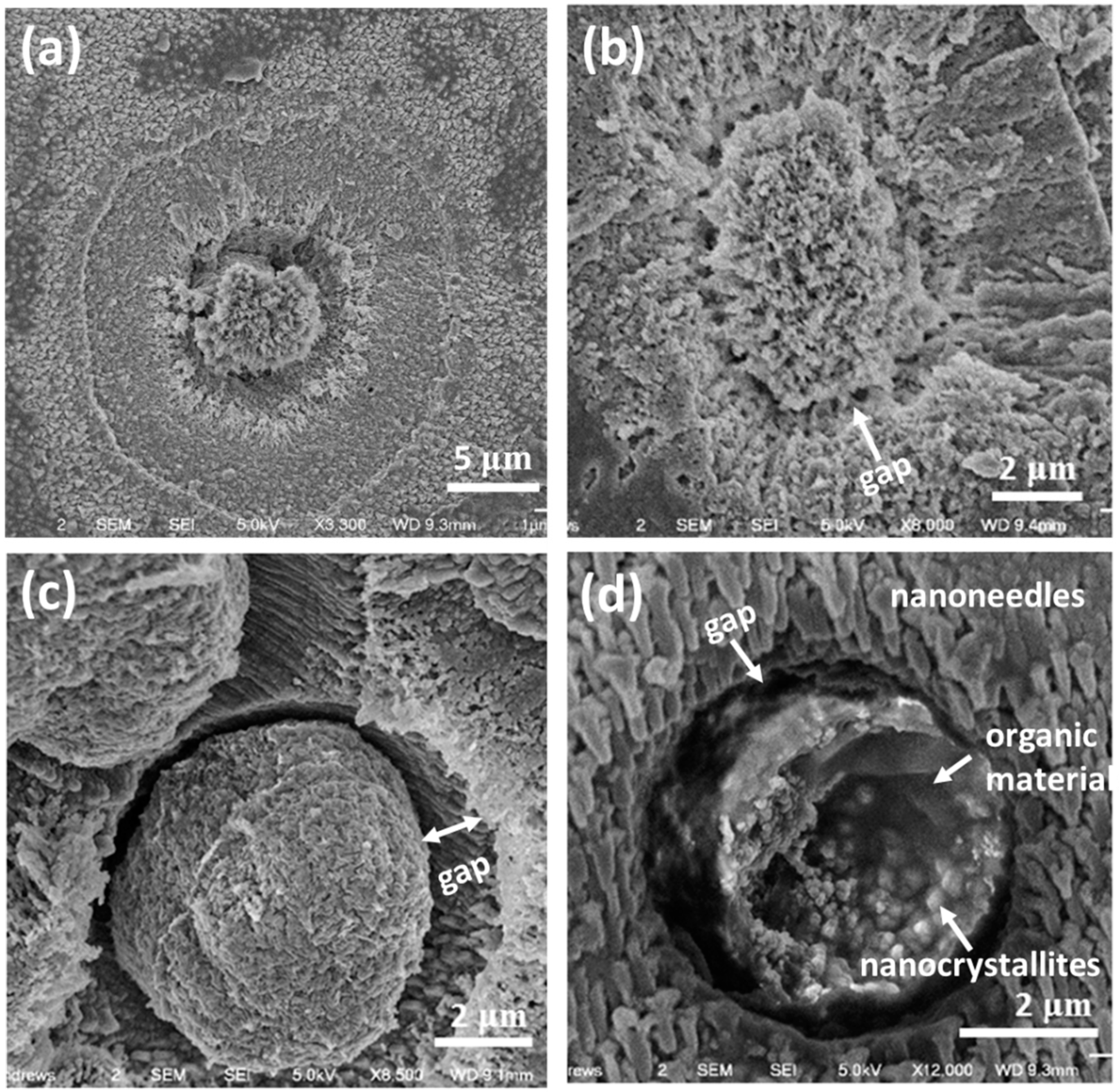
It is believed that the core particles control the morphology and hierarchical structures of the spherulites. To fully understand the formation of the cores, it is useful to look at the cores in early ages when they are small and have a low crystallinity. The particles shown in
Figure 7 are probably some examples of these core particles and surroundings. Such an assumption has to be made since we are unable to observe a real growth of a spherulite with time. Assuming
Figure 7a shows a SEM image of the cross-section of an early stage spherical particle, it is noticed that before the formation of the mesorods, a small core of about 5 μm in diameter is formed. The core is separated from the surroundings, where many nanocrystallites are detected.
Figure 7b shows probably another core particle with a low density. The core with a rough surface looks like a sea urchin. The core particle in
Figure 7c is larger, and denser. The surface is smoother than the cores in
Figure 7a,b, and the gap of about 1 μm between the core and the surroundings is obvious. In
Figure 7d, the broken core has similar features. However, it can be seen that many nanoparticles are embedded in a matrix, which shows a gel-like dark and smooth contrast. On the other hand, the surroundings is consist of many parallel short nanoneedle particles (~1 μm long and 0.4 μm wide).
Some even smaller spherical particles were also detected by TEM and their crystallinity was examined by HRTEM. These samples were quite unstable under electron beam irradiation. This is understandable because the crystallites were embedded in an organic matrix. However, if proper specimen treatments and microscopic operation for a low-dose irradiation are performed, HRTEM images of crystal structures of many beam-sensitive samples can still be possible [
44]. The TEM image in
Figure 8a shows some spherical particles (50 to 200 nm in diameter) found in the M layer in a limpet shell. A closer examination of the particles revealed that most parts of these particles are amorphous (see the inset of
Figure 8a), which could be a mixture of ACC and biological components. However, many randomly-orientated nanocrystallites in the amorphous matrix are also detected in some other regions.
Figure 8b shows lattice fringes of some nanocrystallites. Two kinds of
d-spacings of these nanocrystallites, 2.133 Å and 2.733 Å, were measured and can be indexed to the (004) and (102) planes of vaterite. Similar results were reported in biominerals of earthworm’s calciferous gland by Gago-Duport et al. [
45], where vaterite nanocrystallites were developed in ACC particles.
The short nanoneedle particles in the surroundings near the cores (see
Figure 7d) are monocrystalline with only a very thin amorphous coating.
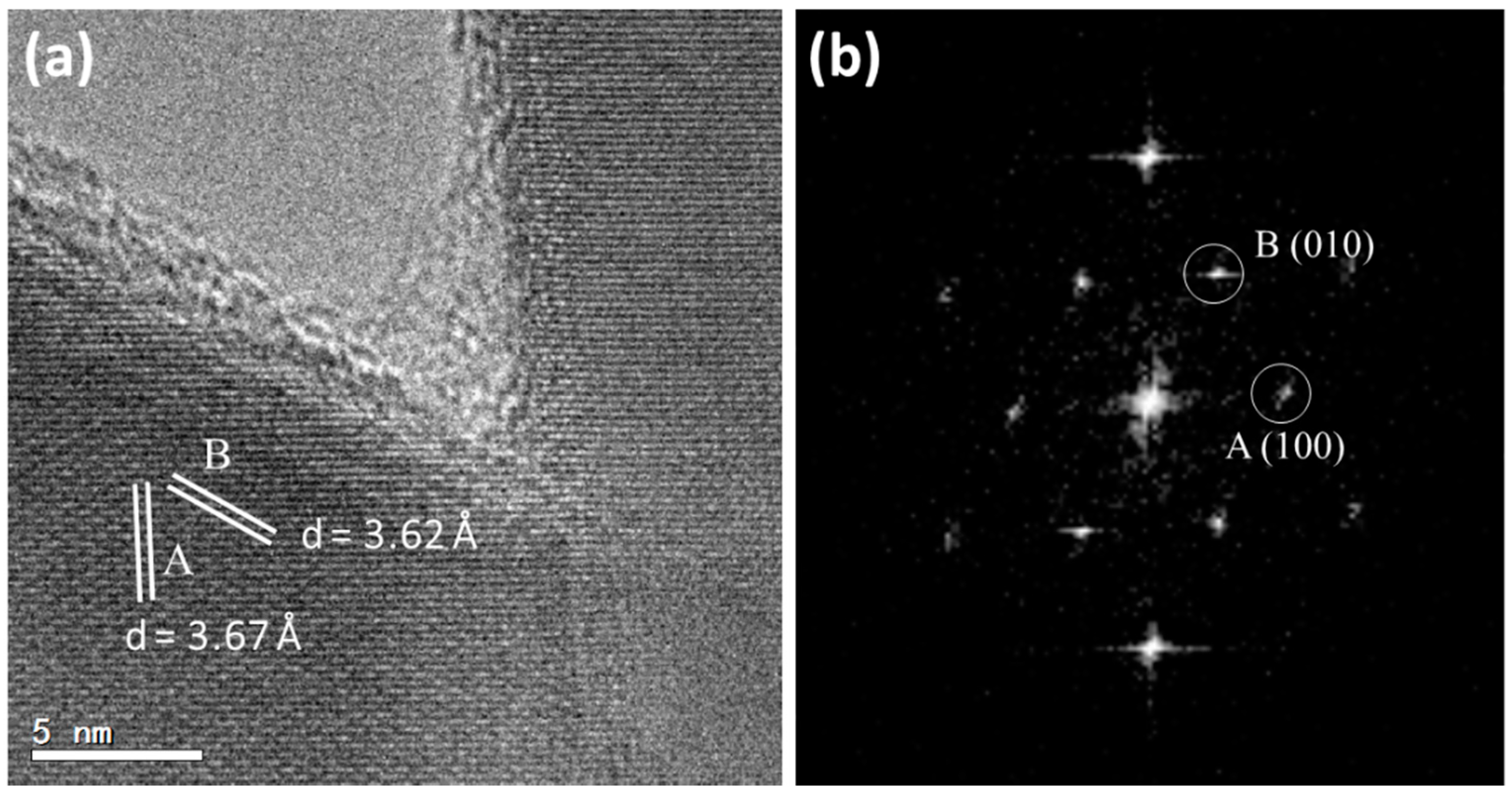
Figure 9 is an HRTEM image of two cross-linked nanoneedles and the corresponding FFT pattern. The measured
d-spacings,
dA = 3.67 Å and
dB = 3.62 Å with an interplane angle of 60°, can be indexed to the (100) and (010) planes of the hexagonal vaterite structure.
3. Discussion
Although a large number of SEM and HRTEM images of the spherulites, growing in limpet shells, have been obtained and analysed, the growth stages of these spherulites cannot be recognized undoubtedly. Drawing an evolution of the spherulites step-by-step as a function of time is difficult. However, a complete series of specimens during spherulite growth via biomimetic synthesis can be easily achieved. These results may help us to understand the time dependant formation of spherulites in a natural environment if the conditions of the latter can be simulated reasonably well.
Vaterite-type CaCO
3 spherulites have been biomimetically synthesized using gelatine as a biological substance [
37]. The selection of gelatine is because it has an isoelectric point in a range of 4.7 to 5.2 [
46], and has similar functions as a base of CaCO
3 deposition with the extracellular polymeric substance (EPS) matrix in biofilms commonly found in the environments for biomineralization. In an aqueous synthetic system containing gelatine (type B), Ca(NO
3)
2·4H
2O, and urea, the growth of CaCO
3 and morphology evolution underwent several distinguished steps: Step 1, the formation of vaterite nanocrystallites, ~5 nm in diameter, embedded in a disordered gelatine matrix. Step 2, aggregation of the nanocrystallites into large spherical particles. Step 3, dipole field driven self-orientation of the nanocrystallites and the development of nanoneedles on a spherical core in a radial arrangement, forming spherulites. Step 4, development of a double-layer disk, i.e., two CaCO
3 plates sandwiching a gelatine layer. This disk supplies a relatively strong and mirror symmetric dipole field, guiding the orientations of all the nanocrystallites, leading to formations of an equatorial notch and “twin-cauliflower”-shaped particles [
37]. It is obvious that, with the increase of growth time, the particle size and ordering of the nanocrystallites increase.
Many structural features observed from the spherulites in the limpet shells are similar with those in the synthetic spherulites [
37], particularly the formation of the double-layer disks. We, therefore, assume that the principles of the formation of these spherulites in both biomineralization and biomimetic synthesis are similar. Dipole field interaction between the crystals is believed to be the most important factor governing the formation of the spherulites.
It has been known that hexagonal vaterite has a permanent dipole moment along the
c-axis. The (001) surface is terminated with a Ca
2+ layer and is positively charged, while the (00
) surface is negatively charged with the top surface layer only containing CO
32− anions. If the distance between two isoelectric terminals is
d, the dipole moment
p can be calculated in Debye units:
where
qi is the charging magnitude and
di is the distance of which the
ith anion/cation held. Forces in a dipole field have correlation with distances and the charging level. For a dipole field, if a small particle is located at
r+ and
r− from positive and negative charges of the dipole, the electric potential
V(
r) is:
where π is a constant, and
is the vacuum permittivity.
If this particle is far enough from the dipole, i.e.,
r2 ≈
r+r− (
r is the distance from the particle to the midpoint of a dipole.
θ is the angle between the dipole and the direction from the midpoint of the dipole to the particle), the potential can be written as:
In the dipole field, we have:
where E(
r) is the intensity of the dipole field at position r [
47]. It can be seen from this equation that the field strength increases remarkably when
r decreases, especially to the nanometer scale.
Based on the above discussion, we can now propose a multi-step formation mechanism for the naturally-occurring spherulites as presented in
Figure 10.
Step 1: The biofilms with extracellular polymeric substance (EPS) in a gap of the M layer in the limpet shell are normally negatively charged and have a power to attract Ca
2+ cations first, followed by attracting CO
32− anions, forming an inorganic/organic composite. When the concentration of the inorganic component is high, some spherical particles (50 to 200 nm in diameter) form, separating from the biofilms. Vaterite nanocrystallites develop from amorphous calcium carbonate (ACC) inside these spheres (
Figure 10a). A typical experimental image of these spherical particles is shown in
Figure 8.
Step 2: When the spheres increase their size and vaterite nanocrystallites grow further, an increase of the density of the central area is faster than the surroundings, and a core particle is separated from the surroundings (
Figure 10b), as seen in
Figure 7a.
Step 3: The vaterite nanocrystallites grow into short nanoneedles. A dipole field is created from each nanoneedle when it forms a regular shape. An interaction between these nanoneedles force them to self-orientate into a parallel arrangement inside the spherical particles (
Figure 10c). The corresponding SEM image is shown in
Figure 7d.
Step 4: The core spherical particle undergoes surface re-crystallization into a double-layer disk sandwiching a biological sheet. The formation of such a double-layer disk via an inorganic/organic phase separation was observed in the biomimetic synthesis of CaCO
3 in the presence of gelatin [
37], and in mirror symmetric growth of ZnO nanoneedles/plates [
48,
49]. It is also regarded as a good example of two-dimensional reversed crystal growth, a non-classical crystal growth route demonstrating a crystal growth direction from a particle surface to its core [
50,
51,
52]. More importantly, the central biological sheet is normally negatively charged. Therefore, the inner surfaces of both vaterite layers are positively charged and the outer surfaces must be negatively charged. Such a structure offers a strong dipole field, which will guide all the nanocrystallites to adjust their orientations along the field force lines (
Figure 10d). The corresponding experimental observation is shown in
Figure 2d.
Step 5: When all the nanocrystallites line up into nanoneedles and, later, mesorods, and all these 1D particles lie along the dipole field force lines created from the central double-layer disk, a spherulite forms with two hemispheres separated by a notch where the field strength is the weakest (
Figure 10e), as seen in SEM images of
Figure 2a,c. Both the spaces between the mesorods and between the hemispherical layers are filled by biological substances. During the above-mentioned process, a phase transformation from vaterite to aragonite takes place. However, the calcite phase was not detected from spherulites, which is the main crystalline phase in other parts of the limpet shells. Another interesting phenomenon is that, although the particles are divided into two hemispheres connected only by a central core, the number of the mesorod layers developed in both hemispheres are the same and the thicknesses of any face-to-face corresponding mesorod layers are uniform (
Figure 5a). The growth is, therefore, mirror symmetric.
Step 6: Finally, as depicted in
Figure 10f, the density of the spherulites increases. The corresponding mesorod layers in the two hemispheres join together across the notch, which is not visible on the outer surface (
Figure 5b). All the mesorod layers in the hemispheres can match the opposite ones and perfectly connect each other. On the other hand, on the both sides of the core disk, further phase separation leads to two hemispherical spaces filled by biological substances, as shown by two dark areas in the core in
Figure 5a,b, as well as
Figure 6.
Nevertheless, the mechanism presented in
Figure 10 is still an assumption, rather than being established unambiguously. Future research is needed to prove all the steps in this mechanism.